Abstract
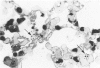
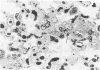
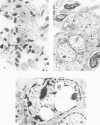
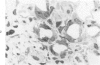
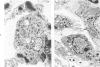
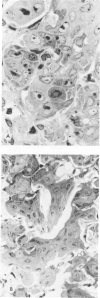
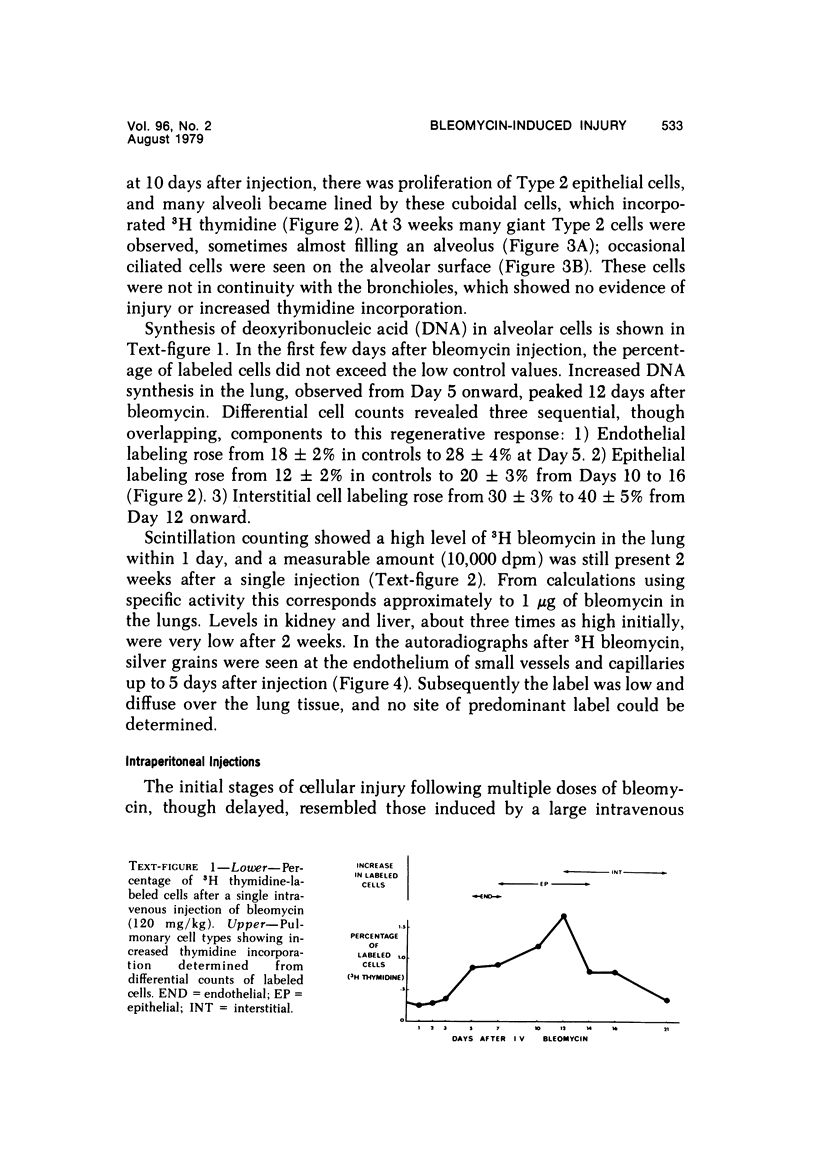
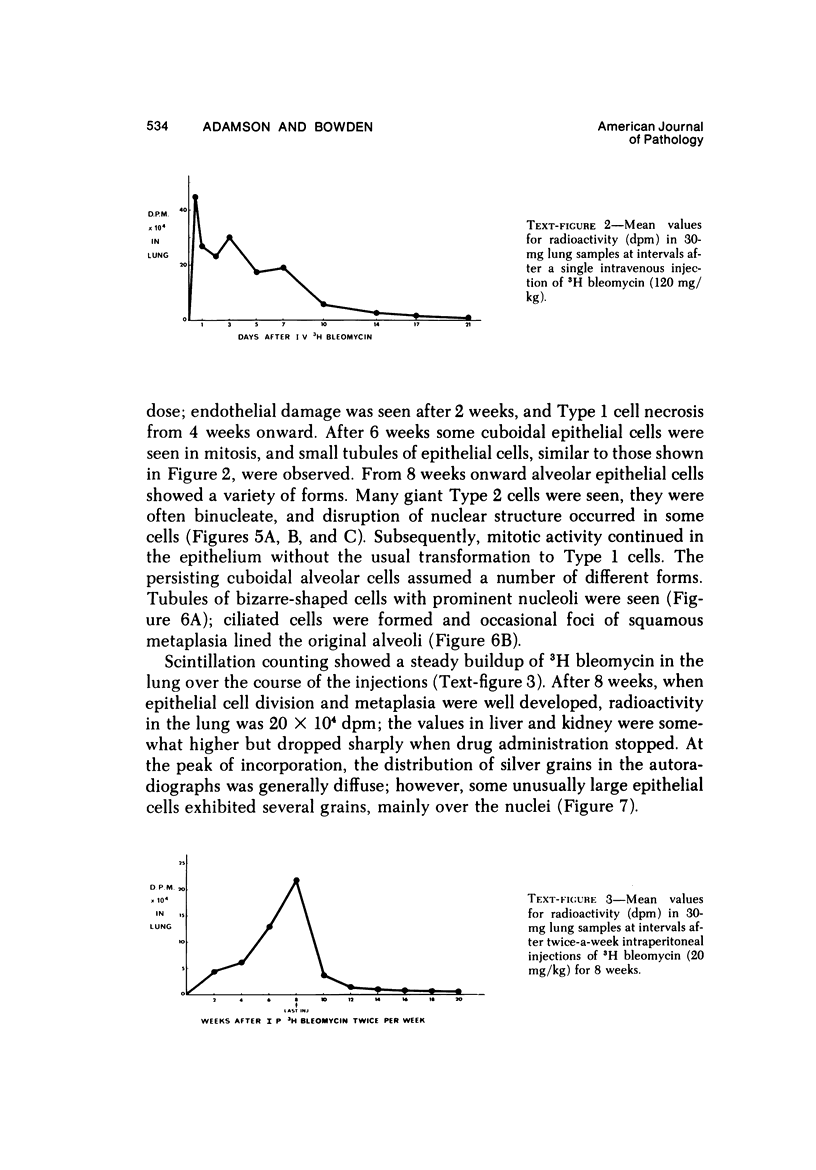
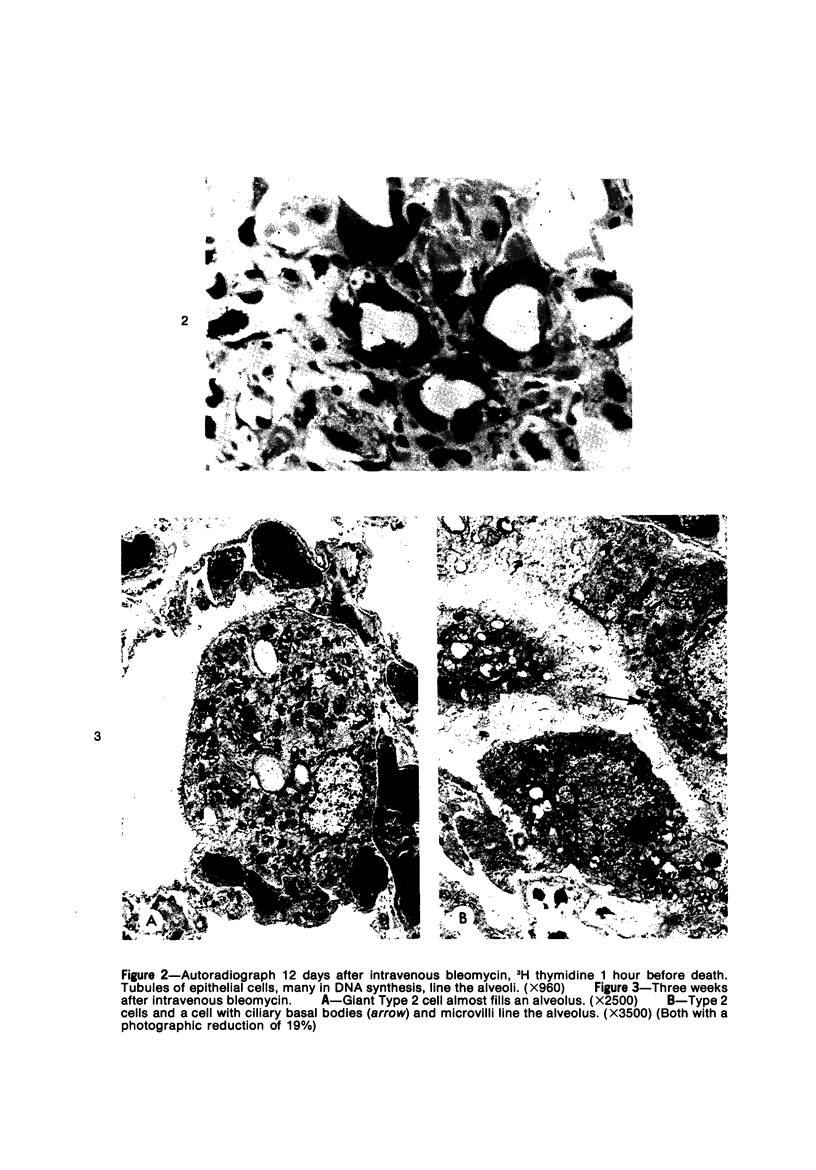
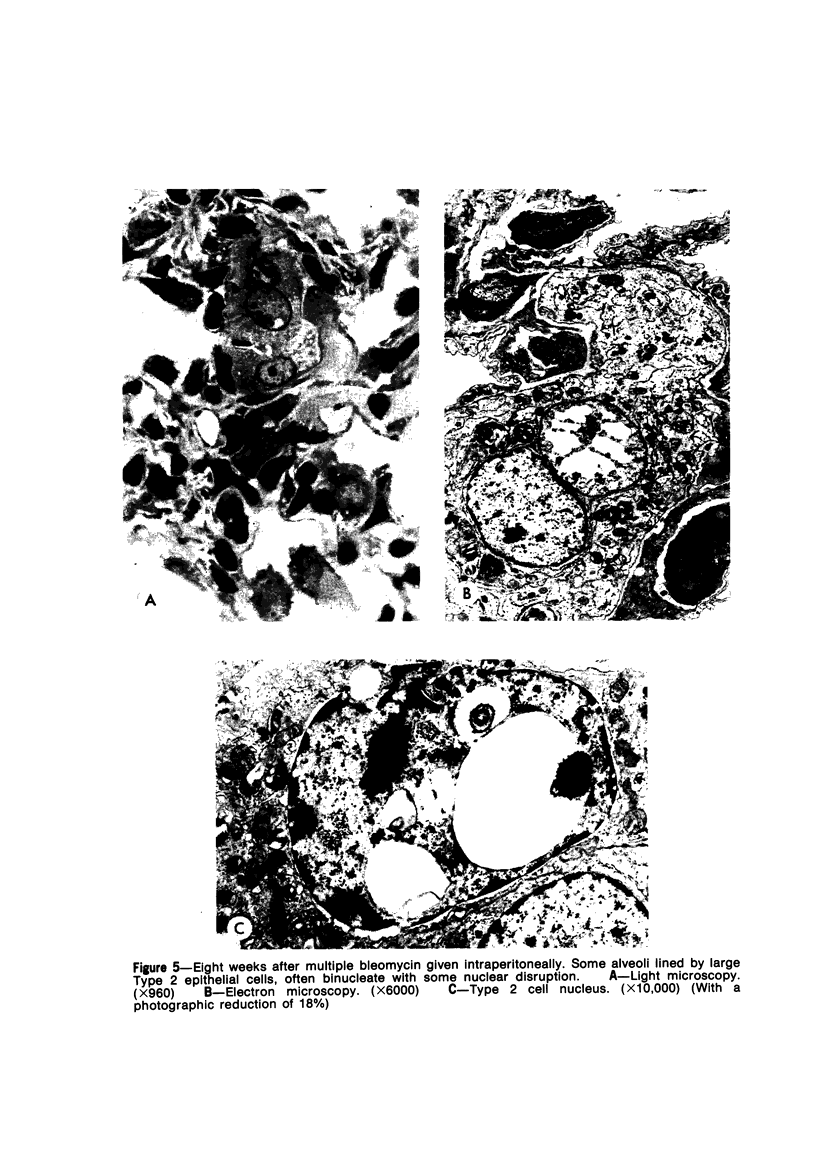
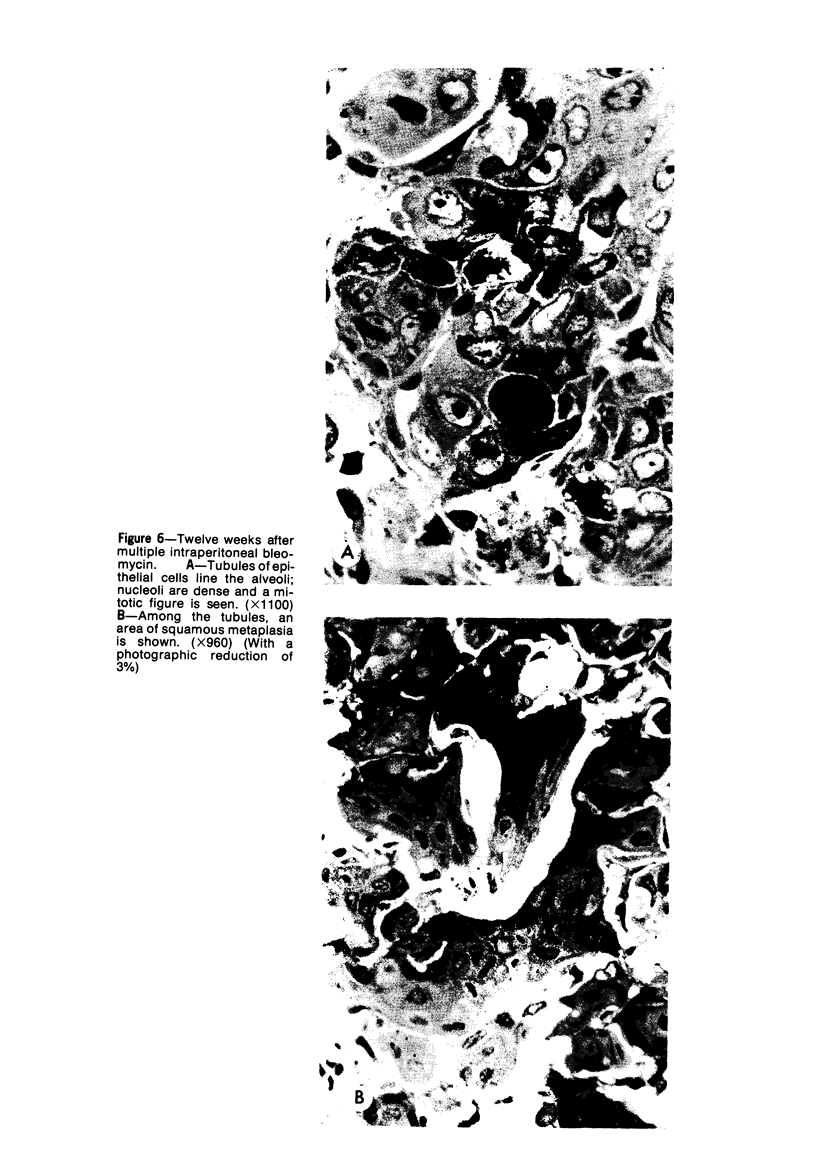
Metaplastic epithelial cells are often observed lining alveoli in bleomycin-induced pulmonary fibrosis. The hypothesis that these cellular changes are induced by the direct action of the drug on differentiating Type 2 cells is now examined in a sequential study to correlate the presence of 3H bleomycin in the lung with the pattern of injury and repair of the alveolar epithelium. A single intravenous dose or multiple small intraperitoneal doses induce focal necrosis of Type 1 epithelial cells followed by Type 2 cell regeneration. At the time of maximal deoxyribonucleic acid (DNA) synthesis in these cells, significant amounts of 3H bleomycin are demonstrable in the lung by scintillation counting; and in autoradiographs, the drug appears to concentrate in epithelial cells. Subsequently many abnormal Type 2 cells are seen. Some are binucleate, and others show nuclear disruption. The usual process of differentiation to Type 1 cells does not occur; instead, a variety of epithelial forms are found, including fetal-like tubular structures and ciliated and squamous metaplastic cells. The correlation of epithelial injury and repair with the direct demonstration of bleomycin in the lung indicates that Type 2 cells are susceptible to injury in the division and differentiation phases of the cell cycle and may then produce a variety of inappropriate alveolar lining cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H., Cote M. G., Witschi H. Lung injury induced by butylated hydroxytoluene: cytodynamic and biochemical studies in mice. Lab Invest. 1977 Jan;36(1):26–32. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Origin of ciliated alveolar epithelial cells in bleomycin-induced lung injury. Am J Pathol. 1977 Jun;87(3):569–580. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974 Nov;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H., Wyatt J. P. Oxygen poisoning in mice. Ultrastructural and surfactant studies during exposure and recovery. Arch Pathol. 1970 Nov;90(5):463–472. [PubMed] [Google Scholar]
- Adamson Y. I., Bowden D. H. Pulmonary injury and repair. Organ culture studies of murine lung after oxygen. Arch Pathol Lab Med. 1976 Dec;100(12):640–643. [PubMed] [Google Scholar]
- Bedrossian C. W., Luna M. A., Mackay B., Lichtiger B. Ultrastructure of pulmonary bleomycin toxicity. Cancer. 1973 Jul;32(1):44–51. doi: 10.1002/1097-0142(197307)32:1<44::aid-cncr2820320106>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Caputo A. Importance of experimental data for the improvement of the therapeutical effect of bleomycin. Prog Biochem Pharmacol. 1976;11:2–17. [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Tosco R., Wheelis R. F., Gould N. S., Kapanci Y. Oxygen pneumonitis in man. Ultrastructural observations on the development of alveolar lesions. Lab Invest. 1972 May;26(5):499–508. [PubMed] [Google Scholar]
- Hirai K. I., Witschi H., Côté M. G. Electron microscopy of butylated hydroxytoluene-induced lung damage in mice. Exp Mol Pathol. 1977 Dec;27(3):295–308. doi: 10.1016/0014-4800(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Iqbal Z. M., Kohn K. W., Ewig R. A., Fornace A. J., Jr Single-strand scission and repair of DNA in mammalian cells by bleomycin. Cancer Res. 1976 Oct;36(10):3834–3838. [PubMed] [Google Scholar]
- Iversen P. H., Clausen O. P., Iversen U. M., Rohrbach R. Some effects of bleomycin on the proliferation, maturation time and protein synthesis of hairless mouse epidermis. Cell Tissue Kinet. 1976 Jan;9(1):77–97. doi: 10.1111/j.1365-2184.1976.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Auger L. T., Saunders G. F., Haidle C. W. Effect of bleomycin on the synthesis and function of RNA. Cancer Res. 1977 May;37(5):1345–1348. [PubMed] [Google Scholar]
- Lown J. W., Sim S. K. The mechanism of the bleomycin-induced cleavage of DNA. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1150–1157. doi: 10.1016/s0006-291x(77)80099-5. [DOI] [PubMed] [Google Scholar]
- Sostman H. D., Matthay R. A., Putman C. E. Cytotoxic drug-induced lung disease. Am J Med. 1977 Apr;62(4):608–615. doi: 10.1016/0002-9343(77)90424-7. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Chemistry and mechanism of action of bleomycin. Fed Proc. 1974 Nov;33(11):2296–2302. [PubMed] [Google Scholar]
- Umezawa H., Ishizuka M., Maeda K., Takeuchi T. Studies on bleomycin. Cancer. 1967 May;20(5):891–895. doi: 10.1002/1097-0142(1967)20:5<891::aid-cncr2820200550>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Ishizuki M., Hori S., Chimura H., Takeuchi T. The distribution of 3H-bleomycin in mouse tissue. J Antibiot (Tokyo) 1968 Nov;21(11):638–642. doi: 10.7164/antibiotics.21.638. [DOI] [PubMed] [Google Scholar]
- Yasuzumi G., Hyo Y., Hoshiya T., Yasuzumi F. Effects of bleomycin on human tongue carcinoma cells as revealed by electron microscopy. Cancer Res. 1976 Oct;36(10):3574–3583. [PubMed] [Google Scholar]