Abstract
BACKGROUND
Cancers can escape immune recognition by means of evading class I major histocompatibility complex (MHC) -mediated recognition by cytotoxic T lymphocytes. However, immunization strategies targeting defined tumor-associated antigens have not been extensively characterized in murine prostate cancer models. Therefore, we evaluated antigen-specific, antitumor immunity after antigen-encoding vaccinia immunization against mouse prostate cancer cells expressing a model tumor-associated antigen (β-galactosidase) and exhibiting partially deficient class I MHC.
METHODS AND RESULTS
Low class I MHC expression in β-galactosidase–expressing D7RM-1 prostate cancer cells was shown by fluorescence activated cell sorting, and deficient class I MHC-mediated antigen presentation was shown in resistance of D7RM-1 to cytolysis by β-galactosidase–specific cytotoxic T lymphocytes (CTL). Despite partially deficient class I MHC presenting function, immunization with vaccinia encoding β-galactosidase conferred antigen-specific protection against D7RM-1 cancer. Antigen-specific immunity was recapitulated in β2m knockout mice (with deficient class I MHC and CTL function), confirming that class I MHC antigen presentation was not required for immunity against tumor partially deficient in class I MHC. Conversely, antigen-specific antitumor immunity was abrogated in Abβ knockout mice (with deficient class II MHC and helper T cell function), demonstrating a requirement for functional class II MHC. Resistant tumors from the otherwise effectively immunized β2m knockout mice (among which tumor progression had been reduced or delayed) showed reduced target antigen expression, corroborating antigen-specificity (and showing an alternative immune escape mechanism), whereas antigen expression (like tumor growth) was unaffected among Abβ knockout mice.
CONCLUSION
Our results demonstrate that class I MHC-restricted antigen presentation and CTL activity is neither necessary nor sufficient for antigen-encoding vaccinia immunization to induce protective immunity against class I MHC-low tumors, whereas host class II MHC-mediated antigen presentation facilitates antigen-specific immunity against prostate cancer in vivo. Reduced expression of the target antigen developed rapidly in vivo as an immune escape mechanism for such cancers.
Keywords: prostate cancer, T lymphocytes, tumor antigens, vaccinia, immunotherapy
INTRODUCTION
One mechanism whereby tumor cells escape immune recognition is by evading class I major histocompatibility complex (MHC) -mediated tumor recognition by cytotoxic T lymphocytes (CTL) [1–3]. Prostate cancer specifically has been implicated as exhibiting class I MHC antigen processing deficiencies in phenotypic and functional analyses, and reduced class I MHC expression in prostate cancer has been found to be associated with advanced stage and grade of such cancers [4–6]. Other human cancers have shown similar deficiencies in class I MHC antigen processing and presentation [1,2]. Preclinical in vivo studies evaluating immune responses against class I MHC-deficient cancers, however, have been rare and have been limited to assessing immune responses of tumor cell vaccines against unidentified tumor-associated antigens (TAA) [3,7,8]. Moreover, animal models previously used to evaluate immune responses, after immunization against specific tumor antigens, have typically exhibited robust class I MHC expression and consequent reliance on class I MHC-directed, cytotoxic T lymphocytes (CTL). In contrast, the effect of TAA-specific immunization has not been extensively characterized in prostate cancer models not requiring interaction between TAA-specific CTL and class I MHC.
Therefore, we sought to determine whether TAA-specific immunity could be induced in a setting of prostate cancer with partially deficient class I MHC function in the target tumor. Due to the growing interest in the clinical development of recombinant poxviruses as TAA-specific vaccines [9,10], we selected vaccinia as the TAA delivery vehicle for these studies. Among various cancers, prostate cancer represents a relevant setting in which micrometastatic, preclinical models relate to clinical stages of prostate cancer in which microscopic cancer burden can serve as a substrate for clinical vaccine trials. Therefore, a syngeneic mouse model of prostate cancer (which we found to exhibit partially deficient class I MHC function) was used as the target for evaluating antitumor effects of TAA-specific immunization [11]. Because no specific native prostate cancer tumor antigen has been isolated from transplantable murine prostate cancer cells, we introduced β-gal to serve as a model TAA for these studies; prior studies have shown the utility of β-gal as a target TAA model in murine systems. Through experiments using RM-1 cells in T cell competent and in CD8 and CD4 knockout mice, we show that TAA-specific immunization with recombinant vaccinia can induce protective antitumor immunity, despite deficient interaction between host CTL and tumor class I MHC and that such immunity requires competent class II MHC function.
MATERIALS AND METHODS
Cell Lines
All cell lines were maintained in Dulbecco's modified Eagle media (Life Technologies, Inc., Grand Island, NY) supplemented with 100 U of penicillin per ml, 100 mg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum (Life Technologies, Inc.) at 37°C in a 5% CO2 incubator unless it is indicated otherwise. Mouse T cell lymphoma lines EL4 (H-2b) and E22 (H-2b) and colon carcinoma cell line CT26.CL25 (H-2d) have been described previously [12]. E22 and CT26.CL25 cells express β-gal and are maintained in 400 µg/ml effective concentration of G418 media. RM-1 (provided by Dr. T. Thompson, Baylor College of Medicine) [11] and D7RM-1 cell lines are prostate cancer cell lines from C57BL/6 mice.
D7RM-1 is a cell line derived from a single cell clone stably expressing β-gal and was generated by transducing RM-1 cells with an amphotropic retroviral vector encoding β-gal as follows: Amphotropic supernatant transmitting LacZ-expressing retroviral vector was generated by calcium phosphate transfection of Phoenix A cells (provided by Dr. G. Nolan, Stanford University) by using ProFection Mammalian Transfection System (Promega, Madison, WI). The complete transfection solution was added onto Phoenix A cells, which were then incubated at 37°C for 8 to 10 hr, after which cell culture medium was exchanged. Viral supernatant was collected from producer cell culture supernatant of days 2–5 filtered through a 0.45-µM filter to remove Phoenix A cells. Protamine sulfate (5 µg/ml) (Sigma, St. Louis, MO) was added to the viral supernatant, which was immediately placed on 105 RM-1 tumor cells that had been plated into each well on a six-well plate 1 day previously. Limiting dilution was performed to generate the single-tumorcell clones, including D7RM-1. LacZ expression by D7RM-1 was measured at 72 hr by X-gal assay, for which cells were fixed in phosphate buffered saline (PBS) with 0.5% glutaraldehyde for 10 min followed by resuspension in complete staining solution (2 mg/ml X-gal, 10 mM potassium ferricyanide, 10 mM potassium ferrocyanide, 4 mM MgCl2), followed by incubated at 37°C in the dark for 2–4 hr, after which detection of blue cells at 100× microscopy confirmed the presence of β-gal.
Fluorescence Activated Cell Sorting Analysis
RM-1 mouse prostate cancer cells, LacZ transduced D7RM-1 cells, or controls were washed twice with fluorescence activated cell sorting (FACS) buffer (PBS supplemented with 0.1% bovine serum albumin and 0.1% sodium azide), and one million cells were incubated with 0.5 µg of fluorescein isothiocyanate (FITC) -conjugated antibody specific for class I MHC (Accurate Chemical & Scientific Corporation, San Diego, CA) or isotype control antibody in a final volume of 50 µl at 4°C for 30–40 min. Cells labeled with isotype-control antibody were used to determine background fluorescence, and total of 10,000 viable cells were analyzed per sample in a FACScan flow microfluorometer (Becton Dickinson, Sunnyvale, CA).
Vaccinia
Recombinant vaccinia viruses rVV-β-gal and V69 were produced as previously described [13,14]; for rVV-β-gal, LacZ gene was driven by the synthetic early/late promoter (pSE/L) [11]. Purified virus was prepared and titered as described by Earl and Moss [15].
Animals
All experiments were approved by the University of Michigan Committee on Use and Care of Animals and were conducted in accordance with National Institutes of Health guidelines. Six- to 8-week-old male C57BL/6 mice were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). β2m and Abβ knockout mice in the C57BL/6 background were purchased from TACONIC, Inc. (Germantown, NY); these strains have been demonstrated to be deficient in the expression of functional class I and class II MHC molecules, respectively [16,17]. Previous studies have shown that CD8+ cells and CD4+ cells are essentially undetectable in the periphery of β2m knockout (CD8+ cells at limits of detection) and Abβ knockout mice (CD4+ cells at limits of detection), respectively, compared with the normal mice [17,18]. For experiments evaluating protection against tumor challenge after immunization, C57BL/6, β2m knockout, and Abβ knockout mice were immunized by intravenous tail vein injection with 107 plague-forming units (pfu) per mouse of rVV-β-gal or V69 (control vaccinia). Three weeks later, 105 β-gal expressing D7RM-1 tumor cells were injected subcutaneously in the right flank. Animals were monitored at least three times weekly for the appearance of measurable tumors, tumor progression, and survival by an individual blinded to the immunization status of the animals. Mice were followed up until death from cancer or were killed when the tumors interfered with the animal's well-being, as shown by ungroomed fur, slow movement, or cachexia. Death was confirmed to be tumor-related by means of postmortem examination.
Generation of CTL and Assay of CTL-Mediated Tumor Cell Cytolysis
Mice were immunized with 107 pfu per mouse of rVV-β-gal or control vaccinia (V69) by means of an intravenous tail vein injection. Splenocytes were harvested 3 weeks later and were stimulated in vitro with 1 mg/ml of β-gal peptide (DAPIYTNV) [19] and 10 U/ml of recombinant IL-2 for 1 week to generate β-gal –specific CD8 CTL. A 6-hr 51Cr-release assay was used to assess the cytotoxic activity of these CTL. Briefly, 106 target cells were labeled with 51Cr for 1–2 hr. Labeled target cells (E22, EL4, CT26.CL25, RM-1, and D7RM-1) were incubated with various numbers of effector cells (effector to target [E:T] = 100, 33, 11, and 3.7) at 37°C for 6 hr. An automated gamma counter (Skatron Instruments, Inc., Sterling, VA) was used to measure chromium release. Percentage specific lysis was calculated from triplicate samples as follows: ([experimental counts per minute {cpm} –spontaneous cpm]/[maximal cpm –spontaneous cpm]) × 100. Data included in this report represent assays in which spontaneous release of labeled target cells was less than 20% of maximal release and standard deviation of triplicate values was less than 15%.
Quantitative Assay of β-gal Expression in Tumor Tissue
Tumors were harvested from killed mice, rinsed, homogenized, and resuspended in protein lysis buffer (5 mM Tris, 120 mM NaCl, 15% IGEPAL, Sigma) with proteinase inhibitors phenylmethyl sulphonyl fluoride, leupeptin, and aprotinin (Boehringer Mannheim, Indianapolis, IN). The tumor lysate suspension was then centrifuged at 14,000g for 15 min. Protein concentration in the supernatant was determined by using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Triplicate samples containing 90 µg of total protein each in 50 µl were added to individual wells in 96-well plates with 100 µl of buffer (2 mM MgCl2, 0.1 mM β-mercapthoethanol, 0.1 M sodium phosphate, pH 7.5) and 50 µl of the substrate onitrophenyl-galactopyranoside (ONPG; 4 mg/ml) and incubated at 37°C before the reaction was stopped by the addition of 100 µl of 1 M Na2CO3. A standard curve was generated with known amounts of β-gal. The colorimetric reaction of the samples was read at 405 nm by using a MRX automated microplate reader (Dynatech Lab, Chantilly, VA).
Statistical Analysis
Differences in tumor-free and overall survival between groups of immunized mice were evaluated for significance by using the log rank test. Differences in β-gal expression as measured by ONPG assay were evaluated for significance by using the t-test. Significance was set at P=0.05 for all comparisons and analyses were performed by using STATISTICA software (Tulsa, OK).
RESULTS
Class I MHC Expression and Function in the Mouse Prostate Cancer Cell Line D7RM-I
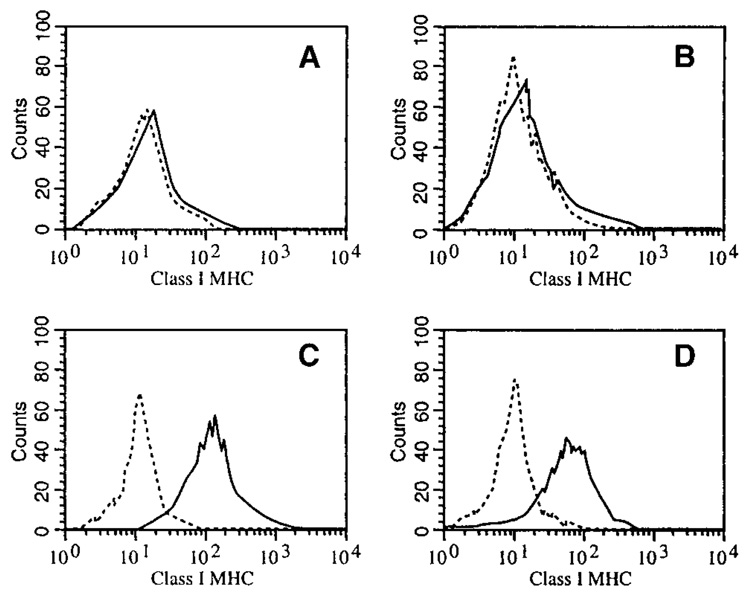
To generate a mouse prostate cancer cell line expressing β-gal as model tumor-associated antigen (TAA), the mouse prostate cancer cell line RM-1 was transduced with a retroviral vector encoding β-gal (Materials and Methods section), and limiting dilution was used to isolate β-gal expressing clones (D7RM-1) after screening for β-gal expression by X-gal assay. The cell surface expression of class I MHC by D7RM-1 cells was evaluated by FACS analysis by using a FITC-conjugated mouse class I MHC framework antibody (Fig. 1). A T-lymphoma cell line, EL4, and its β-gal expressing subclone, E22, were used as positive controls for class I MHC expression in these analyses. Like the parental RM-1 cells (Fig. 1A), D7RM-1 cells are deficient in class I MHC expression (Fig. 1B). To determine possible effects of β-gal expression on the tumorigenicity of RM-1 cells, D7RM-1 cells (expressing β-gal) and untransduced RM-1 were injected subcutaneously in groups of C57BL/6 mice. The mice were followed at least three times weekly for the appearance of measurable tumors, tumor progression, and survival. No significant difference in tumor growth and progression in vivo was found between D7RM-1 and its parental cell line, RM-1 (data not shown).
Fig. 1.
Class I major histocompatibility complex (MHC) expression in D7RM-1 and RM-1 prostate cancer cell lines as measured by flow cytometry. RM-1 (A) and D7RM-1 (B) cells were stained with a fluorescein isothiocyanate–conjugated class I MHC-specific antibody. The dashed lines represent samples stained with isotype control antibodies. EL4 cell line (C) and the derivative β-gal expressing E22 cell line (D) were stained as controls showing abundant class I MHC expression.
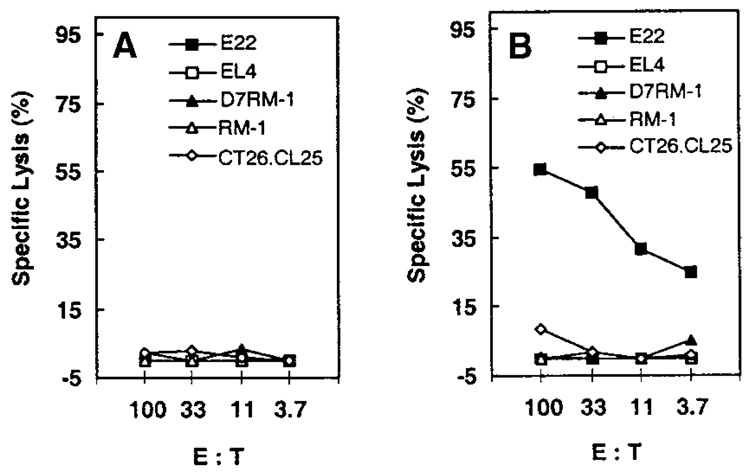
We next sought to evaluate the ability of D7RM-1 prostate cancer cells to present the model TAA (β-gal) to CTL with specificity against this model TAA. Such class I MHC-restricted antigen presentation was evaluated, by measuring the susceptibility of D7RM-1 prostate cancer cells to cytolysis by β-gal–specific CTL (induced by means of recombinant vaccinia immunization). CTL were derived by immunizing C57BL/6 mice with vaccinia control (V69, Fig. 2A) or rVV-β-gal (Fig. 2B). Specificity of rVV-β-gal immunization-derived CTL for the model TAA was confirmed by β-gal–specific CTL-mediated cytolysis of β-gal expressing E22 (H-2b) target cells that have abundant class I MHC, whereas β-gal negative, syngeneic EL-4 cells were not lysed (Fig. 2B). These β-gal–specific CTL (from C57BL/6 mice with H-2b class I MHC) did not lyse β-gal expressing, CT26.CL25 targets (from Balb-c mice with H-2d class I MHC), indicating that the observed β-gal–specific CTL activity was class I MHC restricted. In contrast, neither β-gal–negative RM-1 cells nor β-gal–positive D7RM-1 cells were lysed by β-gal–specific CTL induced by rVV-β-gal (Fig. 2B), indicating that D7RM-1 failed to present the model TAA (β-gal), by means of class I-MHC, to CTL specific for the model TAA.
Fig. 2.
D7RM-1 prostate cancer cells are functionally deficient in antigen presentation to class I major histocompatibility complex (MHC)-restricted cytotoxic T lymphocytes (CTL). C57BL/6 mice (H-2b) were immunized with 107 plaque forming units control vector (V69, A) or β-gal–encoding recombinant vaccinia virus (rVV-β-gal, B). Splenocytes were prepared 3 weeks later and stimulated with β-gal peptide in vitro for a week to generate β-gal–specific CTL. The ability of target tumor cells to present antigen in a class I MHC-restricted manner to these β-gal–specific CTL was evaluated by means of chromium release cytotoxicity assay as described in the Materials and Methods section. Tumor target cells included those with β-gal (E22, D7RM-1) or without β-gal (EL4, RM-1). β-gal–expressing CT26.CL25 cell line (H-2d) was used as a control for class I MHC-restricted antigen presentation. E:T, effector to target.
In Vivo Efficacy of Recombinant Vaccinia Immunization as Prevention Against RM-1 Prostate Cancer Growth and Progression
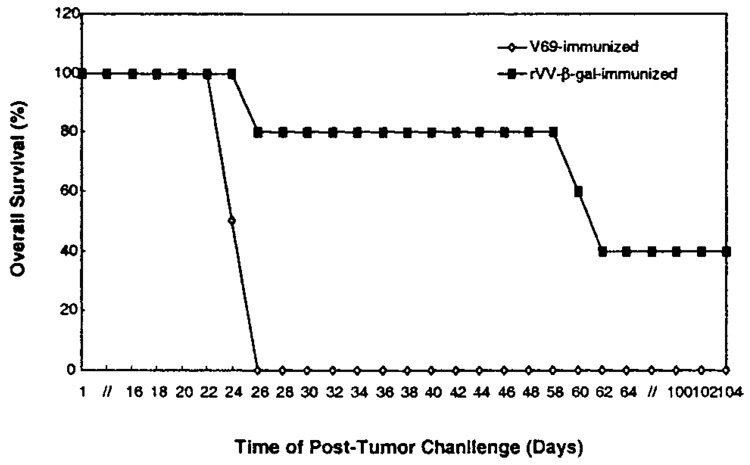
To characterize the in vivo antitumor efficacy of TAA-specific recombinant vaccinia immunization against growth and progression of class I MHC-deficient tumors, C57BL/6 mice were next immunized with rVV-β-gal followed by subcutaneous challenge of β-gal TAA–expressing, D7RM-1 tumor cells. Although 100% of the control vaccinia V69-immunized mice developed D7RM-1 tumor early and died within 1 month of tumor challenge, approximately 40% of the mice immunized with rVV-β-gal were tumor-free and alive 3 months or longer after tumor challenge (Fig. 3; P = 0.002). Therefore, TAA-specific immunization, with recombinant vaccinia virus, induced TAA-specific immunity against class I MHC-deficient D7RM1 prostate cancer cells in vivo (Fig. 3), despite the demonstrated deficiency of these tumor cells in class I MHC-mediated antigen presentation (Fig. 2).
Fig. 3.
Recombinant vaccinia encoding a model TAA (β-gal) induces TAA-specific immunity in vivo against RM-1 prostate cancer cells. C57BL/6 mice were immunized with 107 plaque forming units/mouse of rVV-β-gal (filled symbols) or control vector V69 (open symbols).Three weeks later, immunized mice were challenged with 105 D7RM-1 tumor cells subcutaneously and followed up for tumor-related death (n=10 mice per therapy group; P=0.002). Similar findings confirmed in three separate experiments.
Role of Class I MHC and Class II MHC Function in the Prostate Cancer Host Animal
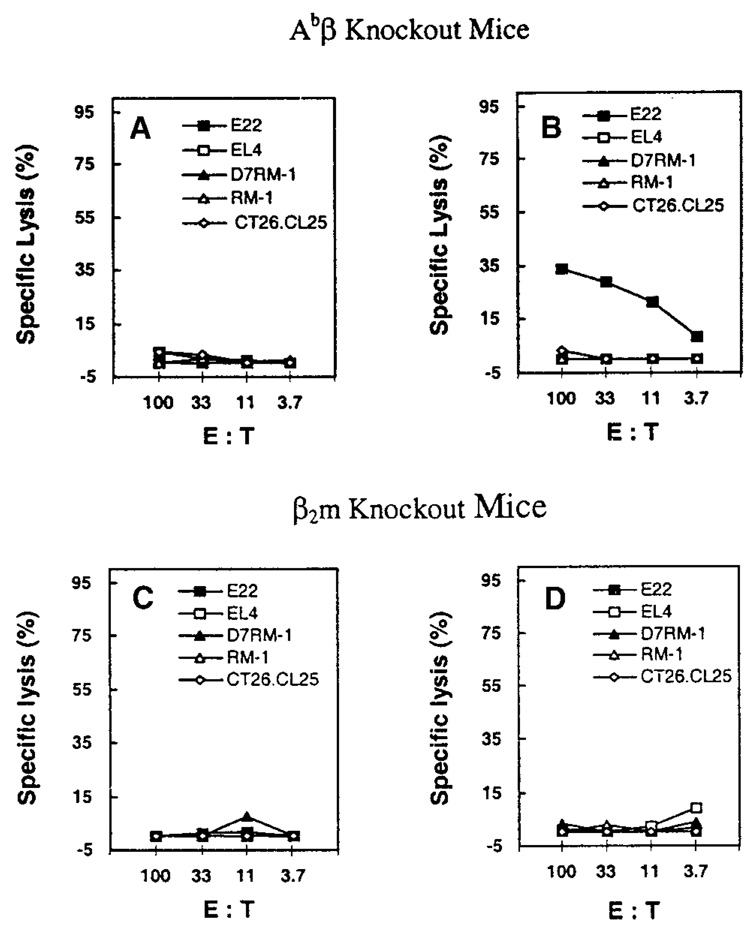
To further ascertain that antigen presentation to class I MHC-restricted CTL was not involved (in the observed protection against D7RM-1 tumor after rVV-β-gal immunization), immunization and D7RM-1 challenge experiments were repeated in class I MHC knockout β2m−/− mice. As expected, rVV-β-gal immunization of β2m−/− mice failed to induce β-gal–specific CTL (Fig. 4). However, despite the absence of β-gal–specific CTL, βm−/− mice immunized with rVV-β-gal were protected against D7RM-1 tumor growth in an antigen-specific manner (Fig. 5D), demonstrating that TAA-specific protection can be conferred, independent of class I MHC-restricted antigen presentation by either tumor or host in vivo.
Fig. 4.
Immunization with recombinant vaccinia virus induces tumor antigen-specific cytotoxic T lymphocytes (CTL) activity in Abβ knockout (class II major histocompatibility complex [MHC] nonfunctional) mice but not in β2-microglobulin (β2-m) knockout (class I MHC nonfunctional) mice. Abβ knockout and β2-microglobulin knockout mice were immunized with 107 plaque forming units V69 (A,C) or rVV-β-gal (B,D). To detect elicited, β-gal–specific CTL, splenocytes were harvested 3 weeks after immunization, stimulated with β-gal peptide, and evaluated by chromium release CTL assay as described in the Materials and Methods section. Targets for distinguishing β-gal–specific class I MHC-restricted CTL activity included tumor targets with (E22, D7RM-1) or without (EL4, RM-1) the expression of β-gal. β-gal–expressing CT26.CL25 cell line (H-2d) was used as a control for MHC restriction.
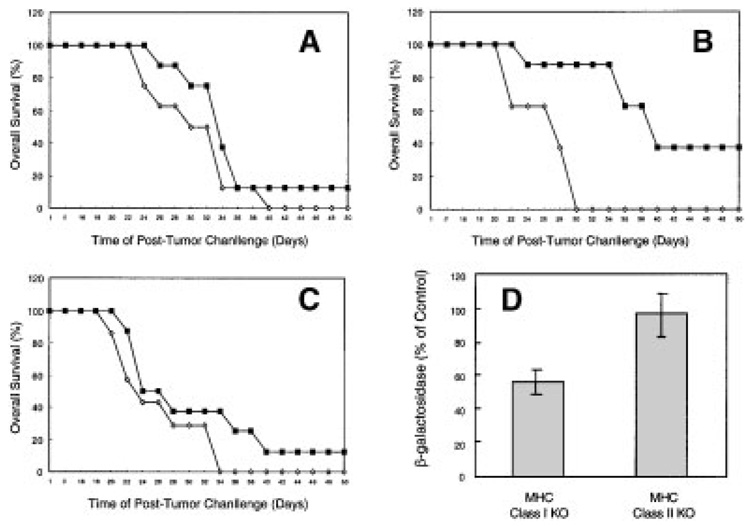
Fig. 5.
Class II major histocompatibility complex (MHC) (but not class I MHC) -restricted antigen presentation is required for effective tumor antigen-specific immunization and consequent reduced TAA levels in immunization-resistant tumors. Class II MHC knockout mice (A), class I MHC knockout mice (B), and interferon-γ knockoutmice (C) were immunized with 107 plaque forming units of either V69 (open symbols) or rVV-β-gal (filled symbols). Three weeks after immunization, mice were challenged by 105 D7RM-1 tumor subcutaneously and followed for tumor-related death. Significant protection in vivo against tumor challenge after immunization was observed only in class I MHC knockout mice (B;P=0.007), whereas antigen-specific protection against tumor challenge was abrogated (not significant) in class II MHC as well as interferon-γ knockout mice (A,C). D: To determine whether rVV-β-gal immunization led to robust β-gal–specific immune response in vivo, expression of β-gal in refractory, progressive (immunization-resistant) tumors was evaluated by o-nitrophenyl-galactopyranoside (ONPG) assay. β-gal expression in tumors from class IMHC knockout (KO) mice immunized with rVV-β-gal were significantly lower than in control mice (P=0.03), whereas β-gal levels in class II MHC knockout mice were unaffected by rVV-β-gal immunization (β-gal levels in tumor tissue from rVV-β-gal immunized mice are shown as a percentage of β-gal levels found in tumor tissue from V69-immunized control mice).
The requirement for competent class II MHC function for induction of effective, TAA-specific immunity against class I MHC-deficient D7RM1 tumor was next assessed by means of vaccinia immunization of class II MHC knockout (Abβ−/−) mice. Of interest, the ability to induce β-gal–specific class I MHC-restricted CTL activity by rVV-β-gal immunization was present in class II MHC knockout mice (Fig. 4). However, despite induction of class I MHC restricted β-gal–specific CTL activity, immunization with recombinant vaccinia encoding the target tumor antigen (rVV-β-gal) did not induce effective, antigen-specific, immune protection against tumor growth and progression in class II MHC knockout mice (Fig. 5A). Abrogation of effective immunity was also noted in interferon-gamma (IFN-γ) knockout mice (Fig. 5C), suggesting that the class II MHC-mediated response required for effective immunity against class I MHC-deficient tumors may occur by means of Th1 pathways. These observations suggest that class I MHC-restricted TAA recognition is neither necessary, nor sufficient, for the induction of effective, TAA-specific immunity against class I MHC-deficient cancer cells.
We next sought to determine whether TAA expression (among immunity-resistant D7RM1 tumors) corroborated the presence of effective, TAA-specific immunity in class I MHC knockout mice compared with deficient antigen-specific immunity in class II MHC knockout mice. We hypothesized that, if potent TAA-specific immunity is present, then immunity-resistant tumors could be expected to show reduced levels of TAA expression compared with tumors from control mice. To test this hypothesis, model TAA (β-gal) immunization-resistant tumors from class I knockout and class II knockout mice were evaluated for levels of β-gal expression by ONPG assay. Tumors that eventually grew in class I MHC knockout mice immunized with rVV-β-gal showed reduced β-gal expression compared with tumors from control mice, whereas β-gal expression in resistant outgrowing tumors from class II MHC knockout mice immunized with rVV-β-gal were unaffected (Fig. 5D). These findings corroborate that TAA-specific immunization can specifically eliminate TAA-expressing tumors in the absence of class I MHC-mediated antigen presentation but that reduced TAA expression represents a mechanism for possible escape, by such tumors, from immune recognition.
DISCUSSION
We have demonstrated the ability of recombinant vaccinia virus encoding a model tumor-associated antigen (TAA) to induce effective, TAA-specific immunity in vivo that is independent of interaction between CTL and class I MHC presenting the target TAA. Through in vivo studies using knockout mice (lacking class I MHC, or class II MHC, or IFN-γ), we found that class I MHC-restricted TAA recognition is neither sufficient, nor necessary, for the induction of such TAA-specific immunity against RM-1 prostate cancer cells. In contrast, both class II MHC-mediated T cell help and IFN-γ were required. Limitations of this study include use of a single cell line with a model TAA that is not a native prostatic TAA.
Our findings complement and extend the prior work of Smyth et al. and Griffith et al., who studied nonspecific, natural killer (NK) cell-mediated immunity by using the RM-1 prostate cancer model [7,8,20,21]. Smyth et al. evaluated immunity against RM-1 cells and class I MHC-deficient lymphoma tumor lines, but evaluated neither class I MHC-mediated CTL nor class II MHC-mediated CD4 helper T cell activity in studies that focused largely on NK responses against unidentified antigens [7,8,21]. Griffith et al. similarly focused on NK-mediated responses in the setting of unidentified TAA, and showed that neither CD8 CTL nor class II MHC-mediated CD4 T helper function were required for such nonspecific, NK-mediated antitumor activity [20]. Neither Smyth et al. nor Griffith et al. reported mechanisms of resistance that were associated with tumors refractory to immunotherapy. The studies described herein complement these prior works by focusing on antigen-specific immune responses, such as would be relevant to TAA-specific tumor vaccines and immunotherapy. Our findings indicate that mechanisms of TAA-specific immunity may differ from those previously reported as required for NK-mediated immunity lacking a defined TAA target. Our use of a model expressing a defined TAA also showed the potential for loss of TAA expression in vivo to provide a resistance mechanism against TAA-specific immunotherapy. Such TAA-loss mechanisms of immune evasion have not been extensively characterized previously by in vivo models.
There is published controversy regarding the adequacy of class I MHC expression in RM-1 cells: Smyth et al. reported absent class I MHC with no change after stimulation with IFN-γ in vitro, whereas Griffith et al. reported low but detectable class I MHC and increased expression after stimulation with IFN-γ in vitro [7,8,20]. Our observations were consistent, in part, with both prior reports: like Smyth et al., we could detect no class I MHC by flow cytometry in the absence of IFN-γ stimulation in vitro (Fig. 1); however, consistent with the findings of Griffith et al., we observed that class I MHC could be detected by flow cytometry after supra-physiological IFN-γ stimulation in vitro (data not shown). We extended beyond the prior observations of Griffith et al. and of Smyth et al. by showing that the low class I MHC expression by RM-1 is associated with defective antigen presentation to antigen-specific CTL (in the absence of IFN-γ) as measured by chromium release assay (Fig. 2). Our findings suggest that the conflicting prior reports regarding class I MHC in RM-1 cells can be reconciled by concluding that RM-1 cells have variably deficient class I MHC function that can be augmented by IFN-γ under some conditions: In sum, class I MHC function in RM-1 cells can show variable deficiency but is not completely absent.
Irrespective of the severity of class I MHC function deficiency in RM-1 prostate cancer cells, studies with class I MHC knockout mice showed that mechanisms requiring interaction between CTL and class I MHC are not necessary for effective, antigen-specific antitumor immunity in vivo (Fig. 5B). Lack of the ability to elicit β-gal–specific CTL from class I MHC knockout mice (after rVV-β-gal immunization) demonstrated that retaining TAA-specific antitumor immunity in class I MHC knockout mice was not due to persistent functional CTL in the class I MHC knockout mice: we were unable to detect any significant, antigen-specific, CTL activity either from splenocytes (Fig. 4) or from tumors (data not shown) of such mice. This finding contrasts with a prior report which showed that CTL specific for allogeneic class I MHC could be elicited from allogeneic tumors in these class I MHC knockout mice [22]. Our findings suggest that emergence of CTL (despite absent β2-microglobulin) in class I MHC knockout mice may depend on the model tumor antigen target and can be absent when allogeneic MHC is not the tumor antigen. Moreover, we found that the ability to induce TAA-specific CTL was not sufficient to eliminate the class I MHC-deficient tumor in vivo, as demonstrated by the absence of protection against D7RM-1 growth in class II MHC knockout mice, despite β-gal–specific CTL induction (Fig. 4, Fig. 5). In our model for inducing a nonself, TAA-specific immune response by recombinant poxvirus immunization, CTL responses restricted by class I MHC-mediated antigen presentation were neither required nor sufficient to generate effective, antigen-specific antitumor immunity against class I MHC-deficient tumor.
In contrast, class II MHC function was required (in the tumor-bearing host) for effective TAA-specific immunization against class I MHC-deficient D7RM-1 tumor, as demonstrated by TAA-specific immunity being abrogated in Abβ knockout mice. The class II MHC-mediated function can be ascribed to host cells (rather than the target tumor) because, as in most tumor cells, D7RM-1 cells do not express class II MHC molecules (data not shown). Mechanisms whereby class II MHC-mediated TAA processing and presentation by host antigen-presenting cells can induce effective TAA-specific immunity include Th1 cell-mediated activation of CTL, natural killer (NK) cells, or macrophages [3,23–27]. The ability of CTL to confer TAA specificity in the effector phase of TAA-specific responses is well characterized; however, our data suggest that CTL were not the principal effector cells against class I MHC-deficient tumor. NK cells and macrophages may be involved in killing of the β-gal expressing, class I MHC-deficient tumor in a non– tumor antigen-specific manner. However, the reduced TAA expression we observed in tumors refractory to TAA immunization (Fig. 5D) corroborated that elimination of class I MHC-deficient tumor was associated with antigen specificity in vivo.
The mechanism of antigen-specificity in the effector component of immune responsiveness against class I MHC-deficient tumor, such as observed herein, remains elusive. In our model, the target tumor antigen was cytosolic, rendering it unlikely that antigen-specific antibodies were pivotal; however, a possible role for humoral effectors, such as tumor antigen-specific antibody that others have found to contribute in class I MHC abundant models, has not been excluded. [28] Other possible mechanisms include models whereby Th1 cells promote antigen-specific tumor cell death consequent to their interaction with antigen-presenting cells that present tumor antigen in the vicinity of class I deficient, tumor antigen-expressing tumor cells. Examples of the latter are Fas-mediated, antigen-restricted cytolysis by CD4+ T lymphocytes [29,30] or stimulation of NK cells or macrophages due to antigen-specific secretion of IFN-γ such as by TAA-specific Th1 cells [21,23]. Of interest, others have also found that immunogenicity in the mouse prostate reconstitution model (from which RM-1 cells were derived) has been shown to be associated with resistance to CTL-mediated lysis [31]. We found that antigen-specific immunity in our class I MHC-deficient model was abrogated in mice lacking IFN-γ. This observation indirectly implicates Th1 cells as important for induction of CTL-independent cellular effectors against class I MHC-deficient tumor cells. A similar requirement for IFN-γ has been shown previously for tumor models with abundant class I MHC [23].
Our findings demonstrate that TAA-specific immunity against low class I MHC prostate cancers can be induced by means of immunization with TAA-encoding vaccinia. Absence of functional class II MHC in the vaccine recipient resulted in a profound defect in effective, TAA-specific, immunity induction by recombinant vaccinia virus against class I MHC-deficient tumor. The lack of protection (in absence of effective host class II MHC function) was associated with unaffected levels of tumor antigen expression in progressive tumors. Although the effector mechanism responsible for the elimination of the class IMHC low tumor remains undefined and under investigation, the requirement of both a competent class II MHC pathway and IFN-γ suggest that class II MHC-mediated antigen presentation, and possibly Th1 cells, can serve a pivotal role in vaccinia virus-induced, antigen-specific protection against class I MHC-deficient cancers in vivo. These findings support efforts to develop vaccine and immune therapies that do not rely exclusively on immune responses mediated by means of class I MHC antigen presentation [32,33].
ACKNOWLEDGMENTS
The authors thank Dr. James J. Mule for helpful discussion, Linda Charles and Marvin Eng for technical assistance, and Jennifer Vida for assistance in manuscript preparation.
Grant sponsor: NCI; Grant number: R01-CA82419-01; Grant sponsor: American Cancer Society; Grant number: Career Development Award 96-77.
REFERENCES
- 1.Cordon-Cardo C, Fuks Z, Drobnjak M, Moreno C, Eisenbach L, Feldman M. Expression of HLA-A,B,C antigens on primary and metastatic tumor cell populations of human carcinomas. Cancer Res. 1991;51:6372–6380. [PubMed] [Google Scholar]
- 2.Eisenbach L, Feldman M. Tumor cell surface determinants in host immunity against metastases. Semin Cancer Biol. 1991;2:179–188. [PubMed] [Google Scholar]
- 3.Levitsky HI, Lazenby A, Hayashi RJ, Pardoll DM. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994;179:1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46:681–687. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 5.Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, Moy P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate. 1997;33:233–239. doi: 10.1002/(sici)1097-0045(19971201)33:4<233::aid-pros2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cellmediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 9.Mastrangelo MJ, Eisenlohr LC, Gomella L, Lattime EC. Pox-virus vectors: orphaned and underappreciated. J Clin Invest. 2000;105:1031–1034. doi: 10.1172/JCI9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lathe R, Kieny MP, Gerlinger P, Clertant P, Guizani I, Cuzin F, Chambon P. Tumour prevention and rejection with recombinant vaccinia. Nature. 1987;326:878–880. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- 11.Baley PA, Yoshida K, Qian W, Sehgal I, Thompson TC. Progression to androgen insensitivity in a novel in vitro mouse model for prostate cancer. J Steroid Biochem Mol Biol. 1995;52:403–413. doi: 10.1016/0960-0760(95)00001-g. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Chen PW, Bronte V, Rosenberg SA, Restifo NP. Antitumor activity of cytotoxic T lymphocytes elicited with recombinant and synthetic forms of a model tumor-associated antigen. J Immunother Emphasis Tumor Immunol. 1995;18:139–146. doi: 10.1097/00002371-199510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 14.Smith TL, Jennings R, Potter CW. Use of single radial haemolysis for assessing antibody response to influenza virus vaccines in animals. Med Microbiol Immunol (Berl) 1987;176:329–339. doi: 10.1007/BF00194892. [DOI] [PubMed] [Google Scholar]
- 15.Earl PL, Cooper N, Moss B. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith LA, editors. Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley Interscience; 1991. pp. 16.16.1–16.16.17. [Google Scholar]
- 16.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 17.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 18.Spriggs MK, Koller BH, Sato T, Morrissey PJ, Fanslow WC, Smithies O, Voice RF, Widmer WB, Maliszewski CR. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for "self" antigens. Methods. 1997;12:117–123. doi: 10.1006/meth.1997.0461. [DOI] [PubMed] [Google Scholar]
- 20.Griffith TS, Kawakita M, Tian J, Ritchey J, Tartaglia J, Sehgal I, Thompson TC, Zhao W, Ratliff TL. Inhibition of murine prostate tumor growth and activation of immunoregulatory cells with recombinant canarypox viruses. J Natl Cancer Inst. 200;93:998–1007. doi: 10.1093/jnci/93.13.998. [DOI] [PubMed] [Google Scholar]
- 21.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 22.Apasov SG, Sitkovsky MV. Development and antigen specificity of CD8+ cytotoxic T lymphocytes in beta 2-microglobulinnegative, MHC class I-deficient mice in response to immunization with tumor cells. J Immunol. 1994;152:2087–2097. [PubMed] [Google Scholar]
- 23.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn M, Sugawara H, McGowan P, Okuno K, Nagoya S, Hellstrom KE, Hellstrom I, Greenberg P. CD4+ T cell clones specific for the human p97 melanoma-associated antigen can eradicate pulmonary metastases from a murine tumor expressing the p97 antigen. J Immunol. 1991;146:3235–3241. [PubMed] [Google Scholar]
- 27.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61:880–883. [PubMed] [Google Scholar]
- 29.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–59. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 30.Katsumoto Y, Monden T, Takeda T, Haba A, Ito Y, Wakasugi E, Wakasugi T, Sekimoto M, Kobayashi T, Shiozaki H, Shimano T, Monden M. Analysis of cytotoxic activity of the CD4+ T lymphocytes generated by local immunotherapy. Br J Cancer. 1996;73:110–116. doi: 10.1038/bjc.1996.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927–1933. [PubMed] [Google Scholar]
- 32.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–214. [PubMed] [Google Scholar]
- 33.McNeel DG, Nguyen LD, Ellis WJ, Higano CS, Lange PH, Disis ML. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate. 2001;47:222–229. doi: 10.1002/pros.1066. [DOI] [PubMed] [Google Scholar]