Abstract
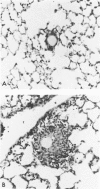
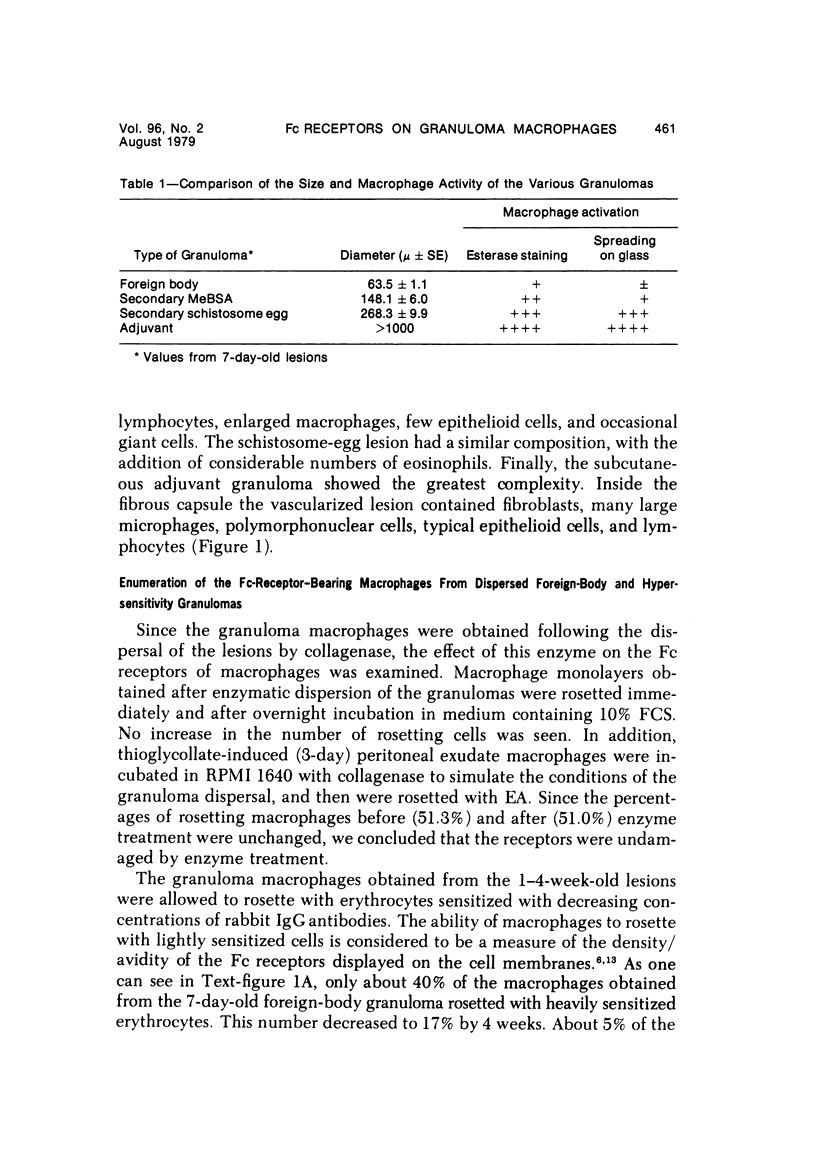
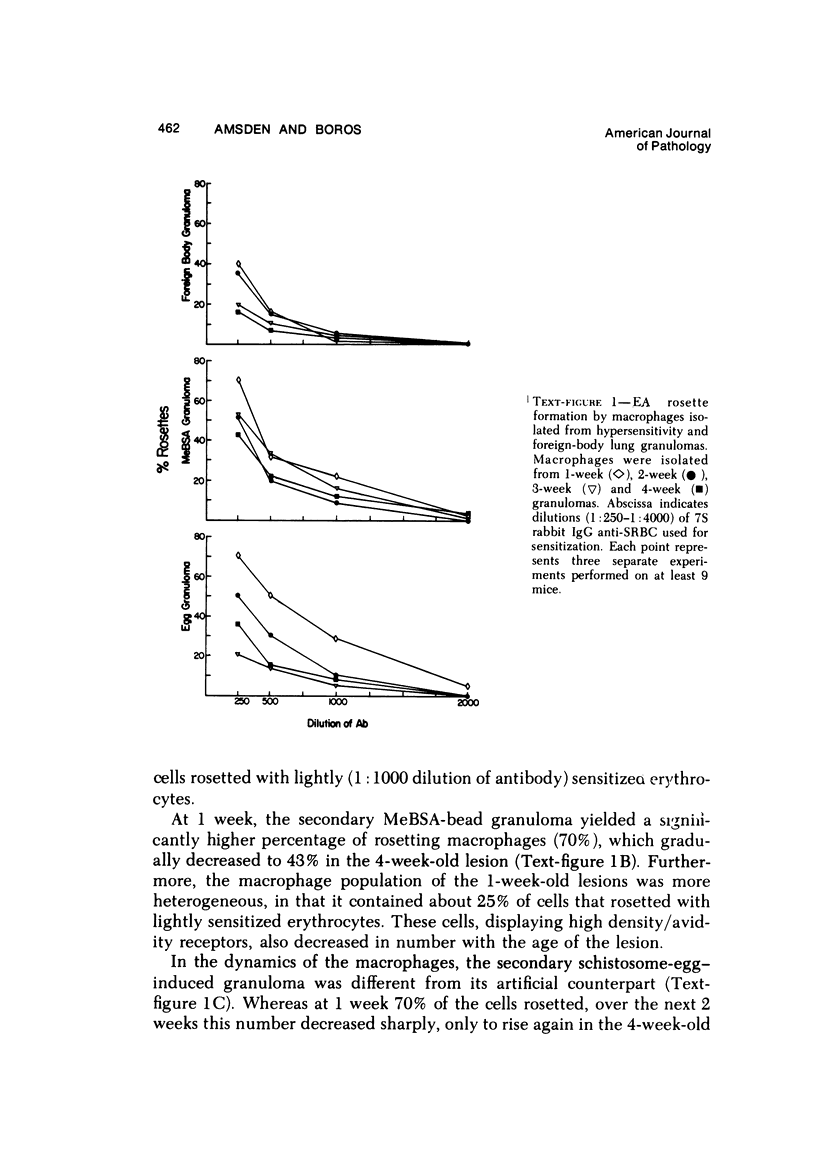
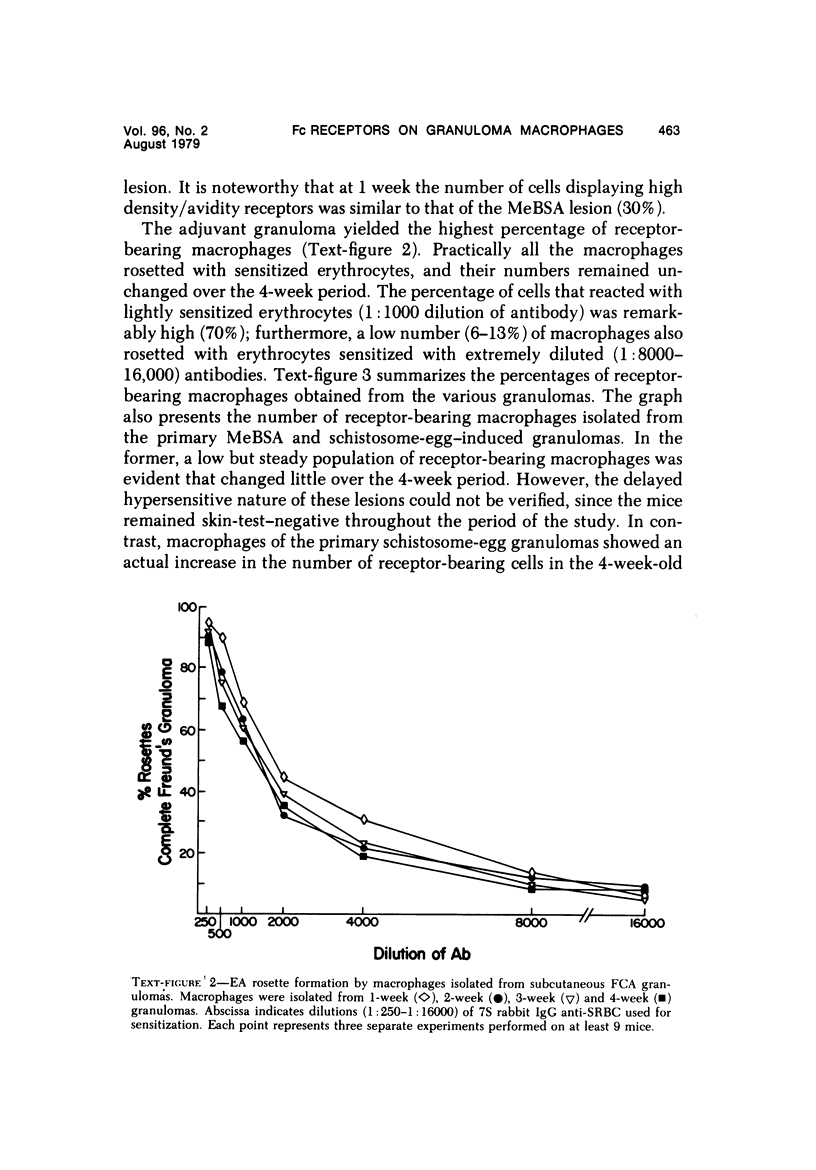
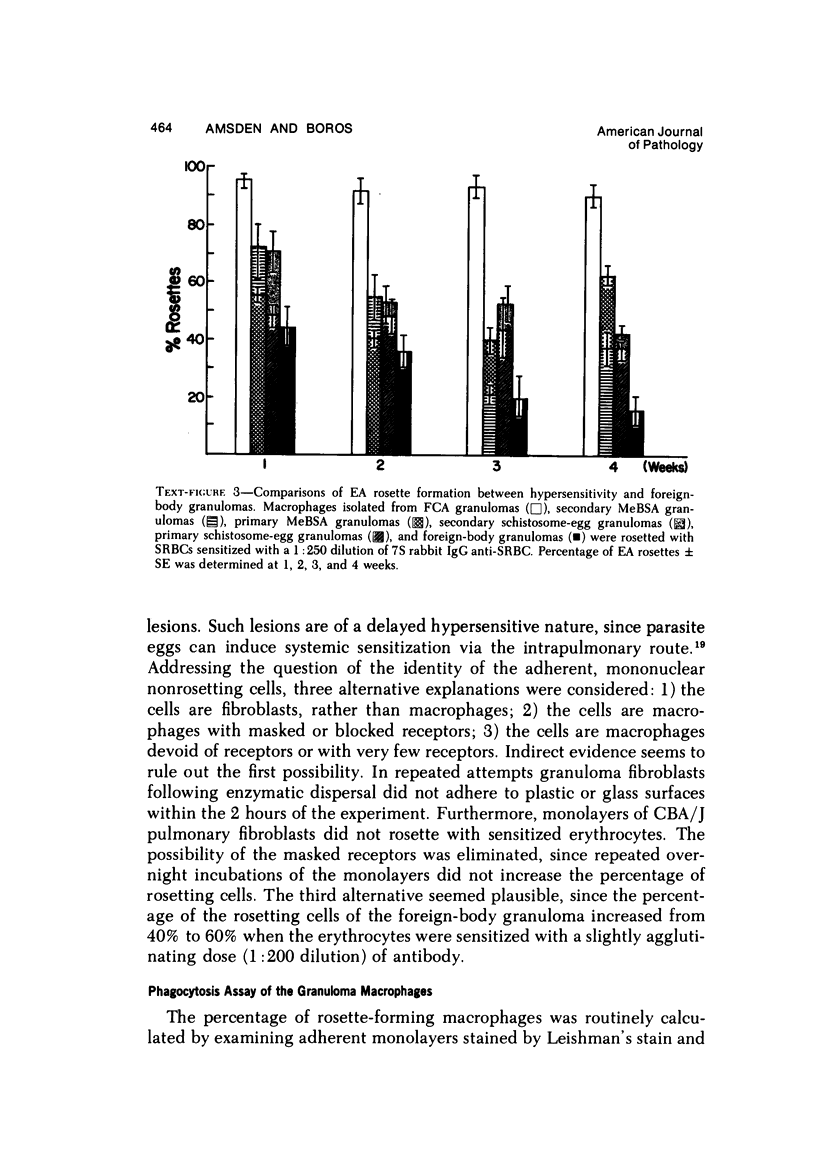
Foreign-body and delayed hypersensitivity granulomas were induced in mice; and the dynamics of macrophages isolated from dispersed, 1--4-week-old lesions was delineated. The size and histologic complexity of the lesions increased as shown: adjuvant greater than schistosome egg greater than methylated bovine serum albumin greater than bead. Esterase staining, spreading on glass, and the percentage of Fc-receptor--bearing macrophages present in the various granulomas reflected the same gradient. The Fc receptors were examined by rosetting with rabbit-antibody--SRBC complex (EA). Whereas more than 90% of the population of macrophages of the dermal adjuvant granuloma contained undiminished numbers of receptor-bearing macrophages throughout the 4 weeks, the percentage of macrophages that displayed receptors in pulmonary foreign-body (40%) and delayed hypersensitivity granulomas (70%) peaked at 1 week and subsequently declined. The EA rosetting of the foreign-body and delayed hypersensitivity granuloma macrophages was strongly inhibited by monomeric IgG2a-specific and weakly by aggregated IgG2b-specific mouse myeloma proteins. Also, macrophages of the delayed hypersensitivity granulomas rosetted in higher percentages with SRBCs coupled with monomeric IgC2a than with those coupled with aggregated IgG2b myeloma proteins. Macrophages of the foreign-body lesion did not react with aggregated IgG2b--SRBC. Rosetting with monomeric IgG2a--SRBC or aggregated IgG2b--SRBC could not be cross-inhibited by the myeloma proteins. Both the monomeric IgG2a--SRBC and aggregated IgG2b--SRBC complexes were readily phagocytized. Trypsin treatment of the macrophages inhibited rosetting with EA or myeloma-protein--coupled SRBCs. The display of Fc receptors on the granuloma macrophages seems to be related to the etiology of the lesion and the intensity and duration of the inflammatory reaction.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Biesecker J. L., Koss L. G. The activation of mononuclear phagocytes in vitro: immunologically mediated enhancement. J Reticuloendothel Soc. 1973 Dec;14(6):550–570. [PubMed] [Google Scholar]
- Arend W. P., Mannik M. In vitro adherence of soluble immune complexes to macrophages. J Exp Med. 1972 Sep 1;136(3):514–531. doi: 10.1084/jem.136.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J. Cytophilic antibodies in mice contact-sensitized with oxazolone. Immunochemical characterization and preferential binding to a trypsin-sensitive macrophage receptor. Immunology. 1974 Oct;27(4):563–576. [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Schwartz H. J. Effect of carrageenan on the development of hypersensitivity (Schistosoma mansoni egg) and foreign body (divinyl-benzene copolymer beads and bentonite) granulomas. Int Arch Allergy Appl Immunol. 1975;48(2):192–202. doi: 10.1159/000231305. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Effect of antimacrophage serum on hypersensitivity (Schistosoma mansoni egg) and foreign body (divinyl-benzene copolymer bead) granulomas. J Immunol. 1971 Aug;107(2):534–539. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. The bentonite granuloma. Characterization of a model system for infectious and foreign body granulomatous inflammation using soluble mycobacterial, histoplasma and schistosoma antigens. Immunology. 1973 Mar;24(3):511–529. [PMC free article] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Davey M. J., Asherson G. L. Cytophilic antibody. I. Nature of the macrophage receptor. Immunology. 1967 Jan;12(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972 Feb;24(2):89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- Diamond B., Bloom B. R., Scharff M. D. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978 Oct;121(4):1329–1333. [PubMed] [Google Scholar]
- Dorrington K. J. Properties of the Fc receptor on macrophages. Immunol Commun. 1976;5(4):263–280. doi: 10.3109/08820137609044280. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Daughaday C. C. Kinetics of monocyte receptor activity for immunoproteins in patients with sarcoidosis. Ann N Y Acad Sci. 1976;278:190–200. doi: 10.1111/j.1749-6632.1976.tb47029.x. [DOI] [PubMed] [Google Scholar]
- Galindo B. Antigen-mediated fusion of specifically sensitized rabbit alveolar macrophages. Infect Immun. 1972 Apr;5(4):583–594. doi: 10.1128/iai.5.4.583-594.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N., Love S. H. A macrophage agglutinating factor produced during a pulmonary delayed hypersensitivity reaction. J Reticuloendothel Soc. 1975 Nov;18(5):295–304. [PubMed] [Google Scholar]
- Gordon S. Macrophage neutral proteinases and chronic inflammation. Ann N Y Acad Sci. 1976;278:176–189. doi: 10.1111/j.1749-6632.1976.tb47028.x. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Silverstein S. C. Segmental response of the macrophage plasma membrane to a phagocytic stimulus. J Exp Med. 1974 Feb 1;139(2):323–336. doi: 10.1084/jem.139.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L. M., Warren K. S., Boros D. L. Schistosoma mansoni: antigenic secretions and the etiology of egg granulomas in mice. Exp Parasitol. 1974 Apr;35(2):288–298. doi: 10.1016/0014-4894(74)90035-6. [DOI] [PubMed] [Google Scholar]
- Heusser C. H., Anderson C. L., Grey H. M. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977 May 1;145(5):1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Kasdon E. J., Schlossman S. F. An experimental model of pulmonary arterial granulomatous inflammation. Am J Pathol. 1973 Jun;71(3):365–372. [PMC free article] [PubMed] [Google Scholar]
- Kellermeyer R. W., Warren K. S. The role of chemical mediators in the inflammatory response induced by foreign bodies: comparison with the schistosome egg granuloma. J Exp Med. 1970 Jan 1;131(1):21–39. doi: 10.1084/jem.131.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotkes P., Pick E. Studies on the inhibition of macrophage migration induced by soluble antigen-antibody complexes. Clin Exp Immunol. 1975 Jan;19(1):105–120. [PMC free article] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of Fc and C3 receptors on myeloid leukemic cells. J Immunol. 1976 Aug;117(2):580–586. [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Mariano M., Nikitin T., Malucelli B. E. Immunological and non-immunological phagocytosis by inflammatory macrophages, epithelioid cells and macrophage polykaryons from foreign body granulomata. J Pathol. 1976 Nov;120(3):151–159. doi: 10.1002/path.1711200304. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Garvin A. J., Hardin D. H., Spicer S. S. Immune complex receptors on cell surfaces. II. Cytochemical evaluation of their abundance on different immune cells: distribution, uptake, and regeneration. Am J Pathol. 1976 Sep;84(3):437–456. [PMC free article] [PubMed] [Google Scholar]
- Moore D. L., Grove D. I., Warren K. S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977 Jan;121(1):41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. I. Relation between Fc receptors, C'3 receptors and surface immunoglobulin. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):47–63. doi: 10.1098/rspb.1974.0060. [DOI] [PubMed] [Google Scholar]
- Parks D. E., Weiser R. S. The role of phagocytosis and natural lymphokines in the fusion of alveolar macrophages to form Langhans giant cells. J Reticuloendothel Soc. 1975 Apr;17(4):219–228. [PubMed] [Google Scholar]
- Rabellino E. M., Metcalf D. Receptors for C3 and IgG on macrophage, neutrophil and eosinophil colony cells grown in vitro. J Immunol. 1975 Sep;115(3):688–692. [PubMed] [Google Scholar]
- Rhodes J. Macrophage heterogeneity in receptor activity: the activation of macrophage Fc receptor function in vivo and in vitro. J Immunol. 1975 Mar;114(3):976–981. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Monocyte IgG receptor activity, dynamics, and modulation-normal individuals and patients with granulomatous diseases. J Lab Clin Med. 1977 Feb;89(2):332–340. [PubMed] [Google Scholar]
- Segal D. M., Titus J. A. The subclass specificity for the binding of murine myeloma proteins to macrophage and lymphocyte cell lines and to normal spleen cells. J Immunol. 1978 Apr;120(4):1395–1403. [PubMed] [Google Scholar]
- Shima K., Dannenberg A. M., Jr, Ando M., Chandrasekhar S., Seluzicki J. A., Fabrikant J. I. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. I. Studies involving their incorporation of tritiated thymidine and their content of lysosomal enzymes and bacilli. Am J Pathol. 1972 Apr;67(1):159–180. [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969 May;98(1):31–39. doi: 10.1002/path.1710980105. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Willoughby D. A. The origin of mononuclear cells in chronic inflammation and tuberculin reactions in the rat. J Pathol Bacteriol. 1968 Oct;96(2):389–399. doi: 10.1002/path.1700960217. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of particulate horseradish peroxidase (HRP)-anti HRP immune complexes with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Dec;55(3):616–634. doi: 10.1083/jcb.55.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. The presence of two Fc receptors on mouse macrophages: evidence from a variant cell line and differential trypsin sensitivity. J Exp Med. 1977 Apr 1;145(4):931–945. doi: 10.1084/jem.145.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Separate Fc-receptors for immunoglogulins IgG2a and IgG2b on an established cell line of mouse macrophages. J Immunol. 1976 Apr;116(4):911–914. [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Furth R. Macrophage activity and clinical immunology. Origin and kinetics of mononuclear phagocytes. Ann N Y Acad Sci. 1976;278:161–175. doi: 10.1111/j.1749-6632.1976.tb47027.x. [DOI] [PubMed] [Google Scholar]