Abstract
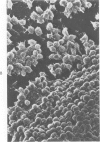
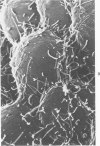
N,N-dimethylacetamide, hexamethylene bisacetamide, and Polybrene induced rapid and extensive differentiation in vitro in an otherwise slowly differentiating subline of embryonal carcinoma cells. The type of differentiated cell induced was dependent on the spatial organization of the stem cells during drug treatment. In monalayer culture "epithelial" cells were produced exclusively. However, treatment of aggregated suspension cultures yielded predominantly "fibroblast-like" cells. The undifferentiated embryonal carcinoma cells and the two differentiated cell types were morphologically distinct when examined by light microscopy, scanning electron microscopy, and transmission electron microscopy; and they had differences in cell surface antigens. Both differential cell types produced large amounts of fibronectin, whereas the embryonal carcinoma cells produced only minimal amounts. This system provides a convenient way to induce relatively synchronous differentiation of embryonal carcinoma cells into specific differentiated cell types.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdwell C. R., Strauss J. H. Replication of Sindbis virus. IV. Electron microscope study of the insertion of viral glycoproteins into the surface of infected chick cells. J Virol. 1974 Aug;14(2):366–374. doi: 10.1128/jvi.14.2.366-374.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974 Oct 1;140(4):1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A. E., Estes L. E., Shinozuka H., Braginski J., Lorz C., Chung C. A. Morphological and biochemical observations on cells derived from the in vitro differentiation of the embryonal carcinoma cell line PCC4-F. Cancer Res. 1977 Jul;37(7 Pt 1):2072–2081. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Evans M. J. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol. 1972 Aug;28(1):163–176. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- Greenwalt T. J., Steane E. A. Quantitative haemagglutination. 6. Relationship of sialic acid content of red cells and aggregation by Polybrene, protamine and poly-L-lysine. Br J Haematol. 1973 Aug;25(2):227–237. doi: 10.1111/j.1365-2141.1973.tb01734.x. [DOI] [PubMed] [Google Scholar]
- HENNIG A., MEYER-ARENDT J. R. Microscopic volume determination and probability. Lab Invest. 1963 Apr;12:460–464. [PubMed] [Google Scholar]
- Herbert M. C., Graham C. F. Cell determination and biochemical differentiation of the early mammalian embryo. Curr Top Dev Biol. 1974;8:151–178. doi: 10.1016/s0070-2153(08)60608-0. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jakob H., Dubois P., Eisen H., Jacob F. Effets de l'hexaméthylènebisacétamide sur la différenciation de cellules de carcinome embryonnaire. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jan;286(1):109–111. [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Kahan B. W., Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. J Natl Cancer Inst. 1970 May;44(5):1015–1036. [PubMed] [Google Scholar]
- Kemler R., Babinet C., Eisen H., Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Wiley L. M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977 Dec;61(2):230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- McBurney M. W. Clonal lines of teratocarcinoma cells in vitro: differentiation and cytogenetic characteristics. J Cell Physiol. 1976 Nov;89(3):441–455. doi: 10.1002/jcp.1040890310. [DOI] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. F., Dubois P., Jakob H., Gaillard J., Jacob F. Tératocarcinome de la souris: différenciation en culture d'une lignée de cellules primitives a potentialités multiples. Ann Microbiol (Paris) 1975 Jan;126(1):3–22. [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M. D., Wishnow R. M., Sato G. H. In vitro growth and differetiation of clonal populations of multipotential mouse clls derived from a transplantable testicular teratocarcinoma. J Natl Cancer Inst. 1970 May;44(5):1001–1014. [PubMed] [Google Scholar]
- Rossant J. Investigation of the determinative state of the mouse inner cell mass. II. The fate of isolated inner cell masses transferred to the oviduct. J Embryol Exp Morphol. 1975 Jul;33(4):991–1001. [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers W. C., Lehman J. M. Increased susceptibility of murine teratocarcinoma cells to Simian virus 40 and polyoma virus following treatment with 5-bromodeoxyuridine. J Cell Physiol. 1976 Jul;88(3):297–305. doi: 10.1002/jcp.1040880305. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Levy J., Terada M., Breslow R., Rifkind R. A., Marks P. A. Induction of erythroid differentiation in murine virus infected eythroleukemia cells by highly polar compounds. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1003–1006. doi: 10.1073/pnas.72.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBerg S. R., Ludwin S. K., Herman M. M., Bignami A. In vitro astrocytic differentiation from embryoid bodies of an experimental mouse testicular teratoma. Am J Pathol. 1976 Apr;83(1):197–212. [PMC free article] [PubMed] [Google Scholar]
- Wartiovaara J., Leivo I., Virtanen I., Vaheri A., Graham C. F. Appearance of fibronectin during differentiation of mouse teratocarcinoma in vitro. Nature. 1978 Mar 23;272(5651):355–356. doi: 10.1038/272355a0. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Stenman S., Vaheri A. Changes in expression of fibroblast surface antigen (SFA) during cytodifferentiation and heterokaryon formation. Differentiation. 1976 Jun 4;5(2-3):85–89. doi: 10.1111/j.1432-0436.1976.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., McNutt N. S. Current concepts. cell junctions. N Engl J Med. 1972 Mar 9;286(10):521–524. doi: 10.1056/NEJM197203092861006. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Martin G. R. Expression of a high molecular weight cell surface glycoprotein (LETS protein) by preimplantation mouse embryos and teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2324–2328. doi: 10.1073/pnas.75.5.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]