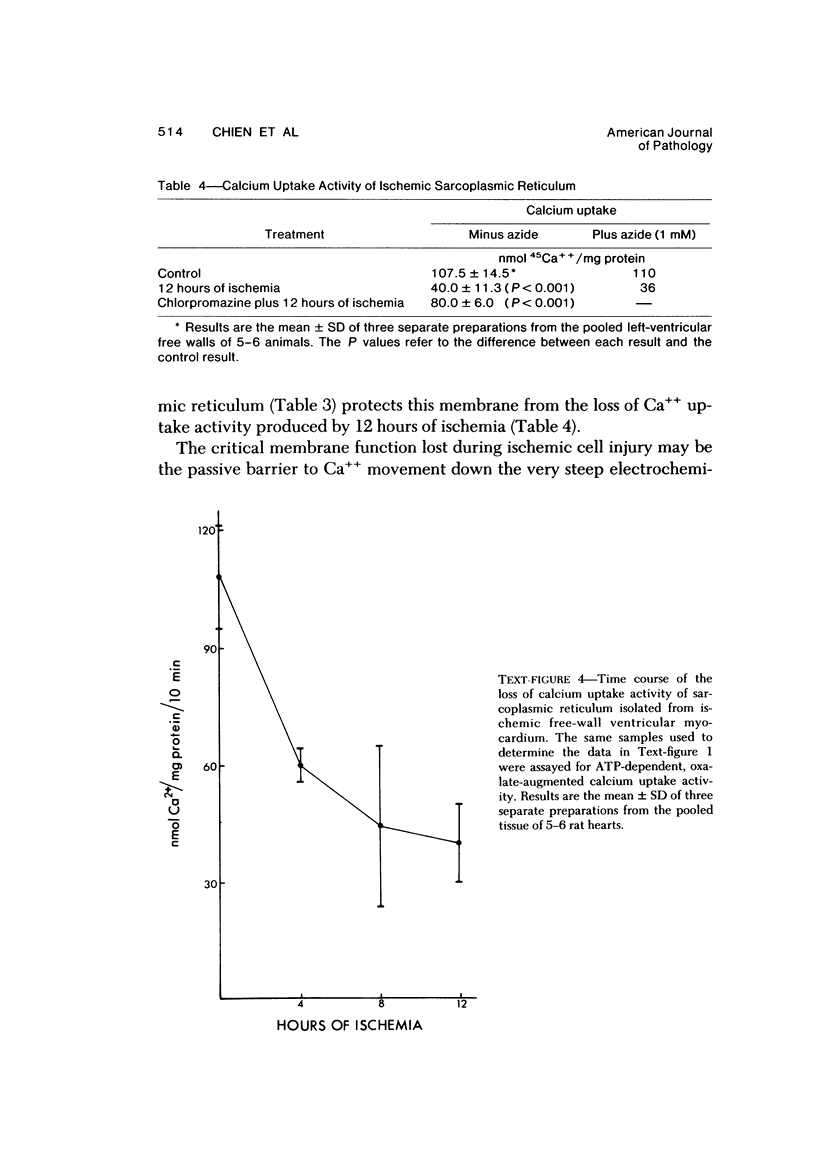
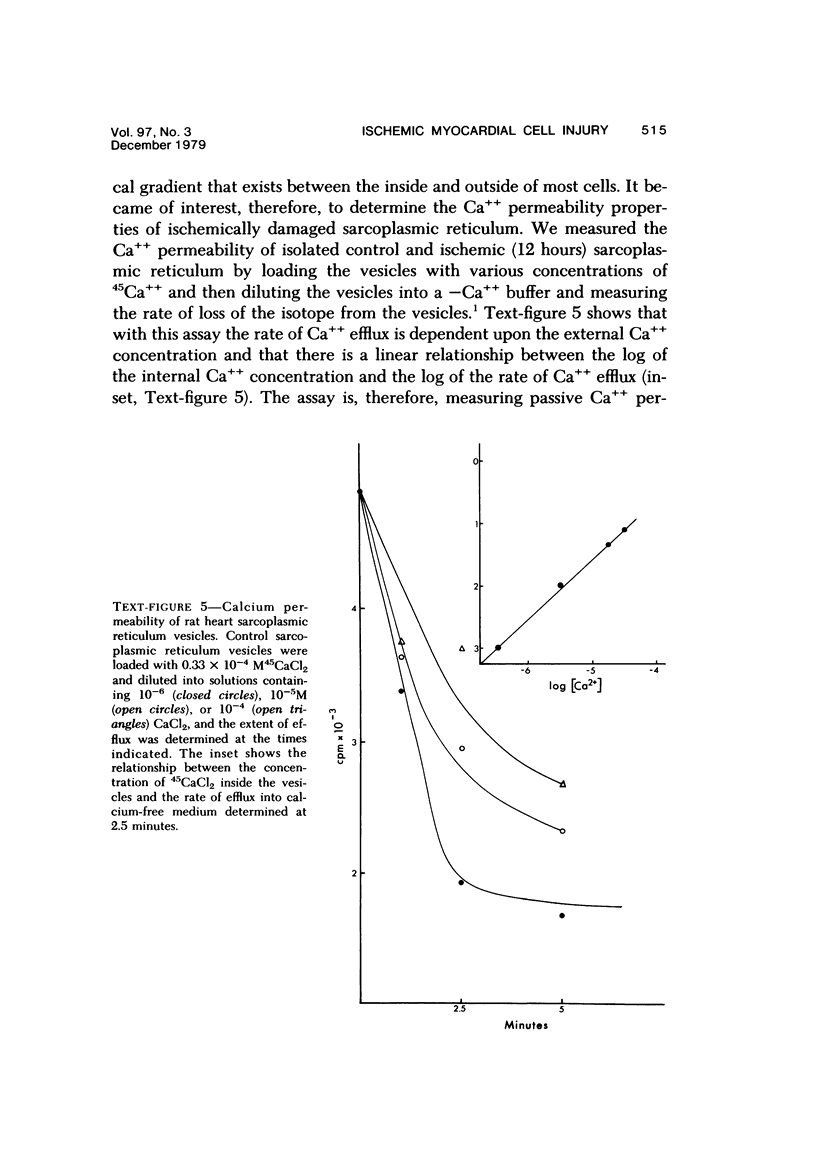
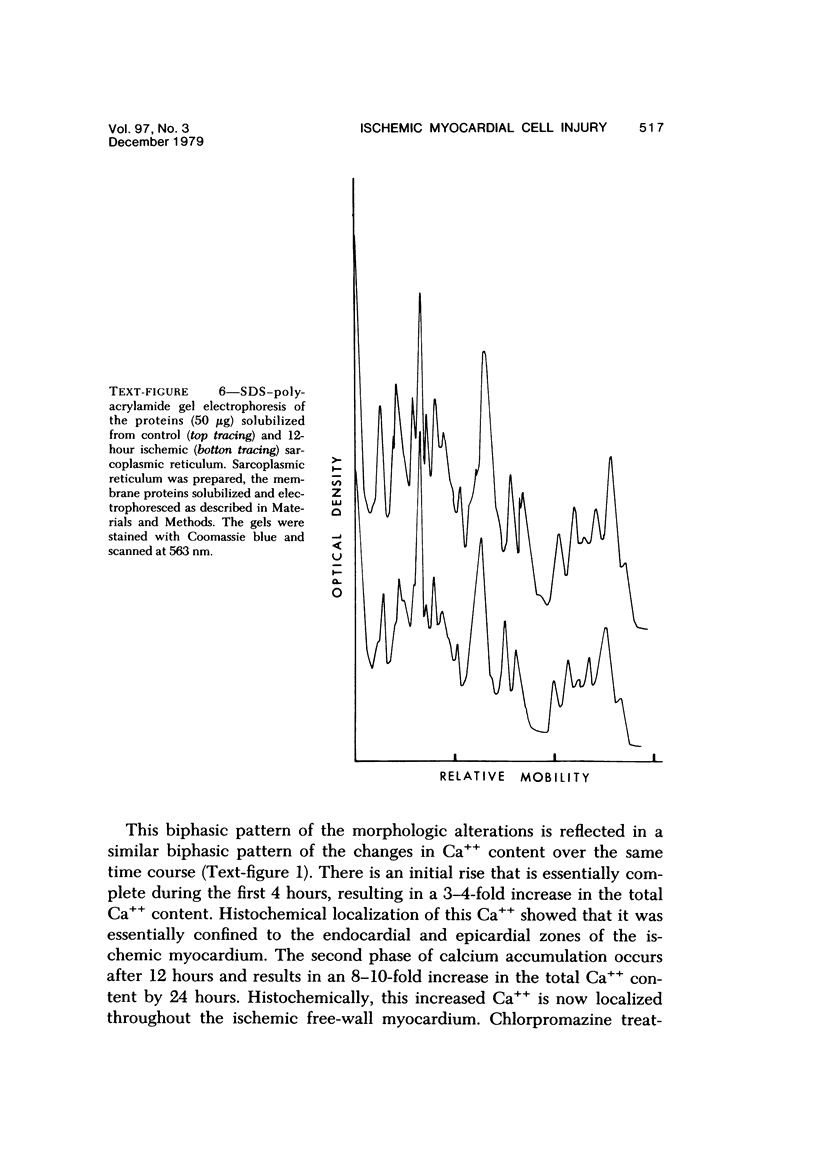
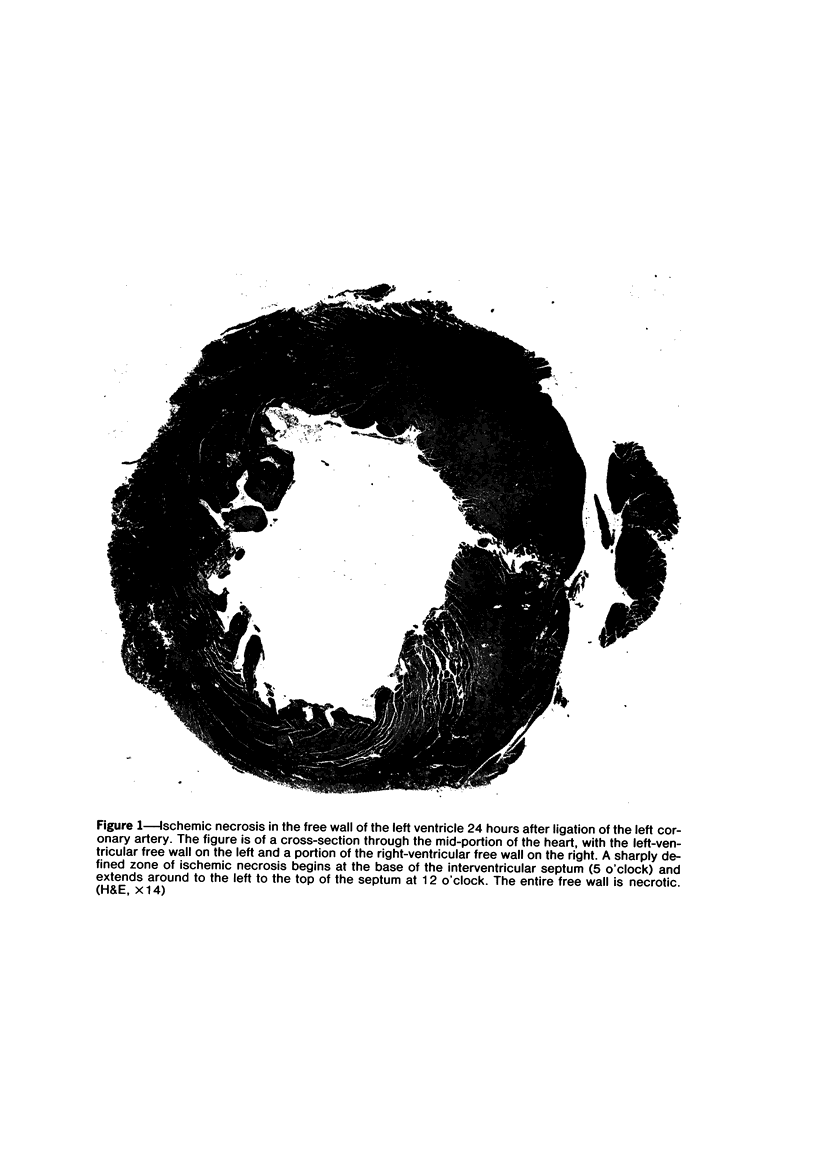
Abstract
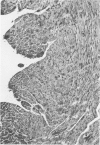
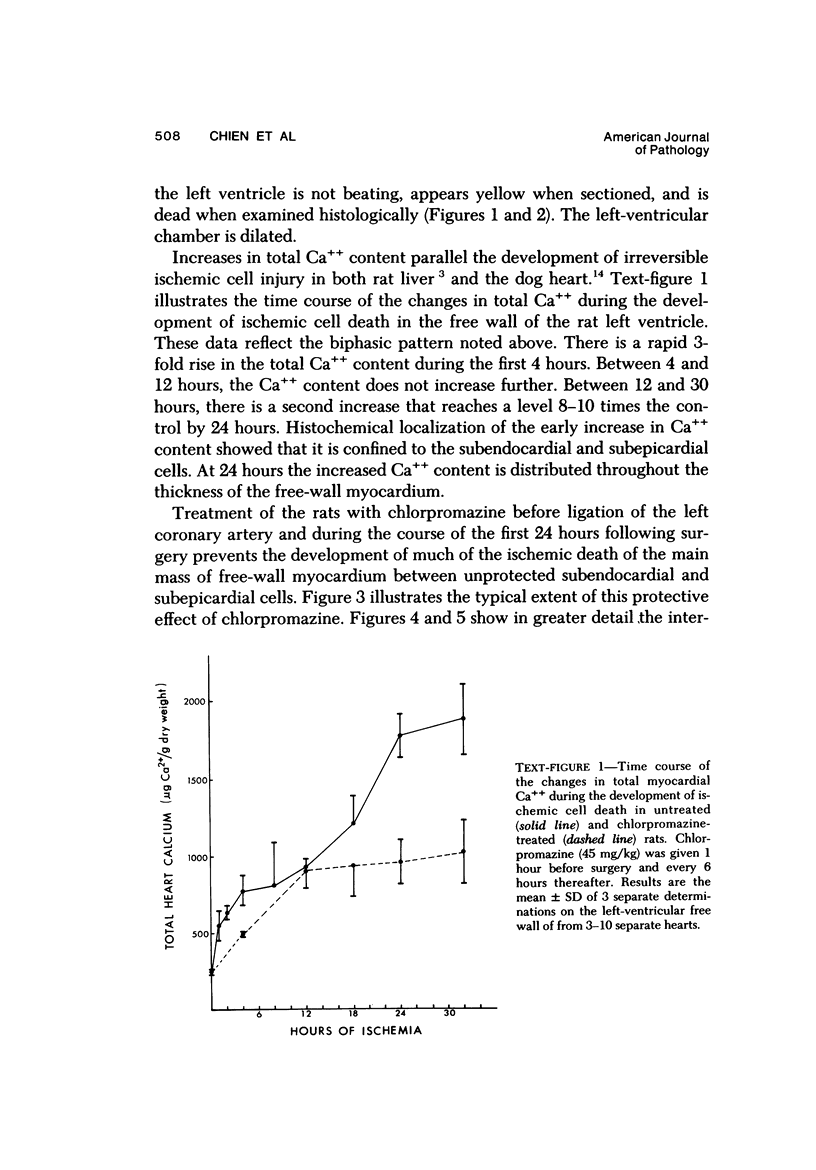
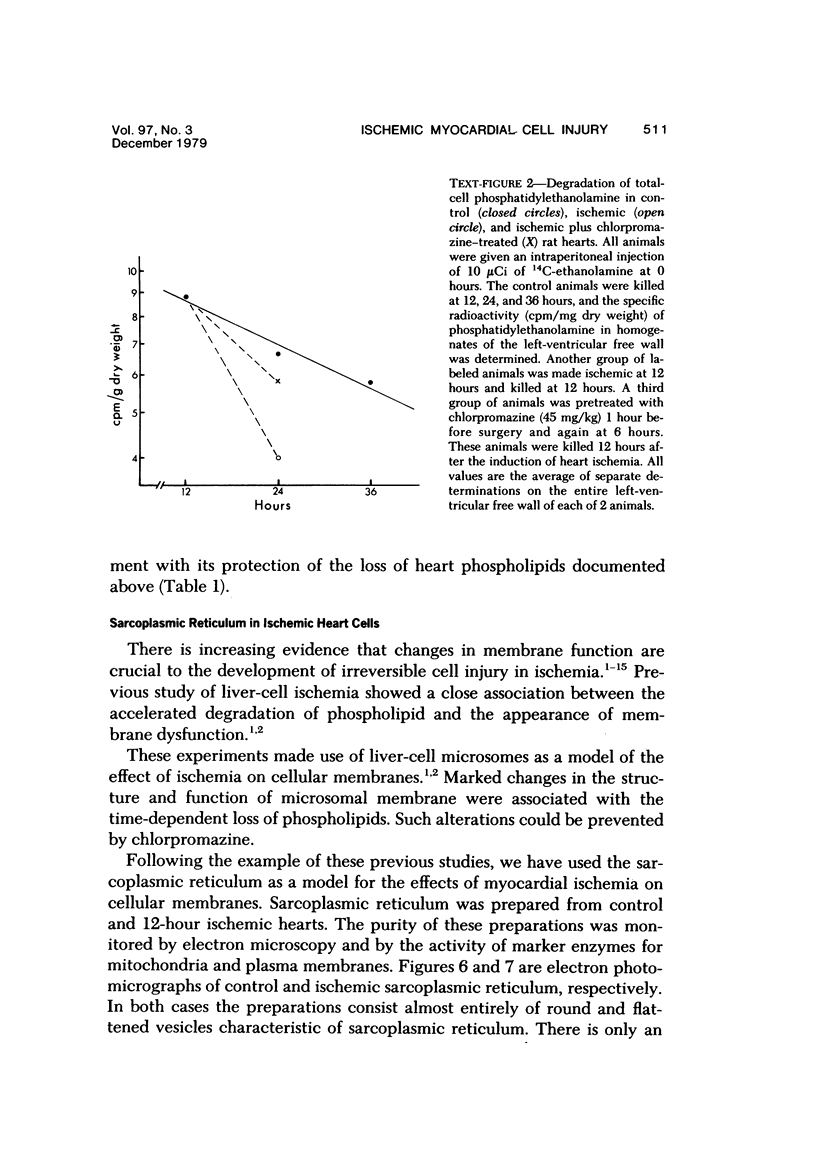
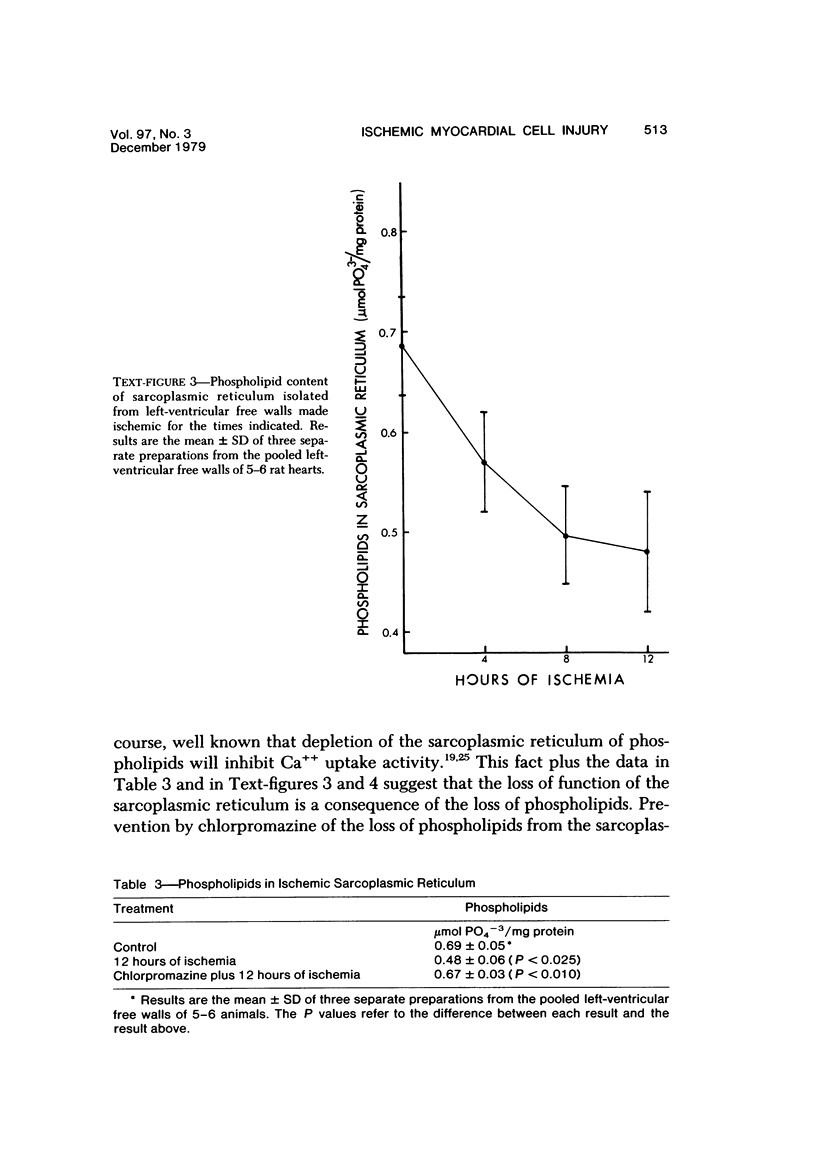
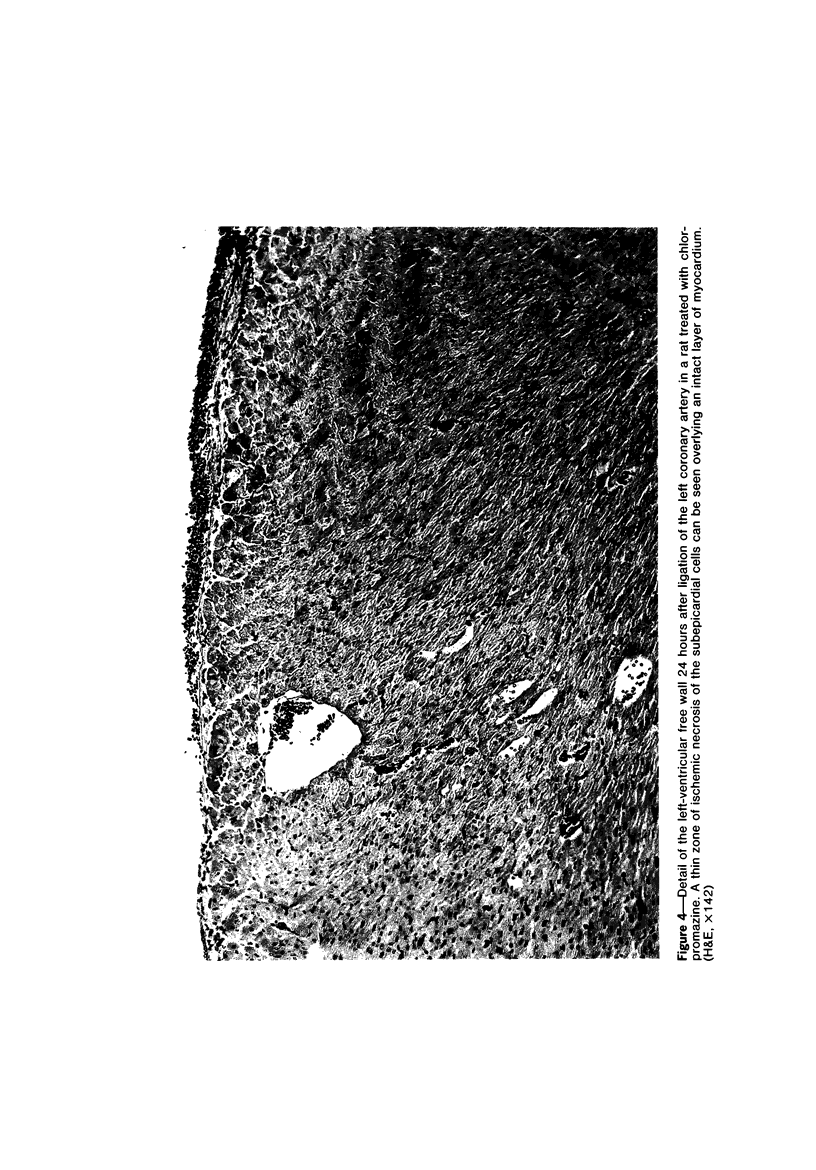
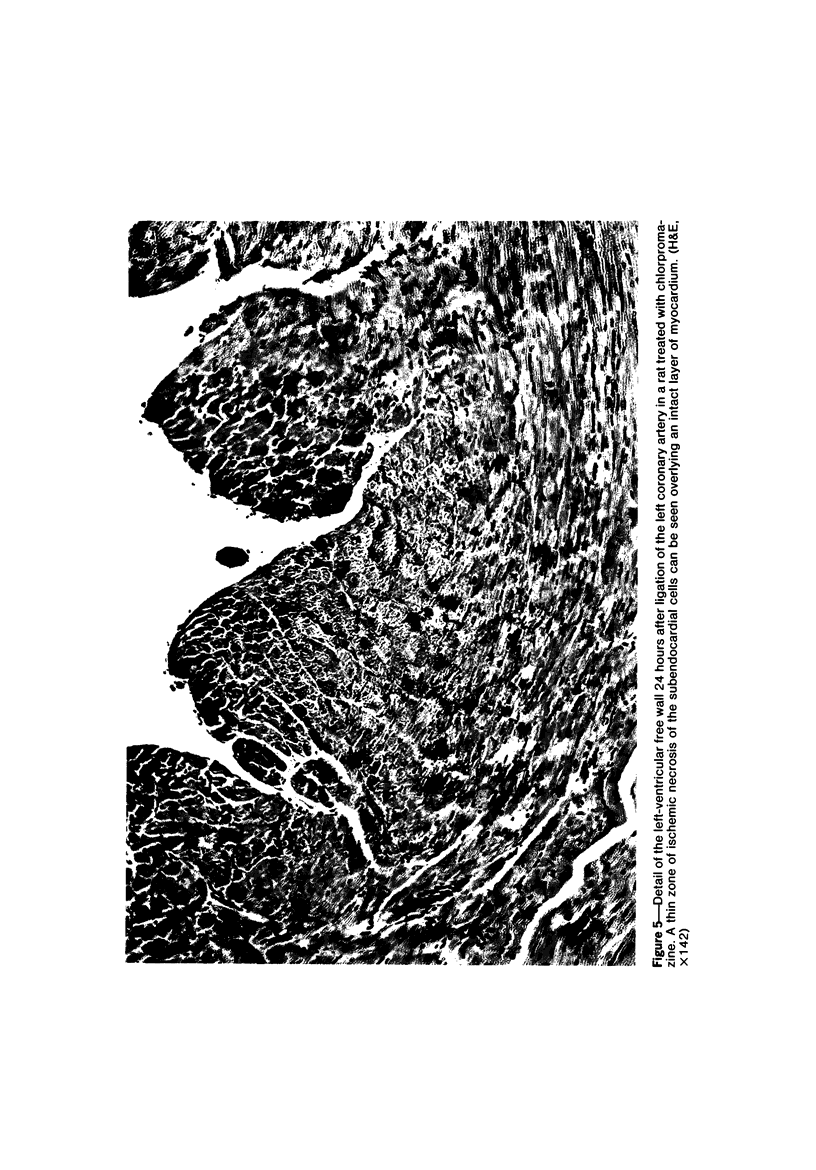
Ligation of the left coronary artery of an adult rat heart results in the reproducible ischemic cell death of the entire free wall of the left ventricular myocardium. The time course of the development of the cellular changes is biphasic. The subendocardial and subepicardial cells die within the first few hours. The main mass of free-wall myocardium reacts more slowly, with morphologic evidence of irreversible cell injury developing after 12 hours. Measurement of the increases in total free wall Ca++ reflected this biphasic pattern. There was a rapid 3-fold rise in total Ca++ during the first 4 hours. Between 4 and 12 hours the Ca++ was constant. Between 12 and 30 hours there was a second increase that reached a level some 8-10 times the control value. Treatment with chlorpromazine before and subsequent to surgery prevented the appearance of ischemic cell death in the main portion of the free-wall myocardium for at least 24 hours without affecting the reaction of the subepicardial and subendocardial cells. Chlorpromazine also inhibited the second phase of Ca++ accumulation. An accelerated degradation of phospholipids was observed with a 33% decrease in total phospholipids by 12 hours. Phosphatidylethanolamine was reduced by 50% and phosphatidylcholine by 25% without increases in the corresponding lysophospholipids. Chlorpromazine prevented the accelerated degradation and consequent loss of phospholipid. Isolated sarcoplasmic reticulum showed a time-dependent loss of phospholipid with a parallel loss of active Ca++ uptake that reach 60% with a total lipid depletion from these membranes of 33% by 12 hours. Twelve-hour ischemic sarcoplasmic reticulum exhibited a 6--7-fold increase in passive permeability to Ca++. Chlorpromazine protected against the loss of phospholipids, the inhibition of Ca++ uptake, and the increased Ca++ permeability of the sarcoplasmic reticulum. These observations indicate that rat myocardial cells react to lethal doses of ischemia in a manner similar to the reaction of liver cells described previously. In both cases the evidence implies that a disturbance in phospholipid metabolism and its associated membrane dysfunction is the critical alteration that produces irreversible cell injury in ischemia.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashraf M., Halverson C. A. Structural changes in the freeze-fractured sarcolemma of ischemic myocardium. Am J Pathol. 1977 Sep;88(3):583–594. [PMC free article] [PubMed] [Google Scholar]
- Beller G. A., Conroy J., Smith T. W. Ischemia-induced alterations in myocardial (Na+ + K+)-ATPase and cardiac glycoside binding. J Clin Invest. 1976 Feb;57(2):341–350. doi: 10.1172/JCI108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstad P. Phospholipase activity in rat-liver microsomes studied by the use of endogenous substrates. Biochim Biophys Acta. 1966 Jun 1;116(3):500–510. [PubMed] [Google Scholar]
- Bjørnstad P., Bremer J. In vivo studies on pathways for the biosynthesis of lecithin in the rat. J Lipid Res. 1966 Jan;7(1):38–45. [PubMed] [Google Scholar]
- Burton K. P., Hagler H. K., Templeton G. H., Willerson J. T., Buja L. M. Lanthanum probe studies of cellular pathophysiology induced by hypoxia in isolated cardiac muscle. J Clin Invest. 1977 Dec;60(6):1289–1302. doi: 10.1172/JCI108888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Farber J. L. Microsomal membrane dysfunction in ischemic rat liver cells. Arch Biochem Biophys. 1977 Apr 15;180(1):191–198. doi: 10.1016/0003-9861(77)90025-x. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., Duggan J., Hackett R. L. Plasma membrane changes in freeze-fractured rat kidney cortex following renal ischemia. Lab Invest. 1976 Jul;35(1):63–70. [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Sequential lysosomal alterations during cardiac ischemia. II. Ultrastructural and cytochemical changes. Lab Invest. 1978 Jun;38(6):662–673. [PubMed] [Google Scholar]
- El-Mofty S. K., Scrutton M. C., Serroni A., Nicolini C., Farber J. L. Early, reversible plasma membrane injury in galactosamine-induced liver cell death. Am J Pathol. 1975 Jun;79(3):579–596. [PMC free article] [PubMed] [Google Scholar]
- Fiehn W., Hasselbach W. The effect of phospholipase A on the calcium transport and the role of unsaturated fatty acids in ATPase activity of sarcoplasmic vesicles. Eur J Biochem. 1970 Apr;13(3):510–518. doi: 10.1111/j.1432-1033.1970.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978 Jan;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Frega N., Leaf A. Cell volume regulation and ischemic tissue damage. J Membr Biol. 1972 Dec 29;10(3):331–343. doi: 10.1007/BF01867864. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Weissmann G., Hirsch J., Streuli F., Fox A. C. Cytochemical localization of lysosomal enzyme activity in normal and ischemic dog myocardium. Am J Pathol. 1975 May;79(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., KALTENBACH J. P., WEST J. J. ELECTROLYTE ALTERATIONS IN ACUTE MYOCARDIAL ISCHEMIC INJURY. Circ Res. 1964 Mar;14:260–269. doi: 10.1161/01.res.14.3.260. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973 Sep;48(3):455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Shug A. L., Thomsen J. H., Folts J. D., Bittar N., Klein M. I., Koke J. R., Huth P. J. Changes in tissue levels of carnitine and other metabolites during myocardial ischemia and anoxia. Arch Biochem Biophys. 1978 Apr 15;187(1):25–33. doi: 10.1016/0003-9861(78)90003-6. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglicki W. B., Waite M., Sisson P., Shohet S. B. Myocardial phospholipase A of microsomal and mitochondrial fractions. Biochim Biophys Acta. 1971 May 4;231(3):512–519. doi: 10.1016/0005-2760(71)90119-6. [DOI] [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Whitmer J. T., Idell-Wenger J. A., Rovetto M. J., Neely J. R. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. 1978 Jun 25;253(12):4305–4309. [PubMed] [Google Scholar]
- Wildenthal K., Decker R. S., Poole A. R., Griffin E. E., Dingle J. T. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978 Jun;38(6):656–661. [PubMed] [Google Scholar]
- Wood J. M., Bush B., Pitts B. J., Schwartz A. Inhibition of bovine heart Na+, K+-ATPase by palmitylcarnitine and palmityl-CoA. Biochem Biophys Res Commun. 1977 Jan 24;74(2):677–684. doi: 10.1016/0006-291x(77)90356-4. [DOI] [PubMed] [Google Scholar]