Abstract
Environmental constraints in aquatic habitats have become topics of concern to both the scientific community and the public at large. In particular, coastal and freshwater habitats are subject to dramatic variability in various environmental factors, as a result of both natural and anthropogenic processes. The protection and sustainable management of all aquatic habitats requires greater understanding of how environmental constraints influence aquatic organisms. Locomotion and predator–prey interactions are intimately linked and fundamental to the survival of mobile aquatic organisms. This paper summarizes the main points from the review and research articles which comprise the theme issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’. The articles explore how natural and anthropogenic factors can constrain these two fundamental activities in a diverse range of organisms from phytoplankton to marine mammals. Some major environmental constraints derive from the intrinsic properties of the fluid and are mechanical in nature, such as viscosity and flow regime. Other constraints derive from direct effects of factors, such as temperature, oxygen content of the water or turbidity, upon the mechanisms underlying the performance of locomotion and predator–prey interactions. The effect of these factors on performance at the tissue and organ level is reflected in constraints upon performance of the whole organism. All these constraints can influence behaviour. Ultimately, they can have an impact on ecological performance. One issue that requires particular attention is how factors such as temperature and oxygen can exert different constraints on the physiology and behaviour of different taxa and the ecological implications of this. Given the multiplicity of constraints, the complexity of their interactions, and the variety of biological levels at which they can act, there is a clear need for integration between the fields of physiology, biomechanics, behaviour, ecology, biological modelling and evolution in both laboratory and field studies. For studies on animals in their natural environment, further technological advances are required to allow investigation of how the prevailing physico-chemical conditions influence basic physiological processes and behaviour.
Keywords: locomotion, predator–prey interactions, temperature, oxygen, turbidity, aquatic organisms
1. The context
The physico-chemical properties of water (e.g. high viscosity, high thermal conductance, low oxygen solubility or the potential for ion–osmotic exchanges) exert constraints upon aquatic organisms which effectively determine the limits and conditions of their distribution ranges. It has been argued that environmental conditions in aquatic systems are generally more stable than in their terrestrial counterparts but, whereas this may be the case for deep or pelagic habitats, it is certainly not the case for most coastal zones or freshwater systems. In such habitats, important environmental parameters can fluctuate to significant extents at a multiplicity of temporal and spatial scales (Abrahams et al. 2007; Claireaux & Lefrançois 2007).
In recent years, concerns about rapidly intensifying anthropogenic impacts upon all aquatic habitats have given greater impetus to studies investigating environmental constraints upon resident organisms. Flow regimes along rivers and coastal areas are continually being modified by dams, harbours and other man-made structures. The expansion of human activities along major rivers, in estuaries and on coastlines is causing a progressive increase in the number and the seriousness of hypoxic episodes in coastal areas (e.g. Pihl et al. 1991; Diaz & Rosenberg 1995). Increases in nutrient loads entering coastal areas can not only cause hypoxia, but also lead to increases in turbidity, through algal blooms (Bonsdorf et al. 1997). At the same time, global warming can be expected to influence all aquatic habitats, including the marine pelagic environment. An unprecedented rate of increase of ocean temperatures is already exerting profound impacts upon aquatic organisms, altering distribution ranges, recruitment predictability and yield (Grebmeier et al. 2006).
There is, therefore, an increasing need for research into the constraints that environmental factors, whether natural or anthropogenic, exert upon aquatic organisms. In this special issue, we focus on environmental constraints upon locomotion and predator–prey relationships in mobile aquatic organisms. This topic is of particular interest because locomotion is a major energetic cost for many aquatic animals, and locomotor performance underlies essential functions such as foraging and escape from predators. Thus, locomotion and predator–prey interactions should be fundamental components of the fitness of individuals, the productivity of populations and, therefore, the sustainability of ecosystems.
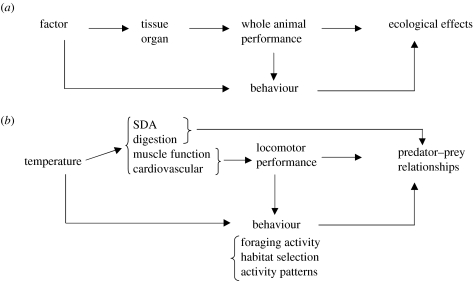
Some major environmental constraints in the aquatic milieu derive from the intrinsic properties of the fluid and are essentially mechanical in nature, such as viscosity and flow regime. Other constraints derive from the direct effects of factors such as temperature, oxygen content of the water or turbidity, upon the mechanisms underlying the performance of locomotion and predator–prey interactions. The effect of these factors on performance at the tissue and organ level (e.g. muscle, heart, eyes) is reflected into constraints upon performance of the whole organism and, consequently, upon ecological performance (figure 1a). For example, the direct effects of temperature on fish muscle may affect their locomotor performance and, consequently, their ability to capture food or escape from predators (figure 1b). Similarly, turbidity reduces the visual range of aquatic organisms and can affect their predator and prey detection.
Figure 1.
(a) The generalized effect of a given environmental factor on whole animal performance, behaviour and ecology in aquatic organisms. A given factor can directly affect the organism's behaviour and its tissue and organ functions, and consequently whole animal performance. This can have effects at the behavioural level as well. Ultimately, altered behaviour and whole animal performance will have ecological effects. (b) The effect of temperature on the locomotion and predator–prey relationships of ectotherms. Temperature has direct effects on digestion and standard dynamic action (SDA) as well as on muscle and heart function. Effects on digestion can cause direct effects on predator–prey relationships. Effects on muscle and heart function have direct effects on swimming performance and, therefore, indirectly upon those predator–prey relationships which are dependent on locomotion. Temperature can also have direct and indirect effects on behaviour, through changes in habitat selection (a direct effect) and activity patterns (an indirect effect through change in locomotor performance). Behavioural changes will ultimately affect predator–prey relationships at the ecological level.
Many of these constraints will influence the performance of different organisms to different extents. For example, the effect of turbulence depends largely upon the size of organisms; oxygen level in the water affects the metabolism of fish, but not that of marine mammals and birds; and turbidity will only affect predator–prey interactions that are based on vision. Temperature can have a profound effect upon the physiology and performance of ectotherms but, in endotherms, these effects are generally limited to the costs of thermoregulation. The differential effects of environmental factors can influence predator–prey relationships if these involve organisms that are constrained to different extents by a given environmental factor. The behaviour of aquatic organisms is also constrained by environmental conditions (figure 1). This modulatory effect is either direct, such as the effect of turbidity on the prey-searching behaviour of fish (Meager & Batty 2007), or indirect, through the effect of temperature on the scope for aerobic activity in fishes, which determines the limits of their behavioural repertoire (Claireaux & Lefrançois 2007).
Given that environmental factors can constrain aquatic organisms at so many different levels, from direct effects upon tissues to effects upon organismal performance and behaviour, the topics of this theme issue embrace a wide variety of approaches. Thus, the articles range from environmental physiology to biomechanics and behavioural ecology, often within an interdisciplinary context, of a diverse range of organisms, from phytoplankton to marine mammals. While environmental physiology investigates the effects of environmental factors on the function of organisms, biomechanics provides insights into the physical mechanisms modulating such effects, and behavioural ecology studies the choices animals can make in response to environmental constraints. Altogether, these articles illustrate that interactions between these disciplines will provide a more integrated understanding of how aquatic environments influence their inhabitants. Recent advances in methodologies to study animal movement in the laboratory, such as the new flow visualization techniques, are providing exciting new information about how locomotion and behaviour are influenced by water viscosity and flow regime. At the same time, many animal physiologists have moved from the laboratory to the field, in large part as a result of recent developments in telemetry, and ecophysiological studies are beginning to unravel how animals in their natural habitats are influenced by environmental factors such as temperature and dissolved oxygen. The contributors to the present theme issue all participated in a symposium entitled ‘Environmental constraints upon the locomotion and predator–prey interactions of aquatic organisms’ held at a meeting of the Society for Experimental Biology, in Barcelona in July 2005.
2. This volume
The effect of environmental constraints may act at various organizational levels (figure 1). Particular constraints and some of their potential ecological effects that are treated in this special issue are listed in table 1. The types of constraints have been loosely organized into mechanical or physiological ones, followed by their interactions with behaviour, although in many cases studies were carried out across these notional boundaries.
Table 1.
Examples of constraints dealt with within this special issue and their potential ecological significance.
| type of constraint | impact | ecological significance | authors |
|---|---|---|---|
| flow regime–turbulence | |||
| impact upon phytoplankton aggregation | planktonic dispersal | Gentien et al. (2007) | |
| impact on feeding and swimming activities in zooplankton | adapted foraging, biotic interactions and motion behaviour | Jiang & Strickler (2007); Strickler & Balázsi (2007) | |
| fish locomotor energetics- activity | fish ability to migrate along rivers | Liao (2007) | |
| depth—light limitation | |||
| ectotherm metabolism | foraging patterns (sit-and-wait versus active hunters) | Seibel & Drazen (2007) | |
| temperature | |||
| direct effects on fish muscle function | power production during swimming and gait transition | Rome (2007) | |
| swimming performance in fishes | breeding success | Wilson et al. (2007) | |
| effects on metabolism and cardio-respiratory performance in fishes | aerobic metabolic scope for activity; swimming abilities; rates of recovery from exhaustion | Claireaux & Lefrançois (2007); Farrell (2007) | |
| distribution of ectothermic prey | marine mammal predator foraging patterns | Bailleul et al. (2007) | |
| thermoregulatory costs in diving endotherms | digestive constraints on foraging patterns | Lovvorn (2007); Rosen et al. (2007) | |
| differential effect of temperature on fish of different sizes | predator–prey interactions | Abrahams et al. (2007) | |
| oxygen availability | |||
| effect of hypoxia on fish anti-predator behaviour and locomotion | predator–prey interactions | Domenici et al. (2007) | |
| hypoxia tolerance | predator–prey interactions | Abrahams et al. (2007) | |
| locomotor patterns in diving mammals | activity and foraging patterns | Davis & Weihs (2007); Rosen et al. (2007) | |
| turbidity | |||
| effect on visual range | activity and foraging patterns in fishes | Meager & Batty (2007) | |
| predator–prey interactions in fishes | Abrahams et al. (2007) | ||
| pollution | |||
| effects on swimming in fishes | ability to colonize polluted habitats successfully | McKenzie et al. (2007) | |
(a) Mechanical constraints
Phytoplankton are at the mercy of the intrinsic physical properties of the water and physical forcing such as wind, currents, upwelling and turbulence can have major effects on these organisms. Using the most common red tide dinoflagellate (Karenia mikimotoi) proliferating in the eastern North Atlantic and around Japan as an example, the article by Gentien et al. (2007) investigates how mortality rate is related to shear rate. Wind events that increase mixing and agitation initiate population declines in this dinoflagellate. Under realistic forcing conditions with a small number of parameters, the authors present a model that reproduces the conditions for the confinement of the population to the pycnocline and the appropriate timing and duration of the recurrent dinoflagellate K. mikimotoi bloom on the Ushant Front (France).
In the water column, planktonic copepods encounter small-scale hydrodynamic disturbances generated by fellow zooplankters. Strickler & Balázsi (2007) investigate whether or not copepods can distinguish between hydrodynamic disturbances created by predators, prey, conspecifics and/or mates. The authors conclude that the information within the hydrodynamic disturbances created by swimming zooplankters is sufficient for differentiated reactions to each type of disturbance. Using a full range of parametric studies based on a hydrodynamic model for active copepod swimming, Jiang & Strickler (2007) provide an integrated picture of how basic flow modes of different flow-field patterns vary systematically and continuously with different basic steady translational swimming behaviours, i.e. sinking, hovering, vertical swimming upward and horizontal swimming. They also speculate about how a free-swimming copepod might interact with the smallest eddies in small-scale oceanic turbulence. The challenge for planktonic organisms such as a copepods may be how to exploit such mechanical energy by staying in particular flow structures, avoiding other flow structures and/or adopting particular unsteady translational/rotational swimming behaviours with suitable body orientations relative to such structures.
Liao (2007) explores how organisms that live at higher Reynolds number than plankton (i.e. in full inertial regime) deal with larger scale turbulence, and reviews the effects of perturbed flow upon fishes. Swimming movements by fishes can be perturbed by complex flows, generated by various abiotic and biotic factors. In general, the evidence indicates that flows with wide fluctuations in velocity are avoided by fish whereas, conversely, perturbed flows that have some component of predictability may attract fishes. Understanding how fish deal with natural and man-made turbulence is of particular relevance for recent river rehabilitation projects, which often aim to increase fish diversity and population density by introducing structures that increase physical heterogeneity.
(b) Physiological constraints
Under natural conditions, environmental factors interact with each other to give rise to constraints with far-reaching effects upon the physiological performance of living organisms, ultimately affecting their ability to grow, survive and reproduce. The proximal effects of the environment on organisms' activities are mediated through metabolism; therefore elucidating the physiological mechanisms involved in environmental adaptation is of fundamental importance to understand the individual performance as well as population demography and dynamics. Our current limitations in understanding the link between environmental conditions and individual fitness in aquatic organisms are due to two main reasons. The first results from the difficulty in unravelling the interplay between the environmental matrix and the pool of the organisms' regulatory mechanisms. The second reason relates to our limited ability to take into account the hierarchical organization of biological systems and how environmental effects propagate through them.
As pointed out by Rome (2007), it is remarkable that ectothermic animals can swim at a range of body temperatures that can exceed 30°C. Locomotion is a complex process that integrates muscular, neural, biomechanical and metabolic components. Since the temperature dependencies of these components vary tremendously, it may seem terribly complex for an investigator to examine the relationship between body temperature and swimming performance. Yet, Rome shows that compared to the 1980s, our current understanding of muscle function and locomotory performance has improved tremendously and we are now in a much better position to appreciate the problems faced by fish swimming at different temperatures. This is particularly true for the relationships between physiological support functions and muscle power production during swimming.
Farrell's (2007) study shows that we also have a clearer understanding of why aerobic performance declines when temperature departs from optimal. Farrell's work also provides evidence that gait transition from steady aerobic swimming to unsteady burst and coast swimming has a strong volitional component and the bioenergetics of swimming cannot be fully understood if disconnected from its behavioural component. Despite significant effort, progress has been slow in placing our understanding of fish physiology within an ecological context. Claireaux & Lefrançois (2007) argue that Fry's 60-year-old monograph (Fry 1947) provides a valuable conceptual basis for linking physiology, ecology and evolutionary biology. Ecotoxicology is certainly one of the fields of environmental sciences that has been particularly successful at considering complex physiological traits of performance and their environmental determinants. McKenzie et al. (2007) demonstrated that complex physiological traits, such as swimming performance and routine metabolic rate, may be valuable biomarkers of the functional integrity of fish exposed to complex mixtures of pollutants in contaminated natural environments. It is the authors' view that, while such complex traits may be less specific than biochemical or molecular biomarkers, they do provide valuable insight into why fish may fail to colonize polluted habitats.
Living in the deep sea involves multiple physiological challenges, such as high hydrostatic pressure, cold temperatures, low food availability, constant darkness and hypoxia. Not surprisingly, it has been assumed that metabolism in the deep sea is universally low and environmentally constrained. Seibel & Drazen (2007) argue, however, that although our knowledge of metabolic rates in aquatic organisms is limited, there is growing evidence that metabolic rates are not typically low and constrained. Although the metabolism of several important animal groups declines strongly with depth, the metabolism of others is similar between abyssal species and ecologically equivalent, shallow-water species. Most notable is the high metabolic demand that results from strong selection for locomotor capacity among visual predators inhabiting the well-lit epipelagic zone and the reduction in metabolic rates that follows relaxation of this selection in darkened bathypelagic waters. As pointed out by Seibel & Drazen (2007) technological limitations play an increasing role in defining our understanding of the relationship between organisms and their environment in deeper waters. Large organisms pose specific challenges to scientific enquiry. They relate, for instance, to the difficulty of obtaining laboratory or even field measurement of the processes of interest. One response to that difficulty has been the development of bioenergetics modelling. The contribution by Lovvorn (2007) illustrates this type of approach in understanding thermoregulation in diving animals. However, Lovvorn also raises the point that it remains very difficult to reconcile the time-scales at which the various subcomponents involved in preserving heat balance proceed, i.e. shivering, heat increment of feeding and thermal substitution of heat produced by exercising muscles.
(c) Interactions with behaviour
Physical and physiological constraints from the aquatic environment can, of course, affect behaviour. In the current issue, the impacts of temperature, dissolved oxygen (hypoxia) and turbidity on aquatic organisms are explored in terms of their effects upon predator–prey interactions, with one paper also investigating how thermal acclimation of swimming ability might interact with breeding behaviour. Other papers focus upon the behaviour of marine mammals: how locomotor patterns can be used to save energy and so extend apnoeic dive durations, how factors such as thermoregulatory demands and digestive capacity can interact with diving physiology to define foraging patterns and, finally, how foraging patterns reflect exploitation of areas where ectothermic prey are concentrated in response to environmental conditions.
Temperature will exert similar effects upon the physiology and metabolism of all ectothermic fishes, but there is little experimental evidence for how this might influence the dynamics of predator–prey interactions within piscine communities. Abrahams et al. (2007) used a theoretical modelling approach to investigate how a sustained increase in water temperature might influence predator–prey interactions between large predatory brown trout (Salmo trutta) and smaller Arctic charr (Salvelinus alpinus) prey in a freshwater lake. Somewhat surprisingly, the model revealed that higher overall metabolic costs of the largest predators would be difficult to sustain at the higher temperature, leading to their starvation and disappearance from the population. This would be beneficial to the prey species, which would exhibit population expansion.
Domenici et al. (2007) demonstrate that aquatic hypoxia exerts a direct limiting effect upon the anti-predator behaviour of a number of fish species. This may expose prey fishes to increased risks of predation by aerial (or terrestrial) predators that do not suffer the same hypoxic effects. These authors also consider how hypoxia can elicit behaviours that increase the risk of predation. For example, many fish species will travel to the surface in hypoxic waters, in an attempt to extract oxygen from the thin layer in contact with the atmosphere (aquatic surface respiration). This response can increase the risk of aerial predation. Indeed, fear of predation inhibits this response in fishes, thereby exacerbating the negative physiological effects of hypoxia. Another example relates to schooling behaviour: hypoxia causes schools to become less dense and this may increase predation risk for school members.
Meager & Batty (2007) found that water turbidity had complex effects upon prey-searching behaviour in cod (Gadus morhua), which use a combination of visual and olfactory cues. In particular, prey-searching behaviour in response to olfactory cues was reduced in waters of low turbidity but then increased significantly in highly turbid conditions. The increased activity in very turbid waters increases encounter rates but incurs a higher energetic cost. Thus, changes in water turbidity clearly have the potential to impact upon energetics of fishes through changes in their behaviour.
Breeding activities are another aspect of behaviour that may be affected by processes of physiological adaptation to the environment. Wilson et al. (2007) explored the effects of prior acclimation to a particular water temperature upon the ability of female mosquito fish (Gambusia holbrookii) to avoid sneaky copulations by males. This avoidance ability is dependent upon swimming performance and that, in turn, is improved in fish acclimated to higher temperatures. Contrary to expectations, although females could swim better at higher temperatures, they actually copulated more often. This was not because swimming performance of males was greater than that of females at the high temperature, but rather that the temperature had changed the propensity of females to mate.
For diving birds and mammals, a great deal of research has investigated how their behaviour patterns might be determined by the limits to aerobic performance during apnoea (Butler & Jones 1997). Much of this research is limited by the availability of technology that can provide adequate physiological and behavioural information on free-ranging animals at sea. One controversy that has emerged is what limits aerobic dive time. A theoretical aerobic dive limit (ADL) is often calculated (cADL) by dividing a species' body oxygen stores by an estimate of diving metabolic rate (Butler & Jones 1997). Direct observation, however, reveals that many endothermic divers regularly exceed their cADL (Boyd & Croxall 1996), suggesting that assumptions about body oxygen stores or metabolic rate must be incorrect. Davis & Weihs (2007) modelled the energetic cost of transit and deep foraging dives in elephant seals to test whether seals can use negative buoyancy to perform gliding descents and so accrue significant energetic savings. Their results show that there is little energetic advantage to transit dives with gliding descent when compared with horizontal swimming beneath the surface. Therefore, other factors such as feeding and predator avoidance may be important factors determining diving to depth during migration. This now requires direct validation on instrumented animals to investigate the extent to which this behaviour contributes to diving patterns in the wild.
Rosen et al. (2007) argue that, despite being the focus of much research, the ADL is only one factor among the physiological constraints within which foraging behaviour must operate in marine birds and mammals. Citing work on pinnipeds and cetaceans, the authors explore how foraging activity can also depend upon the ability to capture and process (digest) prey items. This, in turn, will interact with processes such as thermoregulation. Indeed, the circulatory demands of diving, thermoregulation and digestion may be mutually incompatible under some circumstances. Analysis of these interacting processes is, therefore, essential in developing foraging models and understanding the energetics of these animals in their natural environment.
Southern elephant seals, Mirounga leonina, undertake large-scale oceanic movements to access favourable foraging areas. Bailleul et al. (2007) used a new generation of satellite-relayed telemetry devices on seals to measure and transmit locations with water pressure, temperature and salinity. The authors implied foraging areas from increases in sinuosity of tracks, of dive density and of drift rate (which can indicate body condition). Favoured foraging areas had particular temperature signatures. Some seals targeted colder waters on the sea bottom during benthic dives along the Antarctic continent, while at the Polar Front seals tended to select warmer waters. The authors suggest that, in colder waters, ectothermic fish or squid prey may be easier to catch, while prey such as myctophids (lantern fish) may be most abundant in warmer waters. Thus, the foraging areas of the seals may be determined, at least in part, by the ectothermic physiology of their prey.
3. Future issues
(a) The need for integration
In order to be able to tackle the interactive scheme described in figure 1, and to make progress in mitigating the effects of anthropogenic factors on aquatic ecosystems, further interactions are needed between fields that investigate different levels of biological organization. Some examples of future developments that emerge from the papers presented in this special issue are outlined below.
Integration of modelling, numerical and observational studies are needed in relation to small-scale hydrodynamics interactions between organisms and various flow patterns (Gentien et al. 2007; Jiang & Strickler 2007; Strickler & Balázsi 2007). Novel technologies such as digital particle image visualization can be integrated with modelling to increase our understanding of such complex interactions. The integration of sensory and locomotor processes (Liao 2007) is a promising approach in investigating the effects of perturbed flow on fish populations, i.e. for the design of fish passageways that mitigate the effect of dams on fish migration. Behavioural components (Farrell 2007) also need to be considered when extrapolating laboratory data to field situations (rivers and passageways) and this recent work calls for a re-evaluation of the energetic costs of locomotion under natural conditions.
Full integration of laboratory and fieldwork is necessary to allow us to place environmental constraints within specific behavioural and ecological contexts, as well as to generate predictive environmental scenarios (Abrahams et al. 2007; Claireaux & Lefrançois 2007). This would enhance our ability to predict community changes related to habitat degradation and the current trends of environmental change. Laboratory experiments exploring responses between different species of predators and prey should be integrated with fieldwork to investigate how multiple factors, e.g. temperature or turbidity, affect fish behaviour (e.g. their activity levels) and physiology (e.g. their metabolic scope and sensory performance; Abrahams et al. 2007; Domenici et al. 2007; Meager & Batty 2007). At an applied level, work by McKenzie et al. (2007) shows that complex traits of performance and metabolism are potentially useful physiological biomarkers of sub-lethal aquatic pollution, which should be integrated into programs of ecological risk assessment alongside analyses of a suite of biochemical and molecular markers.
Over the last 40 years or so, many animal species have undergone changes in their behaviour and physiology (e.g. mating, reproducing, developing, habitat choice) as a result of climate changes. These changes are attributed to both phenotypic plasticity and genetic change (Bradshaw & Holzapfel 2006). Organisms can adapt to changes that may occur at different rates through phenotypic plasticity and natural selection. Interest in the ecological consequences of phenotypic plasticity, and the more specific case of thermal acclimation, is rapidly expanding (Miner et al. 2005). More work on the phenotypic plasticity of traits of environmental adaptation is needed. Work by Wilson et al. (2007) shows how conflict between the sexes in mating behaviour with thermal acclimation offers a range of future questions addressing the ecological implications of phenotypic plasticity and their consequences for sexual interactions in nature. With some examples, Claireaux & Lefrançois (2007) have illustrated how unravelling the environmental influences on phenotype performance and adaptive flexibility opens new fields of research, particularly in linking ecophysiology and evolutionary biology. Since environmental factors can affect a whole population, they are believed to be more effective initiators of selectable evolutionary novelties than mutations which initially only affect one individual (West-Eberhard 2005). Selection experiments using model animals in environmental physiology are promising as they can help clarify what the upper limit of a given population might be in response to environmental constraints (Garland & Kelly 2006).
(b) Differential effects on predators and prey
An important issue that needs further attention is the ecological implications of how factors such as temperature (Farrell 2007; Lovvorn 2007; Rome 2007; Rosen et al. 2007) and oxygen (Abrahams et al. 2007; Domenici et al. 2007) can exert different constraints on the physiology of different animal groups; for example, upon ectotherms versus endotherms or water breathers versus air breathers. Most aquatic ectotherms are also water breathers, therefore these effects may be additive. Some of these ecological effects have been studied extensively, such as the effects of hypoxia on the predator–prey relationships between water breathers (e.g. fish) and air breathers (e.g. birds; Kramer 1987; Domenici et al. 2007). In hypoxia, certain bird populations can take advantage of the sluggish behaviour of fish and their tendency to come to the surface to perform aquatic surface respiration (Kersten et al. 1991). However, the extent of this phenomenon is less well known in other predator–prey ‘pairs’ which are constrained to different extents by a given environmental factor. For example, little is known about the extent to which marine mammals and other endotherms take advantage of the negative effects of hypoxia and temperature on their prey (Bailleul et al. 2007).
Turbidity may also affect predator and prey to different extents (Abrahams et al. 2007; Meager & Batty 2007). Turbidity is bound to affect visual organisms, but animals that can rely on other sensory systems may be able to compensate for the decrease in information from vision. Increases in turbidity may cause shifts in the abundance of predator and prey populations with consequences at the ecosystem level, including detrimental effects for biodiversity (Abrahams et al. 2007). More information is needed on the relative importance of sensory systems and their ecological effects in organisms of various taxa.
(c) Technological developments
To answer the questions posed above, and many others within the field of ecophysiology, there is a pressing need for development in technologies to monitor activity of organisms in the field, especially in areas such as deep and pelagic waters where the behaviour of aquatic organisms is poorly described (Bailleul et al. 2007; Davis & Weihs 2007; Seibel & Drazen 2007). This should be approached at three levels to: (i) monitor the physical environment, (ii) monitor the basic physiological processes of free living organisms, and (iii) monitor their behaviour. Recent developments in data logging and telemetry have increased our understanding of the ecological implications of environmental constraints (Daunt et al. 2003; Kawabe et al. 2004; Ropert-Coudert & Wilson 2005; Weng et al. 2005; Wilson et al. 2005; Kuhn et al. 2006; Metcalfe et al. 2006; Sims et al. 2006). Similarly, fieldwork on marine mammals and birds using miniature cameras mounted on the animals is starting to reveal the behaviour of these organisms within their environment (Williams et al. 2000; Gremillet et al. 2006). However, these technologies still require significant improvement. For instance, the size of telemetry tags remains a source of serious potential interference with animal physiology and behaviour. Other limitations are reduced acoustic range and limited autonomy. The recent development of miniaturized data loggers and satellite tags has resolved some of these problems but, because loggers need to be recovered to unload the data, their usage is generally limited to commercially exploited species and life stages. Moreover, although data loggers have provided interesting information about the environmental conditions organisms live in and, to a lesser extent, about their behaviour, information about their physiology awaits further development of appropriate sensors. New technologies to track physiological functions of animals in their natural environment (e.g. telemetry and satellite tracking) can provide important information about how the locomotor apparatus is used and the environment in which it operates (Dewar et al. 1999; Block et al. 2001). Further, by assessing the prevailing temperature ranges, and the time course over which organisms actually experience them, we can design appropriate experiments to investigate processes of acclimatization in animals in their natural environment.
4. Concluding remarks
A primary objective of this issue was to highlight the fact that, despite many difficulties and limitations, integrative research projects can contribute profoundly to our understanding of how environmental factors constrain locomotion and predator–prey interactions in aquatic organisms. Such studies reveal the range and intricacy of regulatory responses that aquatic organisms can implement, and provide valuable information about how the effects of environmental constraints can propagate across levels of biological organization. Understanding the ecological and evolutionary significance of processes of environmental adaptation is essential to our ability to predict and manage anthropogenic impacts upon aquatic ecosystems.
Acknowledgments
The authors thank the Society for Experimental Biology for hosting the session upon which this theme issue is based, the authors of the papers in this issue, the referees and Jay Nelson and Denis Chabot for their useful comments on an earlier draft of this introductory article and James Joseph for his editorial support. Financial support was provided by the European Union, Directorate Fisheries, through contract QLRS-2002-00799 (project ETHOFISH).
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Abrahams M.V, Mangel M, Hedges K. Predator–prey interactions and changing environments: who benefits? Phil. Trans. R. Soc. B. 2007;362:2095–2104. doi: 10.1098/rstb.2007.2102. doi:10.1098/rstb.2007.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul F, Charrassin J.-B, Monestiez P, Roquet F, Biuw M, Guinet C. Successful foraging zones of southern elephant seals from Kerguelen Islands in relation to oceanographic conditions. Phil. Trans. R. Soc. B. 2007;362:2169–2181. doi: 10.1098/rstb.2007.2109. doi:10.1098/rstb.2007.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B.A, et al. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science. 2001;293:1310–1314. doi: 10.1126/science.1061197. doi:10.1126/science.1061197 [DOI] [PubMed] [Google Scholar]
- Bonsdorf E, Blomquist E.M, Mattila J, Norkko A. Long-term changes and coastal eutrophication. Examples from the Åland Islands and the Archipelago Sea, northern Baltic Sea. Oceanol Acta. 1997;20:319–329. [Google Scholar]
- Boyd I.L, Croxall J.P. Dive durations in pinnipeds and seabirds. Can. J. Zool. 1996;74:1696–1705. [Google Scholar]
- Bradshaw W.E, Holzapfel C.M. Climate change—evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. doi:10.1126/science.1127000 [DOI] [PubMed] [Google Scholar]
- Butler P.J, Jones D.R. Physiology of diving of birds and mammals. Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Lefrançois C. Linking environmental variability and fish performance: integration through the concept of scope for activity. Phil. Trans. R. Soc. B. 2007;362:2031–2041. doi: 10.1098/rstb.2007.2099. doi:10.1098/rstb.2007.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunt F, Peters G, Scott B, Gremillet D, Wanless S. Rapid-response recorders reveal interplay between marine physics and seabird behaviour. Mar. Ecol. Prog. Ser. 2003;255:283–288. [Google Scholar]
- Davis R.W, Weihs D. Locomotion in diving elephant seals: physical and physiological constraints. Phil. Trans. R. Soc. B. 2007;362:2141–2150. doi: 10.1098/rstb.2007.2107. doi:10.1098/rstb.2007.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar H, Deffenbaugh M, Thurmond G, Lashkari K, Block B.A. Development of an acoustic telemetry tag for monitoring electromyograms in free-swimming fish. J. Exp. Biol. 1999;202:2693–2699. doi: 10.1242/jeb.202.19.2693. [DOI] [PubMed] [Google Scholar]
- Diaz R.J, Rosenberg R. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Rev. 1995;33:245–303. [Google Scholar]
- Domenici P, Lefrançois C, Shingles A. Hypoxia and the antipredator behaviours of fishes. Phil. Trans. R. Soc. B. 2007;362:2105–2121. doi: 10.1098/rstb.2007.2103. doi:10.1098/rstb.2007.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A.P. Cardiorespiratory performance during prolonged swimming tests with salmonids: a perspective on temperature effects and potential analytical pitfalls. Phil. Trans. R. Soc. B. 2007;362:2017–2030. doi: 10.1098/rstb.2007.2111. doi:10.1098/rstb.2007.2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry F.E.J. Effect of the environment on animal activity. Univ. Toronto Stud. Biol. Ser. 1947;55:1–62. [Google Scholar]
- Garland T, Jr, Kelly S.A. Phenotypic plasticity and experimental evolution. J. Exp. Biol. 2006;209:2344–2361. doi: 10.1242/jeb.02244. doi:10.1242/jeb.02244 [DOI] [PubMed] [Google Scholar]
- Gentien P, Lunven M, Lazure P, Youenou A, Crassous M.P. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae) Phil. Trans. R. Soc. B. 2007;362:1937–1946. doi: 10.1098/rstb.2007.2079. doi:10.1098/rstb.2007.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebmeier J.M, et al. A major ecosystem shift in the Northern Bering sea. Science. 2006;311:1461–1463. doi: 10.1126/science.1121365. doi:10.1126/science.1121365 [DOI] [PubMed] [Google Scholar]
- Gremillet D, Enstipp M.R, Boudiffa M, Liu H. Do cormorants injure fish without eating them? An underwater video study. Mar. Biol. 2006;148:1081–1087. doi:10.1007/s00227-005-0130-2 [Google Scholar]
- Jiang H, Strickler J.R. Copepod flow modes and modulation: a modeling study of the water currents produced by an unsteadily swimming copepod. Phil. Trans. R. Soc. B. 2007;362:1959–1971. doi: 10.1098/rstb.2007.2081. doi:10.1098/rstb.2007.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe R, Naito Y, Sato K, Miyashita K, Yamashita N. Direct measurement of the swimming speed, tailbeat, and body angle of Japanese flounder (Paralichthys olivaceus) ICES J. Mar. Sci. 2004;7:1080–1087. doi:10.1016/j.icesjms.2004.07.014 [Google Scholar]
- Kersten M, Britton R.H, Dugan P.J, Hafner H. Flock feeding and food intake in little egrets: the effects of prey distribution and behaviour. J. Anim. Ecol. 1991;60:241–252. doi:10.2307/5457 [Google Scholar]
- Kramer D.L. Dissolved oxygen and fish behaviour. Environ. Biol. Fish. 1987;18:81–92. doi:10.1007/BF00002597 [Google Scholar]
- Kuhn C.E, McDonald B.I, Shaffer S.A, Barnes J, Crocker D.E, Burns J, Costa D.P. Diving physiology and winter foraging behavior of a juvenile leopard seal (Hydrurga leptonyx) Polar Biol. 2006;29:303–307. doi:10.1007/s00300-005-0053-x [Google Scholar]
- Liao J.C. A review of fish swimming mechanics and behaviour in altered flows. Phil. Trans. R. Soc. B. 2007;362:1973–1993. doi: 10.1098/rstb.2007.2082. doi:10.1098/rstb.2007.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovvorn J.R. Thermal substitution and aerobic efficiency: measuring and predicting effects of heat balance on endotherm diving energetics. Phil. Trans. R. Soc. B. 2007;362:2079–2093. doi: 10.1098/rstb.2007.2110. doi:10.1098/rstb.2007.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie D.J, et al. Complex physiological traits as biomarkers of the sub-lethal toxicological effects of pollutant exposure in fishes. Phil. Trans. R. Soc. B. 2007;362:2043–2059. doi: 10.1098/rstb.2007.2100. doi:10.1098/rstb.2007.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager J.J, Batty R.S. Effects of turbidity on the spontaneous and prey-searching activity of juvenile Atlantic cod (Gadus morhua) Phil. Trans. R. Soc. B. 2007;362:2123–2130. doi: 10.1098/rstb.2007.2104. doi:10.1098/rstb.2007.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J.D, Hunter E, Buckley A.A. The migratory behaviour of North Sea plaice: currents, clocks and clues. Mar. Freshw. Behav. Physiol. 2006;39:25–36. doi:10.1080/10236240600563404 [Google Scholar]
- Miner B.G, Sultan S.E, Morgan S.G, Padilla D.K, Relyea R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005;20:685–692. doi: 10.1016/j.tree.2005.08.002. doi:10.1016/j.tree.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Pihl L, Baden S.P, Diaz R.J. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar. Biol. 1991;108:349–360. doi:10.1007/BF01313644 [Google Scholar]
- Rome L.C. The effect of temperature and thermal acclimation on the sustainable performance of swimming scup. Phil. Trans. R. Soc. B. 2007;362:1995–2016. doi: 10.1098/rstb.2007.2083. doi:10.1098/rstb.2007.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropert-Coudert Y, Wilson R.P. Trends and perspectives in animal-attached remote sensing. Front. Ecol. Environ. 2005;3:437–444. [Google Scholar]
- Rosen D.A.S, Winship A.J, Hoopes L.A. Thermal and digestive constraints to foraging behaviour in marine mammals. Phil. Trans. R. Soc. B. 2007;362:2151–2168. doi: 10.1098/rstb.2007.2108. doi:10.1098/rstb.2007.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel B.A, Drazen J.C. The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Phil. Trans. R. Soc. B. 2007;362:2061–2078. doi: 10.1098/rstb.2007.2101. doi:10.1098/rstb.2007.2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims D.W, Witt M.J, Richardson A.J, Southall E.J, Metcalfe J.D. Encounter success of free-ranging marine predator movements across a dynamic prey landscape. Proc. R. Soc. B. 2006;273:1195–1201. doi: 10.1098/rspb.2005.3444. doi:10.1098/rspb.2005.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J.R, Balázsi G. Planktonic copepods reacting selectively to hydrodynamic disturbances. Phil. Trans. R. Soc. B. 2007;362:1947–1988. doi: 10.1098/rstb.2007.2080. doi:10.1098/rstb.2007.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng K.C, Castilho P.C, Morrissette J.M, Landeira-Fernandez A.M, Holts D.B, Schallert R.J, Goldman K.J, Block B.A. Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science. 2005;310:104–106. doi: 10.1126/science.1114616. doi:10.1126/science.1114616 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B. 2005;304:610–618. doi: 10.1002/jez.b.21071. doi:10.1002/jez.b.21071 [DOI] [PubMed] [Google Scholar]
- Williams T.M, Davis R.W, Fuiman L.A, Francis J, Le Boeuf B.L, Horning M, Calambokidis J, Croll D.A. Sink or swim: strategies for cost-efficient diving by marine mammals. Science. 2000;288:133–136. doi: 10.1126/science.288.5463.133. doi:10.1126/science.288.5463.133 [DOI] [PubMed] [Google Scholar]
- Wilson S.G, Lutcavage M.E, Brill R.W, Genovese M.P, Cooper A.B, Everly A.W. Movements of bluefin tuna (Thunnus thynnus) in the northwestern Atlantic Ocean recorded by pop-up satellite archival tags. Mar. Biol. 2005;146:409–423. doi:10.1007/s00227-004-1445-0 [Google Scholar]
- Wilson R.S, Condon C.H.L, Johnston I.A. Consequences of thermal acclimation for the mating behaviour and swimming performance of female mosquito fish. Phil. Trans. R. Soc. B. 2007;362:2131–2139. doi: 10.1098/rstb.2007.2106. doi:10.1098/rstb.2007.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]