Abstract
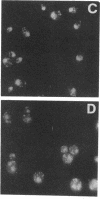
This paper reports a phenotypic characterization of ggp1 mutants. The cloned GGP1 (GAS1) gene, which encodes a major GPI-anchored glycoprotein (gp115) of Saccharomyces cerevisiae of unknown function, was used to direct the inactivation of the chromosomal gene in haploid and diploid strains by gene replacement. The analysis of the null mutants reveals a reduction in the growth rate of 15 to 40%. Cells are round, with more than one bud, and extensively vacuolized. In the stationary phase, mutant cells are very large, arrest with a high percentage of budded cells (about 54 and 70% for haploid and diploid null mutants, respectively, in comparison with about 10 to 13% for control cells), and have reduced viability. The observed phenotype suggests defects in cell separation. Flow cytometric analysis of DNA reveals an increase in the fraction of cells in the G2+M+G1* compartment during exponential growth. Conjugation and sporulation are not affected. The exocellular location of gp115 led us to examine cell wall properties. Cell wall and septum ultrastructure of abnormally budded cells was analyzed by electron microscopy analysis, and no appreciable differences from wild-type cells were found. Microscopic analysis revealed an increase in chitin content and delocalization. In comparison with control cells, ggp1 null mutants are shown to be resistant to Zymolyase during the exponential growth phase. A fivefold overexpression of gp115 does not bring about any effects on cell growth parameters and cell wall properties.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone C., Sommer S. S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J Cell Biol. 1990 May;110(5):1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Riezman H., Desponds C., Bron C. A major 125-kd membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through an inositol-containing phospholipid. EMBO J. 1988 Jul;7(7):2233–2240. doi: 10.1002/j.1460-2075.1988.tb03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel-Mitani M., Flygenring H. P., Hansen M. T. A novel aspartyl protease allowing KEX2-independent MF alpha propheromone processing in yeast. Yeast. 1990 Mar-Apr;6(2):127–137. doi: 10.1002/yea.320060206. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Grandori R., Popolo L., Vai M., Alberghina L. cAMP promotes the synthesis in early G1 of gp115, a yeast glycoprotein containing glycosyl-phosphatidylinositol. J Biol Chem. 1990 Aug 25;265(24):14315–14320. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda M. J., Robbins P. W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991 Oct 15;266(29):19758–19767. [PubMed] [Google Scholar]
- Lipke P. N., Wojciechowicz D., Kurjan J. AG alpha 1 is the structural gene for the Saccharomyces cerevisiae alpha-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol Cell Biol. 1989 Aug;9(8):3155–3165. doi: 10.1128/mcb.9.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Jenö P., Conzelmann A., Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991 Jan;11(1):27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo L., Alberghina L. Identification of a labile protein involved in the G1-to-S transition in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Jan;81(1):120–124. doi: 10.1073/pnas.81.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J. Signal transduction by GPI-anchored membrane proteins. Cell Biol Int Rep. 1991 Sep;15(9):761–767. doi: 10.1016/0309-1651(91)90031-d. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Roy A., Lu C. F., Marykwas D. L., Lipke P. N., Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991 Aug;11(8):4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. A., Mol P. C., Bowers B., Silverman S. J., Valdivieso M. H., Durán A., Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991 Jul;114(1):111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vai M., Gatti E., Lacanà E., Popolo L., Alberghina L. Isolation and deduced amino acid sequence of the gene encoding gp115, a yeast glycophospholipid-anchored protein containing a serine-rich region. J Biol Chem. 1991 Jul 5;266(19):12242–12248. [PubMed] [Google Scholar]
- Vai M., Lacanà E., Gatti E., Breviario D., Popolo L., Alberghina L. Evolutionary conservation of genomic sequences related to the GGP1 gene encoding a yeast GPI-anchored glycoprotein. Curr Genet. 1993 Jan;23(1):19–21. doi: 10.1007/BF00336744. [DOI] [PubMed] [Google Scholar]
- Vai M., Popolo L., Grandori R., Lacanà E., Alberghina L. The cell cycle modulated glycoprotein GP115 is one of the major yeast proteins containing glycosylphosphatidylinositol. Biochim Biophys Acta. 1990 May 8;1038(3):277–285. doi: 10.1016/0167-4838(90)90237-a. [DOI] [PubMed] [Google Scholar]
- Vanoni M., Vai M., Popolo L., Alberghina L. Structural heterogeneity in populations of the budding yeast Saccharomyces cerevisiae. J Bacteriol. 1983 Dec;156(3):1282–1291. doi: 10.1128/jb.156.3.1282-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Iida H., Pardee A. B. Modulation of expression of the stress-inducible p118 of Saccharomyces cerevisiae by cAMP. II. A study of p118 expression in mutants of the cAMP cascade. J Biol Chem. 1988 Jun 25;263(18):8576–8582. [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M. P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J Bacteriol. 1984 Sep;159(3):1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]