Abstract
Background
Increased carotid artery intima-media thickness (IMT) is a non-invasive marker of systemic arterial disease. Increased IMT has been associated with atherosclerosis, abnormal arterial mechanics, myocardial infarction, and stroke. Given evidence of a relationship between cardiovascular health and attention-executive-psychomotor functioning, the purpose of this study was to examine IMT in relation to neuropsychological test performance in patients with a variety of cardiovascular diagnoses.
Methods
One hundred and nine participants, ages 55 to 85, underwent neuropsychological assessment and B-mode ultrasound of the left common carotid artery. IMT was calculated using an automated algorithm based on a validated edge-detection technique. The relationship between IMT and measures of language, memory, visual-spatial abilities and attention-executive-psychomotor functioning was modeled using hierarchical linear regression analyses adjusted for age, education, sex, cardiovascular risk, current systolic blood pressure, and history of coronary artery disease (CAD).
Results
Increased IMT was associated with significantly lower performance in the attention-executive-psychomotor domain (IMT beta = −0.26, p < .01), independent of age, education, sex, cardiovascular risk, current systolic blood pressure, and CAD (F(10,100) = 3.61, p < .001). IMT was not significantly related to language, memory, or visual-spatial abilities.
Conclusions
Our findings suggest that, in patients with cardiovascular disease, IMT may be associated with the integrity of frontal subcortical networks responsible for attention-executive-psychomotor performance. Future studies are needed to clarify the mechanisms by which IMT affects cognition and examine potential interactions between increased IMT and other measures of cardiovascular health such as blood pressure variability, cardiac systolic performance, and systemic perfusion.
Keywords: Cognition, Cardiovascular Diseases, Carotid Arteries, Atherosclerosis, IMT, B-mode Ultrasound
INTRODUCTION
Increasing evidence suggests that cardiovascular disease is associated with diminished cognitive functioning, even in patients who do not meet criteria for vascular dementia [1–4]. A new diagnostic category, referred to as vascular cognitive impairment, has been proposed to include these subtle alterations of cognitive ability [5], that nonetheless appear to be associated with higher incidence of dementia later in life, loss of independence, and increased mortality [6]. Because these cognitive changes may be mediated through compromises in the structural and functional integrity of cerebral blood vessels, it is important to define relationships between markers of vessel health and cognition.
In this study, we examined the relationship between increased carotid artery intima-media thickness (IMT) and neuropsychological functioning in a clinically heterogeneous sample of stable outpatients with cardiovascular disease. IMT, as assessed by B-mode ultrasound, is a reliable, non-invasive measure of disease-related arterial wall changes [7]. Though small increases in IMT may be an adaptive response to changes in blood pressure and flow, there is consensus that IMT levels greater than 0.9 mm are indicative of atherosclerotic vascular disease and end organ damage [8]. Atherosclerosis of the large and medium-sized arteries is associated with plaque formation, inflammation, endothelial dysfunction, thrombosis, and acute or chronic luminal obstruction resulting in abnormal blood flow to target organs [9]. By virtue of cerebral architecture and vasculature, the frontal subcortical networks that govern complex attention, executive functioning and cognitive processing speed are particularly susceptible to ischemic damage resulting from blood flow abnormalities [10].
Impairment of attention-executive-psychomotor functions is a hallmark of both vascular dementia and the milder, yet more prevalent category of vascular cognitive impairment [11]. A relationship between IMT specifically and attention-executive-psychomotor test performance, however, has been inconsistently reported in the previous literature. One community-based study reported a significant association between increased IMT and lower visual attention and psychomotor speed for both men and women [12], while another found a similar relationship only in men with carotid plaques [13]. IMT was found to be a good predictor of cognitive decline over time in stroke patients [14] but not in community dwelling elderly [15]. These differences are likely due to the fact that IMT values in community samples are generally well below the accepted cut off of 0.9 mm indicating vascular damage from atherosclerosis [13;15]. We hypothesized that increased IMT will be related to lower attention-executive-psychomotor performance in patients with cardiovascular disease because they can be expected to exhibit pathological arterial wall changes at rates much higher than those found in the general population.
MATERIALS AND METHODS
Study Sample
Participants between the ages of 55 and 85 were recruited from cardiology outpatient clinics, cardiac rehabilitation programs, and community fliers in the Providence, RI area. Volunteers were screened for participation if they had a documented history of at least one of the following: a diagnosis of coronary artery disease (CAD), angina pectoris, previous myocardial infarction (MI), heart failure, cardiac surgery, arrhythmia or hypertension. Patients were excluded from the study if they had a history of neurological disease (i.e., large vessel stroke, seizure disorder, Parkinson’s disease, clinically significant traumatic brain injury, multiple sclerosis, brain infection/meningitis, or diagnosed dementia), major psychiatric illness (e.g. schizophrenia, bipolar disorder), substance abuse (i.e., diagnosed abuse and/or previous hospitalization for substance abuse), or if they scored below the cut off for dementia (total score < 123) on the Dementia Rating Scale (DRS) [16].
Of 181 screened volunteers, 176 fit the cardiac disease profile. Five participants were excluded for low DRS scores (total score <123). IMT and complete cognitive test data were available on 109 participants (63 men and 46 women). The mean age (SD) of the sample was 69.18 (7.43) years. The mean education level (SD) was 14.40 (2.75) years. The mean total DRS score (SD) was 137.76 (4.67), indicating intact global cognitive functioning according to published norms [16]. Enrollees identified themselves as follows: 90% - Caucasian, 9% - African American, and 1% - Other. Approximately 33% of all participants endorsed at least one cardiovascular risk factor (hypertension, hypercholesterolemia, diabetes, or tobacco use), 28% endorsed two factors, 20% - three factors, 8% - all four factors, and 11% could be classified as low risk with no cardiovascular risk factors. Seventy percent of study participants showed indications of coronary artery disease (CAD) by reporting a diagnosis of CAD, coronary artery bypass surgery (CABG), MI, angina, or angioplasty/stent placement. Mean carotid artery IMT (SD) for the sample was 0.88 (0.13) mm. Approximately 40% of participants registered IMT values equal to or greater than 0.9 mm, levels indicative of atherosclerotic vascular disease according to European Society of Hypertension - European Society of Cardiology guidelines [17]. Ninety two percent of the study participants were currently being treated with antihypertensive medication, 37% with aspirin/antithrombotics, 73% with lipid lowering agents, 33% with vitamins, 25% with gastric acid inhibitors, 16% with hypoglycemics, 37% with vasodilators, and 16% with psychoactive medications. Further details about the demographic and medical characteristics of the sample can be found in Table 1.
Table 1.
Sample Demographic and Medical Information
| Mean | SD | Number of Patients | Frequency | |
|---|---|---|---|---|
| Male | 57% | |||
| Female | 43% | |||
| Caucasian | ||||
| African American | 90% | |||
| Other | 9% | |||
| 1% | ||||
| Age | 69.18 | 7.43 | ||
| Education | 14.40 | 2.75 | ||
| IMT | 0.88 | 0.13 | ||
| Hypertension | 82 | 75% | ||
| Hypercholesterolemia | 48 | 44% | ||
| Diabetes | 27 | 25% | ||
| Tobacco Use | 41 | 38% | ||
| Angina | 25 | 23% | ||
| Angioplasty/Stents | 20 | 18% | ||
| Cardiac Arrest | 6 | 6% | ||
| Coronary Artery Disease | 31 | 29% | ||
| Heart Failure | 18 | 17% | ||
| Myocardial Infarction | 44 | 40% | ||
| CABG | 34 | 31% | ||
| Arrhythmia | 16 | 15% | ||
| Mitral Valve Prolapse | 9 | 8% | ||
| Valve surgery | 10 | 10% | ||
| Pacemaker | 3 | 3% |
Procedures
The local Institutional Review Board approved the study and all volunteers provided written informed consent before enrollment. Participants completed a detailed medical history questionnaire with a focus on cardiovascular disease, and reviewed their answers with a trained research assistant under the supervision of the study PI (RAC). Cardiovascular risk factors (hypertension, hypercholesterolemia, tobacco use, and diabetes), and cardiovascular diagnoses, events, and procedures (e.g., CAD, CABG, MI, angioplasty, angina, arrhythmia, heart failure, valve surgery) were coded as either present or absent according to participants’ self-report. Participants underwent an ultrasound examination of the carotid artery and neuropsychological assessment on separate days.
Ultrasound examination & IMT Quantification
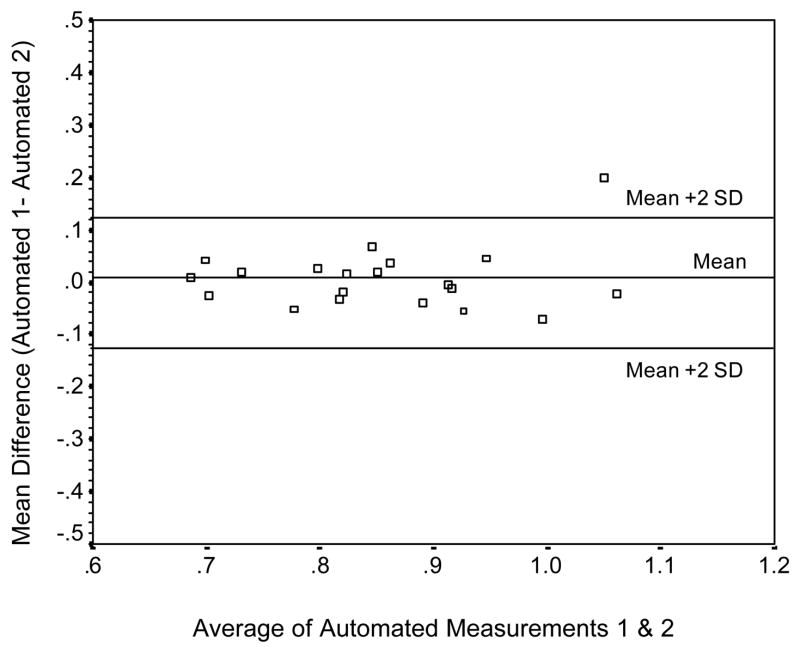
High-resolution B-mode carotid ultrasonography was performed using a 7.5-MHz transducer and an Agilent 5500 machine (Agilent, Andover, MA). IMT was calculated using scans of the far wall of the left common carotid artery approximately 1 cm proximal to the carotid bulb. IMT was defined as the distance between the luminal-endothelial interface and the junction between the media and the adventitia (Figure 1). Automated, objective edge detection software was developed to measure IMT thickness based on a validated technique described previously [18]. The following steps were involved: 1) the user scrolled through the image set and determined the region of greatest vessel wall clarity; 2) a single image was used to orient this extracted region along an axis of symmetry; 3) the program proceeded with edge detection as follows: the region of interest for the entire image set was projected along the axis; candidate edges were identified in this projection by points at which the change in pixel intensity was maximum. A cost function minimized by a Viterbi search, a technique successfully used in speech processing, imposed continuity by simultaneously weighing brightness of the edges and distance of edges from each other and from the image center, and penalizing for large discontinuity in the path through the pair of edges. The detected boundaries for diameter estimation were located in between the lumen-intima and media-adventitia interfaces (Figure 2). Thus, IMT was defines as the distance between the peak intensities above and below the estimated boundaries of the diameter. The final automated IMT measurement was an average of approximately 1400 measurements across the entire image set. The automated method was validated in a subset of participants (n = 20) against a manual IMT measurement completed by a single rater as described by Jegelevicius and Lukoševicius (2002) [19]. The absolute mean difference (SD) between the manual and automated measurements of IMT was .01 (.04) mm for 19 out of the 20 participants in the validation study (95%), and .03 (.06) mm for all 20 participants. The limits of agreement were between −.09 and .07 mm for 95% of the participants, and between −1.15 and .10 mm for all participants. The Bland-Altman plot of agreement between the two measurement methods is represented in Figure 3. The coefficient of repeatability (CR) for the automated measurement was .12 with mean difference (SD) between two automated IMT measurements of .01 (.06) (see Figure 4).
Figure 1.
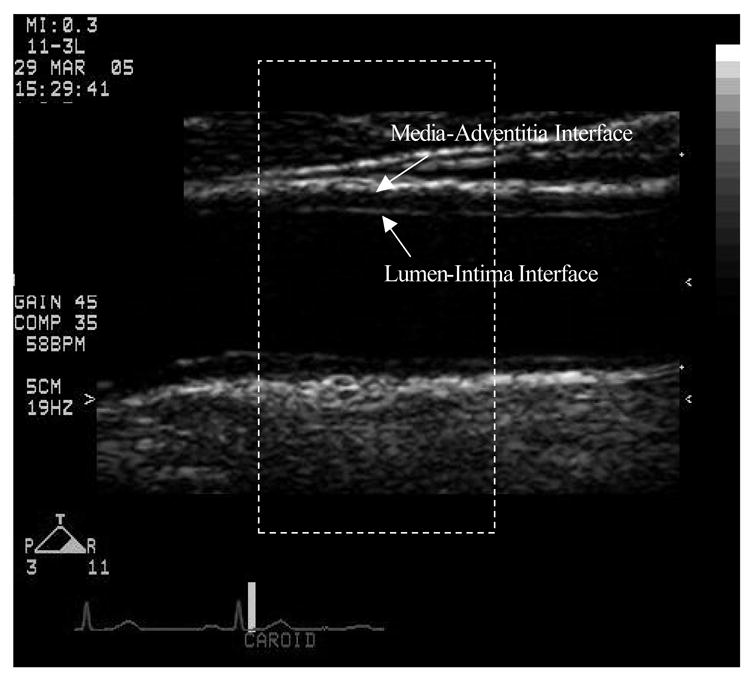
Carotid image of a selected frame of a typical participant. The white arrows mark the lumen-intima and media-adventitia interfaces. The dotted line represents the selected region for analysis.
Figure 2.
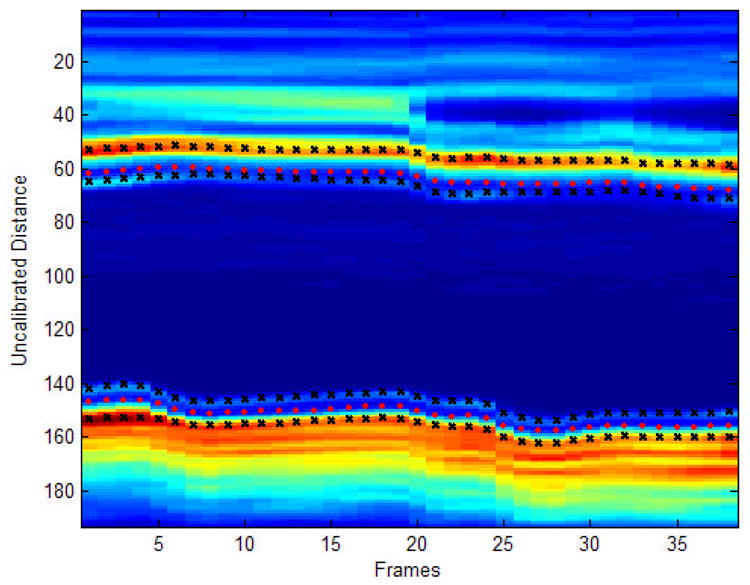
Automated IMT detection. The image represents the signal intensity of the region selected for analysis on far wall of the left common carotid artery for all frames of a typical subject. The orange color indicates high intensity values, and blue color indicates low intensity values. The pink circles represent the estimated boundaries for the carotid diameter. The black ‘x’s represent the estimated lumen-intima and media-adventitia interfaces.
Figure 3.
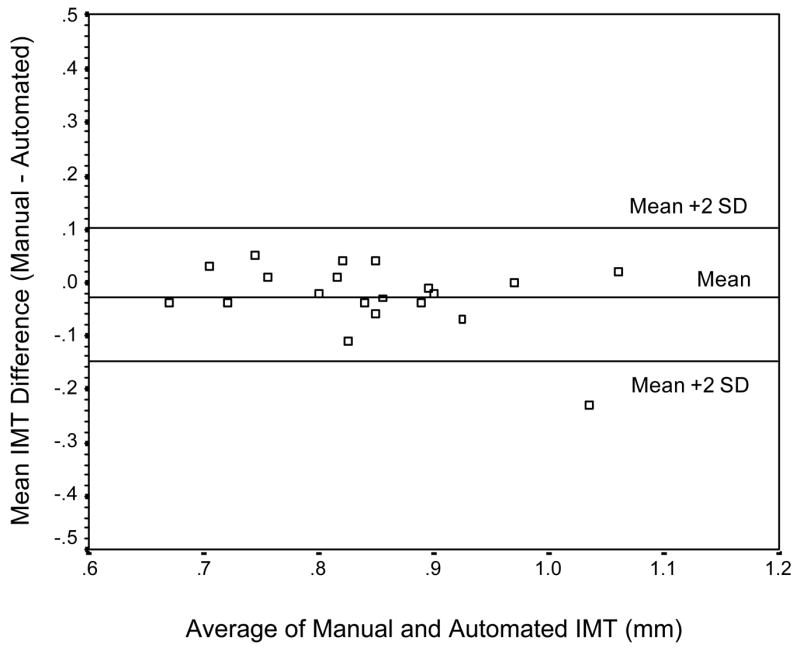
Bland-Altman plot of the agreement between the manual and automated measurements of IMT.
Figure 4.
Bland-Altman plot of the agreement between two automated measurements of IMT.
Neuropsychological Assessment
All participants completed a two-hour neuropsychological assessment including measures of global cognitive functioning, language, memory, attention, executive functioning, psychomotor speed, and visual-spatial ability (see Table 2). The battery included standard clinical neuropsychological instruments with established reliability and validity [20]. All tests were administered and scored by a trained research assistant under the supervision of a licensed clinical psychologist (RAC), using standard administration and scoring criteria.
Table 2.
Cognitive Test Scores, Means and Standard Deviations
| Test Measures by Domain | Sample Mean Score (SD) |
|---|---|
| Global Cognitive Functioning | |
| Mini Mental Status Exam (MMSE) | 28.55 (1.59) |
| Dementia Rating Scale (DRS) | 137.76 (4.67) |
| Language | |
| Boston Naming Test (BNT) | 54.83 (4.26) |
| Category Fluency for Animals (Animals) | 20.18 (5.07) |
| Visual-Spatial | |
| Hooper Visual Organization Test (HVOT) | 23.82 (3.41) |
| Complex Figure Test (CFT-Copy) | 30.51 (5.58) |
| WAIS-III Block Design Subtest (Block Design) | 33.23 (11.27) |
| Memory | |
| California Verbal Learning Test (CVLT) | |
| Immediate Recall | 9.21 (3.15) |
| Delayed Recall | 9.51 (3.25) |
| Recognition Discrimination | 92.00 (6.12) |
| Complex Figure Test (CFT) | |
| Immediate Recall | 15.05 (8.01) |
| Delayed Recall | 14.79 (7.85) |
| Recognition Discrimination | 19.66 (2.66) |
| Brief Visual Memory Test-Revised (BVMT-R) | |
| Delayed Recall | 7.32 (3.11) |
| Recognition Discrimination | 5.10 (1.01) |
| Attention-Executive-Psychomotor | |
| Trail Making Test A, Time (Trails A) | 38.47 (13.17) |
| Trail Making Test B, Time (Trails B) | 96.17 (44.11) |
| Letter Search, Time | 92.66 (27.76) |
| Letter Search, Errors | 1.53 (2.23) |
| Stroop-Word, Correct in 45 seconds | 91.55 (14.36) |
| Stroop-Color, Correct in 45 seconds | 65.22 (11.26) |
| Stroop-Word/Color, Correct in 45 seconds | 31.54 (9.80) |
| Controlled Oral Word Association Test (COWAT) | 40.19 (13.03) |
| Grooved Pegboard, Dominant Hand (Pegs-D) | 94.25 (26.24) |
| WAIS-III Digit Span Subtest (Digit Span) | 17.87 (3.67) |
| WAIS-III Digit-Symbol Coding Subtest (Coding) | 56.09 (13.14) |
Data Analyses
Neuropsychological measures were grouped into one of five cognitive domains: 1) global cognitive functioning, 2) language functions, 3) visual-spatial abilities, 4) memory functions, and 5) attention-executive-psychomotor functions. The following test scores were included in each domain, and raw total scores were utilized unless otherwise stated: 1) global: Mini Mental Status Exam (MMSE) [21], Dementia Rating Scale (DRS) [16]; 2) language: Boston Naming Test (BNT) [22], Category Fluency for Animals (Animals) [23]; 3) visual-spatial: WAIS-III Block Design Subtest (Block Design) [24], Hooper Visual Organization Test (HVOT) [25], Complex Figure Test (CFT) [20] copy; 4) memory: California Verbal Learning Test (CVLT) [26] immediate recall, delayed recall, and recognition discrimination, Complex Figure Test (CFT) [20] immediate recall, delayed recall, and recognition discrimination, Brief Visual Memory Test-Revised (BVMT-R) [27] delayed recall and recognition discrimination; 5) attention-executive-psychomotor functions: Trail Making Test A and B (Trails) [28] time to completion, Stroop Test (Stroop) [29] word, color, and color/word, Controlled Oral Word Association Test (COWAT) [30], Letter Search [20] time to completion and errors, WAIS-III Digit-Symbol Coding Subtest (Coding) [15], WAIS-III Digit Span Subtest (Digit Span) [24], and Grooved Pegboard, Dominant Hand (Pegs-D) [31] time to completion. Participants’ raw test scores were converted to z-scores using the study sample mean and standard deviation. Five composite cognitive domain z-scores were calculated for each participant by averaging the z-scores of all tests within that domain.
The relationship between IMT and level of neuropsychological test performance was analyzed by cognitive domain using hierarchical linear regression analyses where the composite domain z-scores were entered as the dependent variable. In the first step of the regression analyses, the relationship between IMT and cognition was adjusted for the effects of age, education, and sex. In the second step, the models were additionally adjusted for the effects of cardiovascular risk factors such as history of hypertension, hypercholesterolemia, diabetes and tobacco use as well as current level of systolic blood pressure. The independent contribution of IMT to variance in neuropsychological test performance was estimated in the third step. In a secondary analysis, the significant relationship between increased IMT and poorer attention-executive-psychomotor performance was explored further by including patients’ history of CAD as a covariate in the model. History of CAD was coded as present if one of the following diagnoses was endorsed in the health questionnaire: CAD, MI, angina pectoris, CABG, or angioplasty/stent placement. The effect of medication class on attention-executive-psychomotor functioning was also explored in a follow-up analysis. Finally, we tested our hypothesis that a certain level of vascular pathology has to be reached (i.e. IMT = 0.9 mm) before higher IMT consistently relates to poorer cognitive test performance. For the purposes of this analysis, we divided our sample in two groups: a low IMT group (IMT < 0.9 mm), and a high IMT group (IMT = 0.9 mm). Then, we compared the slopes of the linear relationships between IMT and attention-executive-psychomotor performance in the two groups using a Student’s t statistic computed as the difference between the two slopes divided by the standard error of the difference between the slopes on (n – 4) degrees of freedom. Data were analyzed using SPSS 11.0 computer software (SPSS Inc., Chicago, IL). A two-tailed alpha level of .05 was used as the criterion for statistical significance for all primary analyses, and most follow-up analyses. In the final follow-up analysis, a one-tailed alpha level of .05 was used to test the unidirectional hypothesis that the slope of the relationship between IMT and attention-executive-psychomotor test performance in the high IMT group will be steeper than the slope of the same relationship in the low IMT group with higher IMT values relating to poorer neuropsychological test performance.
RESULTS
The raw cognitive test scores for the sample are summarized in Table 2. The fully adjusted models successfully predicted the level of cognitive performance in all five domains: global (F(9,100) = 2.40, p < .05), language (F(9,100) = 4.62, p < .001), visual-spatial (F(9,100) = 2.11, p < .05), memory (F(9,100) = 2.44, p < .05), and attention-executive-psychomotor (F(9,100) = 3.66, p < .001).
Older age was significantly associated with poorer global cognitive functioning (beta = −.23, p < 0.05), weaker language skills (beta = −.24, p < .01), lower attention-executive-psychomotor performance (beta = −.32, p < .001), and a trend toward diminished memory performance (beta = −.19, p = .059).
Higher education was significantly associated with better global cognitive functioning (beta = .32, p < .01), better visual-spatial skills (beta = .30, p < .01), higher attention-executive-psychomotor scores (beta = .21, p < .05), and better memory performance (beta = .32, p < .01).
Independent of the effects of age, education, and sex, neither history of hypertension, hypercholesterolemia, diabetes or tobacco use, nor current systolic blood pressure level accounted for any unique variance in neuropsychological test performance.
Increased IMT was significantly associated with poorer neuropsychological test performance in the attention-executive-psychomotor domain (IMT beta = −.23, p < .05), independent of age, education, sex, cardiovascular risk factors, and current systolic blood pressure. This relationship remained unchanged even when patients’ history of CAD was also included in the model as a covariate (F(10,100) = 3.61, p < .001; IMT beta = −.26, p < .01). IMT was not independently related to performance on measures of global cognitive functioning, language, visual-spatial ability, or memory.
As a secondary aim, we explored the effects of medication on attention-executive-psychomotor performance. We found that, independent of the effects of age and education, none of the medication classes (e.g. antihypertensive, hypoglycemic, lipid lowering, etc.) had a significant effect on neuropsychological test performance (R2 change = .05, F change (9, 82) = .67, p = .73). Therefore, the primary analysis was not repeated with medication class as a covariate.
Finally, we tested our hypothesis that a certain level of vascular pathology has to be reached (i.e. IMT = 0.9 mm) before higher IMT consistently relates to poorer cognitive test performance. We divided our sample in two groups, a low IMT group (IMT < 0.9 mm, n = 64), and a high IMT group (IMT = 0.9 mm, n = 45), and compared the slopes of the linear relationships between IMT and attention-executive-psychomotor performance in the two groups. Our results showed that the slope in the high IMT group was significantly steeper than the slope in the low IMT group (t = 1.77, p < .05) indicating a stronger relationship between higher IMT and poorer neuropsychological test performance in the high IMT group.
DISCUSSION
The present study found that increased carotid artery IMT was associated with lower attention-executive-psychomotor test performance in non-demented patients with a variety of cardiovascular diagnoses. The effect was independent of age, sex, education, cardiovascular risk, current systolic blood pressure, and history of CAD. Our results are consistent with the findings demonstrating a significant relationship between cognition and other measures of large artery structure and function such as pulse wave velocity [32;33]. Our findings are also consistent with Hachinski’s proposed continuum of vascular cognitive impairment ranging from the brain-at-risk to vascular dementia where subtle declines in higher order cognitive skills are observed well before the threshold for dementia is reached.
A relationship between IMT and attention-executive-psychomotor test performance, however, has been inconsistently reported in studies of community dwelling elderly [1;12;13;15]. These differences are likely due to the fact that IMT values in community samples are generally well below the accepted cut off of 0.9 mm indicating vascular damage from atherosclerosis [13;15]. For example, the mean IMT (SD) in the sub-sample of participants in the Auperin et al. study that showed no relationship between IMT and measures of cognition, was 0.69 (0.14) mm as compared to 0.73 (0.14) mm for the sub-sample where a modest relationship was found [13]. In comparison, the mean IMT (SD) in our study was 0.88 (0.13) mm. These results suggest that a certain threshold of pathology may need to be reached before IMT consistently relates to poor neuropsychological test performance, and that the accepted clinical threshold of IMT = 0.9 mm may apply to the risk of cognitive impairment as well as atherosclerotic vascular damage. In support of this threshold hypothesis, we demonstrated that the slope of the linear relationship between IMT and attention-executive-psychomotor performance was significantly steeper in the high IMT sub-group of our study sample (i.e. IMT = 0.9 mm) as compared to the slope of the same relationship in the low IMT sub-group (IMT < 0.9 mm) indicating a stronger relationship between higher IMT and poorer neuropsychological test performance in the high IMT group.
Due to the cross-sectional design of our study, we can only speculate about the nature of the observed relationship between increased IMT and diminished attention-executive-psychomotor functioning. Though it is unlikely that poor cognition causes increases in IMT, it is possible, that both increased IMT and lower neuropsychological test performance may be related to a third variable, not considered in this study. One may hypothesize, however, that atherosclerosis of the small penetrating arteries of the brain as measured by IMT contributes to chronic cerebral hypoperfusion and ischemic damage even in the absence of frank infarctions. This idea is supported by previous reports of impaired attention-executive-psychomotor performance in patients with documented subcortical white matter disease, presumably of vascular origin [34–36].
Though the exact mechanism through which subcortical white matter lesions relate to cognition is unknown, a diaschisis model might be applicable. Such a model would suggest that vascular damage to subcortical white matter disrupts higher order cognitive functions by causing a disconnection between brain regions responsible for storing, rehearsing, and organizing information. Furthermore, mild, diffuse brain damage related to chronic hypoperfusion may affect the brain’s ability to process information quickly and efficiently even in the absence of discrete white matter lesions. A reduction in cognitive processing speed is likely most detrimental to the highest order cognitive functions such as complex attention and planning ability. This hypothesis is supported by our observation of a relationship between IMT and performance on attention-executive-psychomotor tests in patients with intact global cognition, language, and memory.
Interestingly, neither history of hypertension, nor a history of diabetes was significantly related to cognitive performance in our study. A possible explanation for this discrepancy between our results and previous reports linking poor cognition to hypertension [37] and disturbances in glucose regulation [38] is that our sample consisted exclusively of older patients with overt cardiovascular disease resulting in high frequency of endorsement for all cardiovascular risk factors. Thus, there was little variability in these risk factors within the sample. For example, 75% of all study participants reported a history of hypertension, and 44% endorsed a history of hypercholesterolemia.
In summary, we found that increased IMT was associated with lower attention-executive-psychomotor functioning in patients with a variety of cardiovascular diseases independent of age, sex, education, cardiovascular risk and current systolic blood pressure. The heterogeneity of our population, and the fact that the relationship between IMT and cognition remained significant even after adjustment for traditional cardiovascular risk and history of CAD, suggest that IMT has value as an integrative measure of arterial health among patients with a variety of cardiovascular diagnoses. Increased IMT in isolation, however, is likely insufficient to contribute to significant vascular-related pathology in the brain. Future studies should consider IMT in conjunction with other variables known to be related to cognition such as blood pressure variability [39], pulse wave velocity [32;33], and systemic perfusion [40]. Furthermore, the impact of these complex cardiovascular interactions on brain morphology, metabolism, and activity should continue to be explored.
Acknowledgments
The authors thank Drs. Michael Cohen and J. Andrew Taylor for their help in the development of the automated edge detection software used for calculating IMT, and Dr. Makoto Ono for his help with coding the medication data.
This work was supported by National Institute of Health grants AG017975 (RAC), AG 026850 (KFH), HL074568 (JG), MH065857 (RHP), AG022773 & HD043444 (ALJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Johnston SC, O’meara ES, Manolio TA, Lefkowitz D, O’Leary DH, Goldstein S, Carlson MC, Fried LP, Longstreth WT., Jr Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- 2.Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: The Tromso Study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- 3.Paul RH, Gunstad J, Poppas A, Tate DF, Foreman D, Brickman AM, Jefferson AL, Hoth K, Cohen RA. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovasc Dis. 2005;20:129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Exel E, Gussekloo J, Houx P, de Craen AJ, Macfarlane PW, Bootsma-van der Wiel A, Blauw GJ, Westendorp RG. Atherosclerosis and cognitive impairment are linked in the elderly. The Leiden 85-plus Study. Atherosclerosis. 2002;165:353–359. doi: 10.1016/s0021-9150(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 5.Bowler JV, Steenhuis R, Hachinski V. Conceptual background to vascular cognitive impairment. Alzheimer Dis Assoc Disord. 1999;13 (Suppl 3):S30–S37. [PubMed] [Google Scholar]
- 6.Wentzel C, Rockwood K, MacKnight C, Hachinski V, Hogan DB, Feldman H, Ostbye T, Wolfson C, Gauthier S, Verreault R, McDowell I. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;57:714–716. doi: 10.1212/wnl.57.4.714. [DOI] [PubMed] [Google Scholar]
- 7.Bots ML, Dijk JM, Oren A, Grobbee DE. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: current evidence. J Hypertens. 2002;20:2317–2325. doi: 10.1097/00004872-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Van Bortel LM. What does intima-media thickness tell us? J. Hypertens. 2005;23:37–39. doi: 10.1097/00004872-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol. 2000;86:6H–11H. doi: 10.1016/s0002-9149(00)01094-8. [DOI] [PubMed] [Google Scholar]
- 10.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K. Vascular cognitive impairment and vascular dementia. J Neurol Sci. 2002;203–204:23–27. doi: 10.1016/s0022-510x(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 12.Cerhan JR, Folsom AR, Mortimer JA, Shahar E, Knopman DS, McGovern PG, Hays MA, Crum LD, Heiss G. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 13.Auperin A, Berr C, Bonithon-Kopp C, Touboul PJ, Ruelland I, Ducimetiere P, Alperovitch A. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59- to 71-year-olds. The EVA Study Group Stroke. 1996;27:1290–1295. doi: 10.1161/01.str.27.8.1290. [DOI] [PubMed] [Google Scholar]
- 14.Talelli P, Ellul J, Terzis G, Lekka NP, Gioldasis G, Chrysanthopoulou A, Papapetropoulos T. Common carotid artery intima media thickness and post-stroke cognitive impairment. J Neurol Sci. 2004;223:129–134. doi: 10.1016/j.jns.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Mattis S. Dementia Rating Scale (DRS) Odessa, FL: Psychological Assesment Resources; 1988. [Google Scholar]
- 17.2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Stadler RW, Karl WC, Lees RS. New methods for arterial diameter measurement from B-mode images. Ultrasound Med Biol. 1996;22:25–34. doi: 10.1016/0301-5629(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 19.Jegelevicius D, Lukosevicius A. Ultrasonic measurements of human carotic artery wall intima-media thickness. Ultragarsas. 2002;Nr 2:43–47. [Google Scholar]
- 20.Neuropsychological Assessment. 3. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr” Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 23.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1979. [Google Scholar]
- 25.The Hooper Visual Organization Test. Los Angeles: Western Psychological Services; 1983. [Google Scholar]
- 26.Delis D, Kramer J, Kaplan E, Ober BA. Manual: California Verbal Learning Test, Adult Version. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 27.Benedict R. Brief Visuospatial Memory Test-Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 28.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- 29.Golden C. Stroop color and word task: A manual for clinical and experimental uses. Wood Dale, IL: Stoeling; 1978. [Google Scholar]
- 30.Eslinger P, Damasio A, Benton A. The Iowa Screening Battery for Mental. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 31.Klove H. Clinical Neuropsychology. In: FM Forster., editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- 32.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- 33.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 34.Cohen RA, Paul RH, Ott BR, Moser DJ, Zawacki TM, Stone W, Gordon N. The relationship of subcortical MRI hyperintensities and brain volume to cognitive function in vascular dementia. J Int Neuropsychol Soc. 2002;8:743–752. doi: 10.1017/s1355617702860027. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2002;72:217–220. doi: 10.1136/jnnp.72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul RH, Haque O, Gunstad J, Tate DF, Grieve SM, Hoth K, Brickman AM, Cohen R, Lange K, Jefferson AL, Macgregor KL, Gordon E. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Arch Clin Neuropsychol. 2005;20:697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. doi: 10.1161/01.hyp.36.6.1079. [DOI] [PubMed] [Google Scholar]
- 38.Kumari M, Marmot M. Diabetes and cognitive function in a middle-aged cohort: findings from the Whitehall II study. Neurology. 2005;65:1597–1603. doi: 10.1212/01.wnl.0000184521.80820.e4. [DOI] [PubMed] [Google Scholar]
- 39.Gunstad J, Cohen RA, Tate DF, Paul RH, Poppas A, Hoth K, Macgregor KL, Jefferson AL. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Press. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]