Abstract
One potential use for prostate-cancer-associated genes discovered through ongoing genetics studies entails the construction of virus- or plasmid-based recombinant vector vaccines encoding these new tumor-associated antigens (TAA) to induce TAA-specific immune responses for the prevention or therapy of prostate cancer. Clinical trials evaluating prototypes of such recombinant vaccines are under way. TAA-encoding recombinant vector vaccines, however, have not previously been evaluated in a prostate-cancer animal model. For assessment of the potential susceptibility of prostate cancer to genetic immunization strategies using TAA-encoding recombinant vectors, the antitumor efficacy of a model recombinant viral vector encoding a TAA was evaluated in rat Dunning prostate cancer. Recombinant vaccinia was chosen as a prototype virus vector encoding a TAA for these studies, and β-galactosidase was chosen as a model target TAA. Dunning AT-2 cells were transduced with a retroviral vector to express β-galactosidase, and the susceptibility of tumorigenic AT-2-lacZ cells to immunization with vaccinia-lacZ was measured using protection studies in Copenhagen and nu/nu rats. Stably transduced AT-2-lacZ cells expressing β-galactosidase as measured by enzymatic substrate-based assays were found to retain their tumorigenicity in vivo despite abundant expression of rat major histocompatibility complex (MHC) class I. Immunization with model TAA-encoding recombinant vaccinia-lacZ conferred significant protection against subsequent growth of AT-2-lacZ cells in vivo (P = 0.01); however, the efficacy of such immunization was markedly dependent on the volume of tumor challenge. The antitumor efficacy of TAA-encoding recombinant vaccinia immunization was abrogated in nu/nu rats, suggesting a T-cell-dependent mechanism of activity. These studies suggest that prostate cancer may be a suitable target for immunization strategies using TAA-encoding recombinant vectors. Such immunization strategies may be more effective in settings of minimal cancer burden.
Advances in tissue and genetic engineering have opened new avenues for the induction of immune responses to prostate cancer as potential therapy. The susceptibility of prostate cancer to genetic manipulation of the immune response has been demonstrated in preclinical animal models, and clinical trials based on these studies are under way [7, 20, 25, 28, 31]. Initial clinical studies using recombinant and tissue engineering for augmentation of prostate-cancer-specific immunity focused on ex vivo manipulations, such as transduction of tumor cells with immunostimulatory genes or loading of in-vitro-differentiated dendritic cells with prostatic antigens, followed by adoptive transfer of such cells to induce prostate-cancer-specific immunity in the recipients of the adoptive cells [22, 28]. These approaches, however, are limited in their potential for widespread use due to the requirement for individual in vitro growth of patient-derived cells. For example, therapy using gene-modified autologous tumor cells is potentially limited in its applicability to patients with a paucity of cells for procurement, such as men presenting with recurrent prostate cancer following radical prostatectomy. In addition, the requirement for cell culture implies potential costs that may prohibit the widespread use of vaccine therapy with syngeneic patient-derived cancer cells.
Alternative recombinant vaccine therapies have been developed in animal models of nonprostate tumors that circumvent these limitations of patient-derived, cultured-cell-based vaccines. These therapies include the delivery of immunostimulatory genes to primary tumors in situ [1, 17] and the use of recombinant viral or plasmid vaccines encoding tumor-associated antigens (TAA) as targets of therapy [5, 6, 16, 19, 21]. The latter has led to clinical trials using recombinant vaccinia vectors encoding TAA in colon cancer and cervical cancer, with biologic effects being demonstrable in phase I and II studies [3, 30]. Analogous clinical trials using vaccinia vectors encoding TAA have been proposed for human prostate cancer [8, 9, 13, 27].
The ability of recombinant viral or plasmid vectors (encoding specific TAA) to induce an immune response capable of suppressing tumor growth and progression, however, has not previously been evaluated in a prostate-cancer animal model. To assess the suitability of prostate cancer as a potential target for TAA-encoding recombinant vector vaccines, we therefore evaluated the effects of immunization with recombinant vaccinia encoding a model TAA (β-galactosidase) in Dunning rat prostate cancer.
Methods
Retroviral transduction of AT-2 Dunning rat prostate-cancer cells
Retroviral vector (BAG) containing the lacZ gene regulated by a long-terminal-repeat (LTR) promoter and a neomycin-resistance gene regulated by the cytomegalovirus (CMV) promoter were collected in supernatants harvested from established ψCRIP producer cells [23]. Retroviral supernatants were filtered through 0.45-μm filters and frozen at −80 °C until transduction, which was accomplished by the addition of BAG retrovirus supernatant supplemented with DEAE-Dextran (Sigma, St. Louis, Mo.) to subconfluent AT-2 cells (obtained from Dr. K. Pienta, University of Michigan) as described elsewhere [25]. Beginning at 48 h after BAG transduction, retrovirally transduced AT-2-lacZ cells were selected by growth in complete medium [Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS); Gibco-BRL, Gaithersburg, Md.] supplemented with G418 (500 μg/ml).
Fluorescence-activated cell-sorting analyses
For the fluorescence-activated cell-sorting (FACS)-gal assay of β-galactosidase activity, 5 × 105 BAG-transduced or control AT-2 cells were spun to a pellet, to which 50 μl of 37 °C phosphate- buffered saline (PBS)/10% FCS (Gibco-BRL) and 50 μl of 2 mM fluorescein digalactosidase (FDG-Molecular Probes) was added at 37 °C for exactly 1 min, after which 1 ml of ice-cold PBS was added and the tubes were placed on ice. The β-galactosidase activity was then quantified using FACS (FACstar, Becton Dickenson) as previously described [11]. Expression of β-galactosidase by sorted, FACS-gal-positive cells was confirmed by X-gal assay as described elsewhere [25].
For FACS analysis of major histocompatibility complex (MHC) class I expression, samples of 5 × 105 AT-2 cells were spun to a pellet, resuspended in 100 μl of FCS for 5 min at room temperature, rinsed with FACS buffer (2 ml of 1XPBS containing 1% FCS) twice, incubated with 10 μl of monoclonal antibody (mAb) OX18 (anti-rat MHC class I; Sera-Lab, Crawley Down, Sussex, UK) or control mouse IgG1 at 4 °C for 30 min, washed in FACS buffer, and resuspended, and the intensity of fluorescent staining in the resuspended cells was evaluated in the FL1 channel of a FACStar device (Becton Dickenson).
Tumor growth in vivo and effects of immunization using recombinant vaccinia
The growth of parental wild-type Dunning AT-2 prostate-cancer cells was compared with that of lac-Z-transduced AT-2 cells in syngeneic-host Copenhagen rats (Harlan Sprague-Dawley Inc, Indianapolis, Ind.). Animals were injected subcutaneously with transduced or control tumor cells, were monitored for appearance of visible tumors every other day, and tumors were measured in three dimensions. Tumor volumes were determined using the equation volume = 4/3 π[(r1 + r2 + r3)/3]³. Vaccinia-lacZ containing lac-Z under the control of the synthetic MSC-65 promoter [21] was isolated by sucrose-gradient purification of HeLa-cell lysates and titered via infection of BOSC monolayers as previously described [6]. In recombinant vaccinia immunization studies, male Copenhagen rats received 5 × 107 plaque-forming units (pfu) of vac-lacZ or carrier control by tail-vein injection and were challenged with 105 or 106 lacZ-transduced AT-2 cells or control (untransduced) AT-2 cells implanted by subcutaneous injection 26 days later. For evaluation of the role of T-cell-mediated immunity in protection against tumor challenge, this immunization and tumor-challenge protocol was repeated in nu/nu rats (Harlan Sprague-Dawley). All animal experiments and protocols were approved by the University of Michigan Committee on Use and Care of Animals in compliance with NIH guidelines.
Statistical analysis
Statistical analyses were performed using Statistica software (StatSoft, Tulsa, Okla.). Survival data were graphically summarized using Kaplan-Meier plots and were analyzed using the log-rank test. Longitudinal measurements of tumor-volume change were compared using analysis of variance (ANOVA). Determinations of the mean time required for tumors to become palpable were compared using Wilcoxon’s rank-sum analysis.
Results
Dunning AT-2 prostate-cancer cells expressing a transduced, model TAA retain their capacity to form tumors in Copenhagen rats
For evaluation of the susceptibility of prostate-cancer cells to immunization with recombinant vaccinia vectors encoding defined TAA, a syngeneic prostate-cancer cell line encoding a model target TAA was required. The AT-2 line of Dunning tumors was chosen for this purpose, since AT-2 cells grow principally as primary tumors, which facilitates the longitudinal evaluation of tumor growth in vivo because tumor measurement at the site of implantation accurately represents the total tumor burden [14]. In preparation for immunization studies using recombinant poxvirus targeting a specific TAA, AT-2 cells were transduced with a retrovirus vector encoding lacZ such that they would stably express lacZ as a model TAA; lacZ was chosen due to the availability of both retroviral and poxviral vectors encoding this gene. Following BAG-lacZ retroviral vector transduction (the BAG-lacZ vector encodes lacZ as well as neomycin resistance [23]), transduced AT-2 cells were grown in G418 for elimination of nontransduced cells, and enrichment of AT-2 cells expressing significant levels of lacZ was accomplished by FACS sorting of lacZ-expressing cells identified using the FACS-gal assay of β-galactosidase enzymatic activity (Fig. 1a).
Fig. 1.
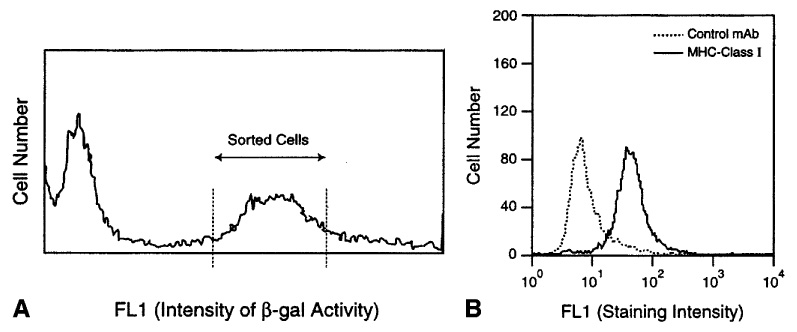
A, B Isolation and characterization of a Dunning AT-2 prostate-cancer cell line transduced to express lacZ as a model TAA. A Following retrovirus tranduction, lacZ expression was measured by FACS-gal assay; cells expressing high levels of β-galactosidase were selected as shown using the cell sorter and were used for subsequent immunization studies. B Class I MHC expression was confirmed in AT-2 cells using mAb OX-18
Expression of β-galactosidase by these positively sorted AT-2-lacZ cells was then confirmed by X-gal assay, which showed that >90% of the cells expressed abundant β-galactosidase at levels detectable by this assay. Transduction of a tumor cell line by retrovirus vector encoding lacZ as a model heterologous (nonself) TAA, however, could potentially alter the baseline growth characteristics of such tumor cells in vivo, particularly as AT-2 cells were also found to express MHC class I at significant levels detectable by FACS (as demonstrated by FACS with the rat MHC class-I-specific monoclonal antibody Ox-18; Fig. 1b). The in vivo growth of AT-2-lacZ cells was therefore compared with that of untransduced AT-2 cells to determine whether the ability of these cells to form tumors was conserved. All rats injected with either AT-2 or AT-2-lacZ formed tumors within 2 weeks of the injection of 105 or 106 cells in the hind leg (n = 12), providing a basis for use of the AT-2-lacZ Dunning prostate-cancer cells for subsequent immunization and tumor-challenge studies.
Prevention of tumor growth by in vivo delivery of a model TAA by recombinant vaccinia is dependent on the tumor volume
Studies evaluating the in vivo susceptibility of prostate cancer to immunization with TAA-encoding recombinant vaccinia vectors were undertaken. The growth of Dunning AT2-lacZ prostate cancers was evaluated in Copenhagen rats immunized with vaccinia vector encoding lacZ (vaccinia-lacZ) and in carrier-control-immunized rats. Tumor-free survival and increases in measured tumor volume after tumor onset were compared between these groups. The effect of the tumor burden on the efficacy of antigen-specific immune protection against subsequent tumor challenge was tested by evaluation of the efficacy of immunization in protecting against a minimal tumor burden (105 AT-2-lacZ cells/rat) and a moderate tumor burden (106 AT-2-lacZ cells/rat).
In the cohort of animals that had received a minimal burden of AT-2-lacZ tumor cells (105 cells/rat), tumor-free survival was significantly improved following vaccinia-lacZ immunization of rats challenged with AT2-lacZ tumor as compared with survival of control rats (Fig. 2a). The protective effect of immunization was specific for the TAA (lacZ) as evidenced by the lack of effect of vaccinia-lacZ immunization on tumor growth of untransduced AT-2 cells lacking the target tumor antigen (data not shown). The protective antitumor effect of antigen-specific immunization with recombinant vaccinia seen in the minimal-tumor-burden cohort, however, was markedly attenuated in animals receiving a 1-log (incrementally) higher burden of tumor cells (106 AT-2-lacZ cells/rat; Fig. 2b.). These findings suggest that in the use of a model TAA as a target of recombinant poxvirus immunization, prevention of Dunning prostate cancer via tumor-antigen-specific immunization is more effective at minimal volumes of tumor burden or tumor challenge.
Fig. 2.
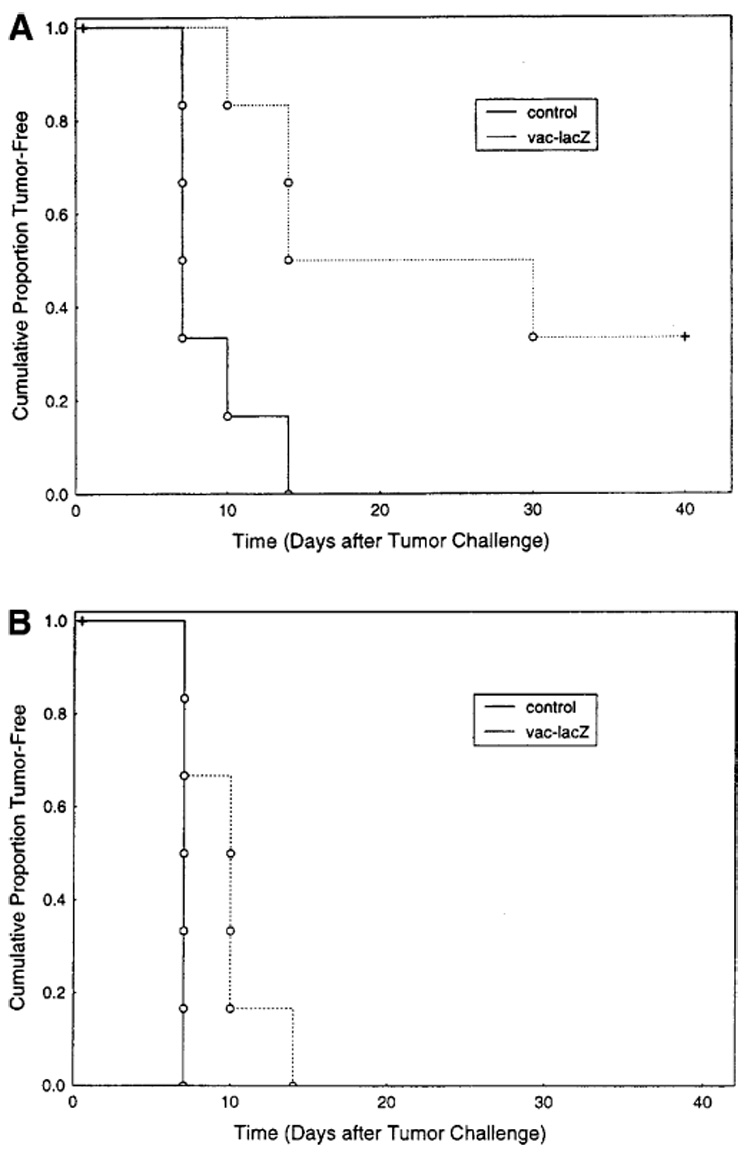
A, B Immunization with recombinant vaccinia targeting a specific TAA prolongs tumor-free survival in rats challenged with Dunning prostate cancer. Copenhagen rats were challenged with AT-2-lacZ tumor cells following immunization with vaccinia-lacZ or the carrier control. A Minimal tumor burden challenge comprising 105 AT-2-lacZ cells/rat (P = 0.01). B Moderate tumor challenge comprising 106 AT-2-lacZ cells/rat (P = 0.05)
The effects of TAA-specific immunization on the progression of AT-2-lacZ cancers was further evaluated by measurement of longitudinal changes in the average volume of palpable and growing AT-2-lacZ tumors in these animals. This end point showed similar responses to TAA-specific immunization as did tumor-free survival, i.e., in the minimal-tumor-burden model, AT-2-lacZ tumors in animals immunized with vaccinia-lacZ were smaller than the corresponding tumors in animals immunized with the carrier control (Fig. 3a). In contrast, the higher-tumor-burden cohort (106 AT-2-lacZ cells/rat) showed no longitudinally detectable difference in tumor volume between rats receiving model TAA-specific immunization and rats immunized with the carrier control (Fig. 3b). These findings support the hypothesis that the antitumor efficacy of immunization with TAA-encoding recombinant poxviruses may be limited to a setting of minimal tumor burden.
Fig. 3.
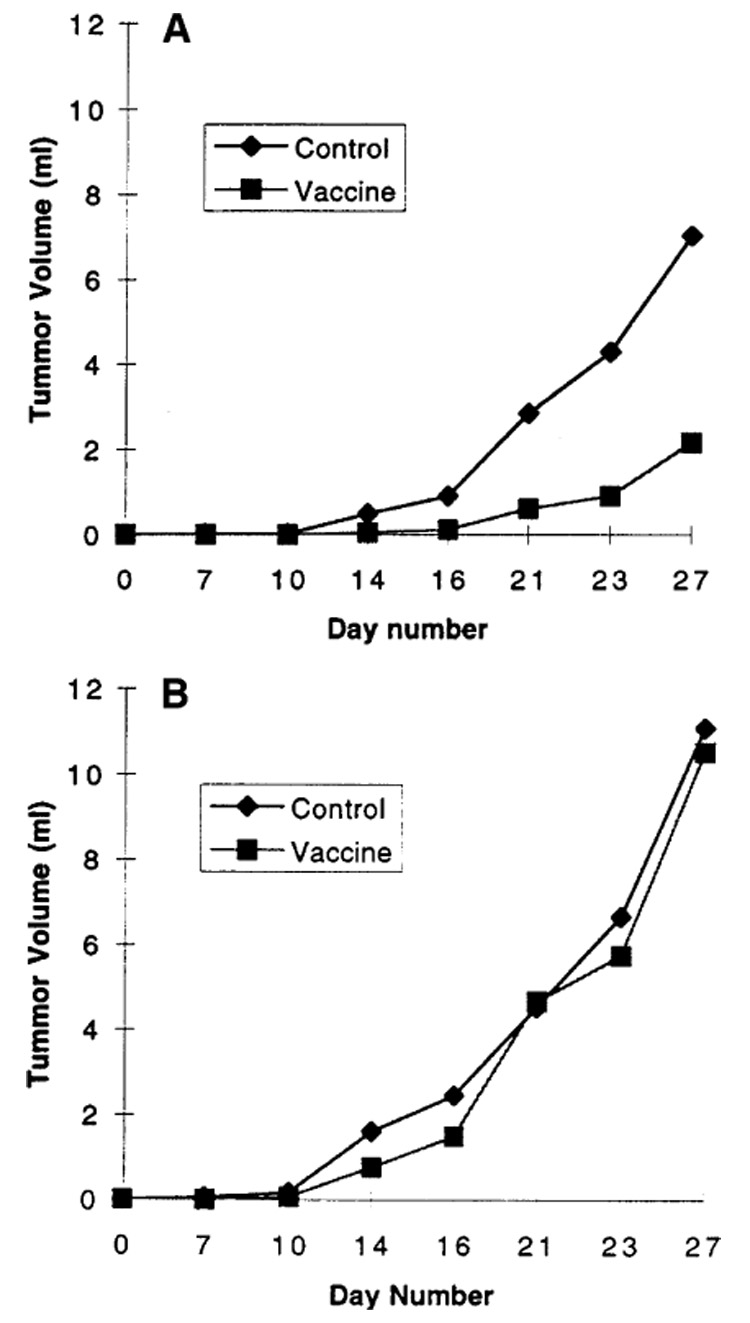
A, B Delay of Dunning prostate-cancer growth and progression following immunization with target antigen-encoding recombinant vaccinia requires a minimal tumor burden. Progression in tumor volume was measured longitudinally in Copenhagen rats that developed tumors refractory to vaccinia-lacZ immunization. A Minimal tumor burden challenge comprising 105 AT-2-lacZ cells/rat (P < 0.05, ANOVA). B Moderate tumor challenge comprising 106 AT-2-lacZ cells/rat (no significant difference)
Requirement of competent host T-cell function for antitumor efficacy of immunization with recombinant vaccinia vectors
To determine whether competent T-cell function was required for the in vivo antitumor efficacy of TAA-specific immunization by recombinant poxviruses in the Dunning prostate-cancer model, immunization and challenge experiments were undertaken using athymic nu/nu rats. The minimal-tumor-burden model (which was responsive to vaccinia-lacZ immunization in immunologically competent Copenhagen rats) was used for these studies. In athymic nu/nu rats, which lack normal T-cell function, no difference in protection against a minimal tumor challenge was seen between rats immunized with lacZ TAA-encoding vaccinia and those immunized with control vaccinia (Fig. 4). These findings indicate that competent host T-cell function was required for TAA-encoding recombinant poxvirus to impart effective protection against the growth and progression of prostate-cancer cells expressing the target TAA.
Fig. 4.
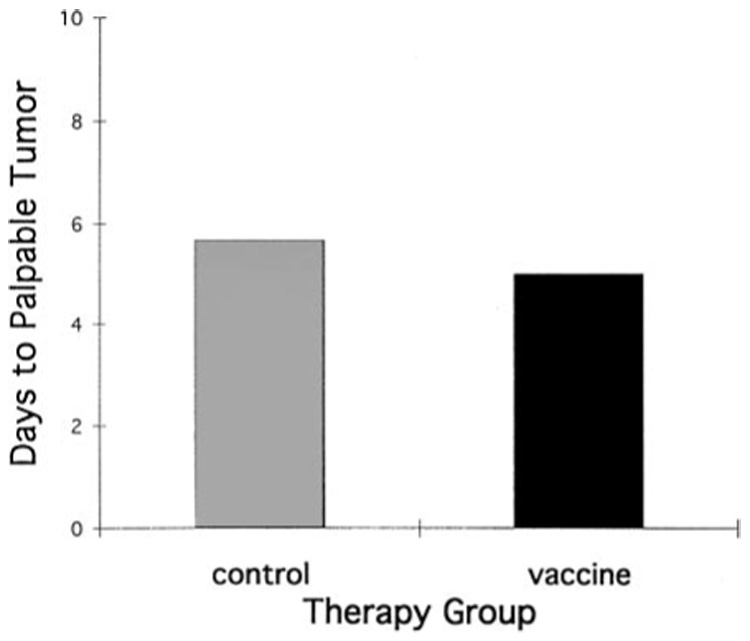
Competent T-cell function is required for the efficacy of recombinant vaccinia immunization against Dunning rat prostate cancer. Mean time (y-axis days) to the development of palpable/detectable tumor in nu/nu rats challenged with 105 AT-2-lacZ cells/rat following immunization with vaccinia-lacZ or control vaccinia. No significant difference in tumor development was seen between vaccinia-lacZ- and control-immunized nu/nu rats
Discussion
Novel approaches to tumor-vaccine therapy are under current preclinical and clinical development as alternative therapeutic strategies for prostate cancer. Efforts to generate effective prostate-cancer vaccines, which had previously been hampered by the limited availability of a tissue-vaccine source and nonspecific adjuvants [4, 12], were rejuvenated by advances in molecular techniques, which have allowed the production of recombinant vaccines designed for further enhancement or better targeting of tumor-specific immune responses [10, 21]. Among the approaches for prostate-cancer vaccine therapy that have recently been explored preclinically (as well as in clinical trials) are cellular vaccines comprising either prostate-cancer cells transduced to produce immunomodulatory gene products or patient-derived adoptive-cell transfer of antigen-presenting cells preloaded with prostate TAA [7, 20, 22, 25, 28, 31]. An emerging alternative tumor-vaccine strategy entails the use of recombinant viral vectors encoding specific TAA. This approach has the potential advantages of directly targeting one or more of the many prostate-cancer-associated gene products identified via differential display and other molecular-science techniques [15, 24, 32] while bypassing costly in vitro culture of patient-derived prostate-cancer or antigen-presenting cells.
Although prostate-cancer animal models, such as the Dunning model, have been evaluated extensively with regard to their susceptibility to genetically engineered tumor-cell vaccines or tumor-cell-lysate vaccines [7, 18, 20, 25, 28, 31], there has been a lack of published data regarding the susceptibility of animal prostate-cancer models to recombinant viral (cell-free) vaccines encoding specific TAA. Recombinant poxviruses are an especially promising molecular tool for the induction of TAA-specific immune responses [19, 21]. The prototype of poxvirus vectors is vaccinia, the contemporary descendent of the first virally based vaccine used in humans by Edward Jenner over 200 years ago. The efficacy of vaccinia as a safe and potent immunogen has been established by its worldwide use in the eradication of smallpox. Subsequent construction of plasmid systems for the introduction of novel genes in vaccinia led, first, to the construction of recombinant vaccinia encoding antigens for the induction of immunity to human immunodeficiency virus (HIV) and other infectious diseases and, more recently, to the construction of model TAA-encoding vaccinia vectors, which have been widely studied as tumor vaccines in nonprostate animal models [6, 16, 19, 21]. On the basis of these prior preclinical studies, recombinant vaccinia encoding carcinoembryonic antigen (CEA), human papillomavirus (HPV) proteins, and other TAA have entered clinical trials in colon, pancreatic, and cervical cancers [3, 30].
As a potential clinical target for recombinant poxvirus vaccines, prostate cancer poses unique opportunities and obstacles to effective vaccine therapy. The unique opportunities include a significant prevalence of microscopic disease burden without an accepted, low-morbidity curative therapy: serologic recurrence following radical prostatectomy. The importance of focusing recombinant vaccine therapy such that it targets a minimal tumor burden (such as the clinical correlate of recurrence of rising prostate-specific antigen (PSA) levels after ablative locoregional therapy) was demonstrated by our observation of differential efficacy for recombinant vaccinia in relation to tumor volume. These findings support a strategy whereby clinical studies conducted for the development of prostate-cancer TAA-encoding recombinant poxvirus prototypes (such as vaccinia-PSA) would focus on postsurgically recurrent prostate cancer [8, 9, 13, 27].
The desirable characteristics of prostate cancer as a target for vaccine therapy, such as the prevalence of men with serologically measurable microscopic tumor burdens as subjects for rational vaccine therapy, are contrasted by potential obstacles to vaccine efficacy related to the poorly immunogenic phenotype of prostate cancer. The Dunning model of prostate cancer is highly relevant in this regard, as Dunning cells have previously been found to be only weakly immunogenic, if at all, in the absence of a genetically engineered immunostimulatory signal [20]. This characteristic distinguishes Dunning prostate cancers from immunogenic tumors, which have been the principal focus of recombinant poxvirus vaccine studies in nonprostate tumors [6, 16, 19]. Characteristics of human prostate cancer that may contribute to its poorly immunogenic phenotype include the expression of transforming growth factor-beta (TGF-β) by prostate-cancer cells; TGF-β can actively suppress T-cell responsiveness to tumors in vivo, thereby indirectly contributing to tumor growth and progression and posing a potential obstacle to vaccine efficacy. As shown in the present study, the ability of recombinant vaccinia vectors to induce specific immune responses capable of preventing or delaying Dunning prostate-cancer progression, despite the previously described abundant TGF-β production and weak immunogenicity of these tumors [20, 29], provides relevant evidence supporting the continued development of recombinant vaccinia vectors as a rational prototype for future recombinant prostate-cancer vaccines.
Another potential obstacle to the antitumor efficacy of recombinant prostate-cancer vaccines is the capacity of human prostate cancers to be deficient in antigen processing via the MHC class I pathway [2, 26]. The Dunning AT2 model used for these studies displayed abundant MHC class I expression as measured by FACS analysis. The possibility therefore remains that MHC class I deficiency can pose a significant obstacle to the efficacy of TAA-specific recombinant viral vaccines, and this possibility has not been addressed by the Dunning model evaluated in these studies due to its competent expression of MHC class I antigen. Other clinically relevant issues, not addressed in the present study but requiring further study, include the roles of TAA-specific tolerance, vector-specific immunity, and loss of TAA expression as potential obstacles to the efficacy of TAA-specific viral vaccines.
Despite these limitations, our findings do provide evidence that TAA-specific T-cell responses induced by TAA-encoding vaccinia can be effective in protecting against tumor growth, even in the setting of a prostate-cancer model known to be poorly immunogenic and harboring immunosuppressive signals such as abundant production of TGF-β [29]. This study also provides evidence indicating the importance of targeting of a minimal tumor volume for optimization of the efficacy of recombinant vaccines against prostate cancer. Therefore, a clinical setting of minimal tumor burden, such as early recurrence of PSA increases following radical prostatectomy, represents a rational clinical target for therapy with recombinant vectors encoding tumor-associated prostate-cancer antigens.
Acknowledgments
This work was supported by NIH grant R29 CA71532-01. M.G.S. is the recipient of an American Cancer Society Clinical Career Development Award
Contributor Information
Linda G. Charles, Department of Surgery/Urology, University of Michigan School of Medicine, 2916 Taubman Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109-0330, USA
Yilin C. Xie, Department of Surgery/Urology, University of Michigan School of Medicine, 2916 Taubman Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109-0330, USA
Nicholas P. Restifo, Surgery Branch, National Cancer Institute, Bethesda, Maryland, USA
Blake Roessler, Department of Medicine/Rheumatology, University of Michigan School of Medicine, Ann Arbor, Michigan, USA.
Martin G. Sanda, Surgery Service, Ann Arbor Veterans Administration Medical Center, Ann Arbor, Michigan, USA
References
- 1.Addison CL, Braciak T, Ralston R, Muller WJ, Gauldie J, Graham FL. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA. 1995;92:8522–8526. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blades RA, Keating PJ, McWilliam LJ, George NJR, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46:681–686. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 3.Borysiewicz LK, Flander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 4.Brannen GE, Gomolka DM, Coffey DS. Specificity of cell membrane antigens in prostatic cancer. Cancer Chemother Rep. 1975;59:127–138. [PubMed] [Google Scholar]
- 5.Bright RK, Beames B, Shearer MH, Kennedy RC. Protection against a lethal challenge with SV40-transformed cells by the direct injection of DNA-encoding SV40 large tumor antigen. Cancer Res. 1996;56:1126–1130. [PubMed] [Google Scholar]
- 6.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carducci MA, Ayyagari SR, Sanda MG, Simons JW. Gene therapy for human prostate cancer: translational research in the hormone refractory Dunning prostate model. Cancer. 1995;75:2013–2020. [Google Scholar]
- 8.Chen AP, Bastian A, Dahaut W, et al. A phase I study of recombinant vaccinia virus (RV) that expresses prostate specific antigen (PSA) in adult patients (PTS) with adenocarcinoma of the prostate. Am Soc Clin Oncol Proc. 1998;17:314A. [Google Scholar]
- 9.Eder JP, Jr, Kantoff PW, Bubler GJ, et al. A phase I trial of recombinant vaccinia virus, Prostvac, that expresses prostate specific antigen (rV-PSA) as a vaccine in men with advanced prostate cancer. Am Soc Clin Oncol Proc. 1998;17:434A. [Google Scholar]
- 10.Fearon ER, Pardol DM, Itaya T, Golumbek P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B, Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 11.Fiering SN, Roederer M, Nolan GP, Micklem DR, Parks DR, Herzenberg LA. Improved FACS-Gal: flowcytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12:291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- 12.Guinan PD, John T, Baumgartner G, Sundar B, Ablin RJ. Adjuvant immunotherapy (BCG) in stage D prostate cancer. Am J Clin Oncol. 1982;5:65–68. [PubMed] [Google Scholar]
- 13.Hodge JW, Schlom J, Donahue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs JT, Heston WDW, Weissman RM, Coffey DS. Animal models of the hormone-sensitive and insensitive prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and R-3327 AT. Cancer Res. 1978;38:4353–4359. [PubMed] [Google Scholar]
- 15.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. [PubMed] [Google Scholar]
- 16.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 17.Kawakita M, Rao GS, Ritchey JK, Ornstein DK, Hudson MA, Tartaglia J, Paoletti E, Humphrey PA, Harmon TJ, Ratliff TL. Effect of canarypox virus (ALVAC)-mediated cytokine expression on murine prostate tumor growth. J Natl Cancer Inst. 1997;89:428–436. doi: 10.1093/jnci/89.6.428. [DOI] [PubMed] [Google Scholar]
- 18.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathe R, Gerlinger P, Clertant P, Guizani I, Cuzin F, Chambon P. Tumor prevention and rejection with recombinant vaccinia. Nature. 1987;326:878–880. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- 20.Moody B, Lazenby A, Ewing C, Isaacs WB. Interleukin-2 transfected prostate cancer cells generate a local antitumor effect in vivo. Prostate. 1994;24:244–251. doi: 10.1002/pros.2990240505. [DOI] [PubMed] [Google Scholar]
- 21.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 22.Murphy G, Tjoa B, Ragde H, et al. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Price J, Turner D, et al. Lineage analysis in the vertebrate nervous system by retrovirus mediated gene transfer. Proc Natl Acad Sci USA. 1997;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanda MG, Ayyagari SR, Jaffee EM, Epstein JI, Clift SL, Cohen LK, Dranoff G, Pardoll DM, Mulligan RC, Simons JW. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 26.Sanda MG, Restifo NP, Walsh J, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (Prostvac) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 28.Simons J, et al. Phase I/II study of autologous human GM-CSF gene transduced prostate cancer vaccines in patients with metastatic prostate carcinoma. Hum Gene Ther. 2000 (in press) [Google Scholar]
- 29.Steiner MS, Zhou ZZ, Tonb DC, Barrack ER. Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 1994;135:2240–2247. doi: 10.1210/endo.135.5.7956947. [DOI] [PubMed] [Google Scholar]
- 30.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 31.Vieweg J, Rosenthal FM, Bannerji R, Heston WDW, Fair WR, Gansbacher B, Gilboa E. Immunotherapy of prostate cancer in the Dunning rat model: use of cytokine gene modified tumor vaccines. Cancer Res. 1994;54:1760–1765. [PubMed] [Google Scholar]
- 32.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17:159–163. [PubMed] [Google Scholar]


