Abstract
Background
Very few studies have reported cancer outcomes of patients referred through different routes, despite the prominence of current UK cancer urgent referral guidance.
Aim
This study aimed to compare outcomes of cancer patients referred through the urgent referral guidance with those who were not, with respect to stage at diagnosis, survival, and delays in diagnosis.
Design of study
Analysis of hospital records.
Setting
One hospital trust in England
Method
The records of 889 patients diagnosed in 2000–2001 with one of four types of cancer were analysed: 409 with lung cancer; 239 with colorectal cancer; 146 with prostate cancer; and 95 with ovarian cancer. Outcome measures were diagnostic stage, survival, referral and secondary care delays.
Results
For lung cancer, urgent referrals had more advanced TNM (tumor, node, metastasis) stage than patients diagnosed through other routes (P = 0.035) and poorer survival (P = 0.020). There was no difference in stage or survival for the other cancers. For each cancer, a higher proportion of urgent referrals was seen within 2 weeks. Secondary care delays for lung and colorectal cancer were shorter for inter-specialty referrals.
Conclusion
For patients with lung cancer, the guidance appears to be prioritising those in the more advanced stages of disease. This was not the case for the other three cancers. Referral delays were shorter for patients urgently referred, as is the intention of the guidance. The avoidance of delays in outpatient diagnostics probably accounts for shorter secondary care delays for inter-specialty referrals.
Keywords: cancer, diagnostic tests, primary health care, referrals and consultation
INTRODUCTION
Cancers are diagnosed at a more advanced stage in the UK compared with other European countries;1 this contributes to poorer survival of patients with cancer in the UK. Reducing diagnostic delays may increase the proportion of early stage cancers, and improve survival. In addition to focusing on screening and public education, UK Government policy aimed at the reduction of diagnostic delays has primarily focused on referral delays.2 The urgent cancer referral guidelines aim to facilitate appropriate referral between primary and secondary care, and were implemented in stages between 1999 and 2000.3 They guarantee that everyone with suspected cancer should be able to see a hospital doctor within 2 weeks of their GP deciding that they need to be seen urgently and requesting an appointment. The signs and symptoms that fulfil the referral criteria for lung, colorectal, prostate, and ovarian cancer are summarised in Box 1.
Box 1. Summary of guidance for urgent referral
Lung
-
▸
Chest X-ray suggestive of lung cancer
-
▸
Persistent haemoptysis in smokers/ex-smokers over 40 years of age
-
▸
Signs of superior vena cava obstruction
-
▸
Stridor
-
▸
History of asbestos exposure and unexplained symptoms or suspicious chest X-ray
Colorectal
-
▸
A definite palpable right-sided abdominal mass (any age)
-
▸
A definite palpable rectal (not pelvic) mass (any age)
-
▸
Rectal bleeding WITH a change in bowel habit to looser stools and/or increased frequency of defecation persistent for 6 weeks (any age)
-
▸
Rectal bleeding persistently WITHOUT anal symptoms (anal symptoms include soreness, discomfort, itching, lumps and prolapse as well as pain) (over 60 years)
-
▸
Change in bowel habit to looser stools and/or increased frequency WITHOUT rectal bleeding and persistent for 6 weeks (over 60 years)
-
▸
Iron deficiency anaemia WITHOUT an obvious cause (Hb<11 g/dl in men and Hb<10 g/dl in non-menstruating women) (any age)
Prostatea
-
▸
An elevated age specific PSA in men with a 10-year life expectancy
-
▸
A high PSA in men with a clinically malignant prostate or bone pain
Ovarianb
-
▸
Palpable pelvic mass not obviously fibroids
-
▸
Suspicious pelvic mass on pelvic ultrasound
aProstate-specific criteria as part of the ‘urological’ guidance. PSA = prostate specific antigen.
bOvarian-specific criteria as part of ‘gynaecological’ guidance.
Limited research into the effectiveness of the guidelines4 demonstrates that the guidance is working to some extent.5,6 A concern is that the guidelines may simply identify the most obvious cancers that GPs would have identified and referred anyway, and which may be so advanced that the diagnosis is of limited value. A further concern is that cancers with atypical presentations may benefit most from early diagnosis.4 There is also emerging evidence that GPs' compliance with the guidance is less than perfect,6,7 and that they may use the guidance as a mechanism to get their patients referred quickly. Few studies have reported clinical outcomes of patients diagnosed through an urgent suspected cancer referral compared with patients diagnosed through other routes. Urgent referrals have been shown to reduce delays between referral and diagnosis in colorectal cancer, although the effect on survival in this study was inconclusive.8 Another study has shown no difference in diagnostic delays or in stage at diagnosis in colorectal cancer.9 No overall change in diagnostic stage has been found in lung cancer before or after the introduction of the guidelines.10 Delays can occur in any part of the cancer diagnostic journey, but the vast majority of delays occur before patients are seen in secondary care.11 Two-thirds of pre-hospital delays are patient delays (time from onset of symptoms to presentation), and one-third are primary care delays (time from presentation of symptom to onward referral or diagnostic investigation).11 However, it is likely that primary care delays are most amenable to intervention to reduce these delays and potentially improve outcomes. The aim of this paper is to compare outcomes of cancer patients referred through the urgent guidance with those who were not, with respect to survival, stage at diagnosis, and delays in diagnosis.
METHOD
Data collection
The process of collecting and cleaning data has been previously reported.6 In summary, data from a 2-year period (2000–2001) for patients with lung, colorectal, prostate or ovarian cancer within one NHS trust were used to identify two groups of patients:
Patients diagnosed with cancer who had been urgently referred by their GP through the 2-week fast-track system using locally agreed processes (‘urgent guideline referrals’).
Patients with cancer diagnosed through other referral pathways (non-urgent guideline GP referrals, inter-speciality referrals, accident and emergency department [A&E] referrals, screening diagnoses, and others). Standard referral letters marked ‘urgent’ were included in this group because such referrals may have been different in terms of symptom presentation, and different in the ways in which they were managed by the trust.
To make analysis as pragmatic as possible, and not simply a study of the guidelines, a sub-analysis of the data was performed combining all urgent referrals (that is, urgent guideline referrals and GP letters marked as urgent) compared with all other referrals.
How this fits in
Very few studies have reported cancer outcomes from patients referred through different routes; despite the prominence of current UK cancer urgent referral guidance. The current findings show that for patients with lung cancer the UK urgent referral guidance is prioritising those with more advanced disease: those who have least to gain in terms of morbidity and mortality from treatment. This was not the case for colorectal, prostate, or ovarian cancer. While urgently referred patients are diagnosed more quickly, the benefits to these patients and the potential detriment to patients diagnosed through non-urgent routes remain unknown.
In this paper data are reported for those patients with cancer whose hospital medical records were available (65% lung, 60% colorectal, 57% prostate, 83% ovarian). The process of retrieving records and the reasons for missing data have been previously reported.6 Patients with ovarian or prostate cancer were allocated to the urgent guideline referral group if they were referred as part of the urgent gynaecological or urological guidance respectively.
Data extraction from medical records
The following data, where available, were extracted from patients' hospital medical records:
Demographic data: age; sex; marital status; and ethnicity.
Source of referral: urgent GP referral; non-urgent GP-referral; inter-speciality referral; A&E; screening; or other (Table 1).
Dates of events: date of referral (taken from the date of GP referral letter or urgent referral form, date of inter-speciality referral, date of A&E attendance, or date of a screening referral); date of the first hospital appointment (from urgent appointment slip, or from clinic notes, and cross checked); date of cancer diagnosis (from specialist's letter to GP, or if unavailable from results letter, multidisciplinary team notes, or histology report); and date of death (taken from the death certificate, or from patient notes, or Patient Administration System database). These dates were used to calculate delays between GP referral and first hospital appointment, and secondary care delay (delay between first hospital appointment and diagnosis).
Stage of cancer at diagnosis: for lung and colorectal cancer the TNM (tumour, node, metastasis) staging system was recorded. For colorectal cancer Duke's stage was also recorded. For lung cancer, the extent of cancer was determined from the TNM stage groupings: limited (cancer cells can be seen only on one lung, in nearby lymph nodes, or in fluid around the lung); or extensive (cancer spread outside the lung within the chest area or to other parts of the body). For ovarian cancer, stage was defined as stage I–IV. For prostate cancer, stage was defined as T1–T3/T4; Gleason scores were also recorded. Additional staging data were obtained from the Bradford Medical Oncology Database.
Detail of GP referral: for urgent guideline referrals, data about the referral were gathered from the urgent referral form, with symptoms being recorded in a tick-box format mirroring the urgent referral guidance. For non-urgent GP, inter-speciality, and A&E referrals, information about referring symptoms was obtained from the referral letter.
Table 1.
Summary of referral routes.
| Diagnosed through other routes | |||||||
|---|---|---|---|---|---|---|---|
| Urgent guideline referrals | Total | Urgent GP letter | Non-urgent GP letter | Inter-specialty | A&E referral | Unknown | |
| Lung | 96 | 313 | 25 | 35 | 148 | 20 | 85 |
| Colorectal | 51 | 188 | 55 | 50 | 40 | 19 | 24 |
| Prostate | 46 | 100 | 10 | 62 | 13 | 9 | 6 |
| Ovarian | 23 | 72 | 6 | 25 | 14 | 4 | 23 |
Statistical methods
Tests undertaken for comparisons between urgent guideline referrals and patients diagnosed through other routes categorical data, were χ2 and t-tests were also undertaken to compare interval data. A Kaplan–Meier analysis with log rank tests was undertaken to compare survival as of June 2003 (the date when data collection commenced). This treats patients as ‘censored’, allowing for patients followed up for various time periods, and whether patients are known to be alive or dead at the point of data collection. All analyses were undertaken using SPSS. A further analysis was undertaken, correcting survival for stage.
RESULTS
Survival
Findings are presented in Table 2, and Figures 1–4. (Data relating to the comparison of all urgent referrals [guideline and letter] compared with all other routes are available from the authors.)
Table 2.
Kaplan–Meier survival analysis.
| Deaths Censored | |||||
|---|---|---|---|---|---|
| n | n | n | Mean survival days at June in 2003 (SE) | 95% CI | |
| Lung | |||||
| Urgent referrals | 81 | 39 | 42 | 458.2 (37.9) | 383.9 to 532.5 |
| Diagnosed through other routes | 222 | 76 | 146 | 591.9 (55.1) | 543.7 to 640.2 |
| Colorectal | |||||
| Urgent guideline referrals | 48 | 13 | 35 | 609.5 (46.0) | 519.4 to 699.6 |
| Diagnosed through other routes | 168 | 55 | 113 | 720.3 (36.2) | 649.3 to 791.3 |
| Prostate | |||||
| Urgent guideline referrals | 45 | 1 | 44 | 755.7 (15.1) | 726.1 to 785.3 |
| Diagnosed through other routes | 91 | 3 | 88 | 808.8 (14.8) | 779.8 to 837.9 |
| Ovarian | |||||
| Urgent guideline referrals | 11 | 5 | 6 | 459.9 (110.4) | 243.6 to 676.4 |
| Diagnosed through other routes | 47 | 16 | 31 | 685.7 (63.9) | 560.5 to 810.8 |
SE = standard error.
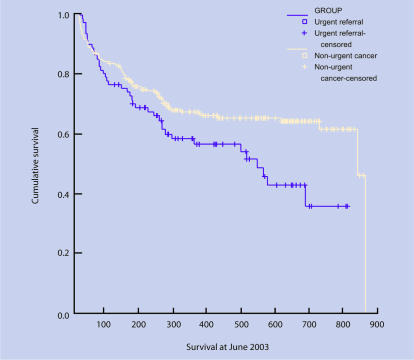
Figure 1.
Survival curves: lung.
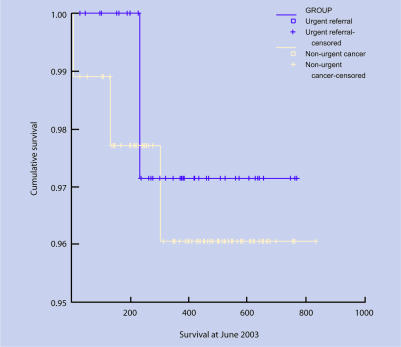
Figure 4.
Survival curves: ovarian.
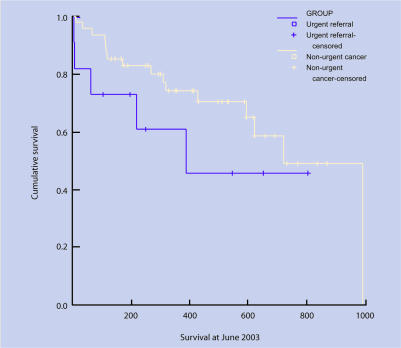
Figure 2.
Survival curves: colorectal.
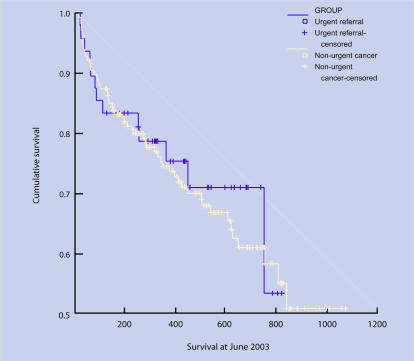
Figure 3.
Survival curves: prostate.
Lung cancer
Data for survival were available for 303 patients (74%). There was a difference in survival rates between urgent guideline referrals and those diagnosed through other routes (log rank = 5.40, df = 1, P = 0.020), with urgent referrals having shorter survival times. Comparison of all urgent referrals (guideline and letter) with other routes also showed a difference (log rank = 11.10, degrees of freedom [df] = 1, P ≤ 0.001).
Colorectal cancer
Data for survival were available for 217 patients (91%). The log rank test showed no difference in survival rates between urgent guideline referrals and those diagnosed through other routes (log rank = 0.11, df = 1, P = 0.735). Comparison of all urgent referrals (guideline and letter) with other routes also showed no difference (log rank = 1.78, df = 1, P = 0.183).
Prostate cancer
Data for survival were available for 136 patients (93%). There was no difference in survival rates between urgent guideline referrals and those diagnosed through other routes (log rank = 0.12, df = 1, P = 0.731). Comparison of all urgent referrals (guideline and letter) with other routes also showed no difference (log rank = 0.32, df = 1, P = 0.573).
Ovarian cancer
Data for survival were available for 58 patients (61%). There was no difference in survival rates between urgent guideline referrals and those diagnosed through other routes (log rank = 0.209, df = 1, P = 0.209). Comparison of all urgent referrals (guideline and letter) with other routes showed a borderline difference (log rank = 3.15, df = 1, P = 0.076).
Correcting survival for stage
For colorectal cancer, correcting using TNM stage still gave no difference (log rank = 0.02, df = 1, P = 0.881); correcting for Duke's stage was not possible due to missing data. For lung cancer, correcting using limited or extensive stages gave no difference (log rank = 3.70, df = 1, P = 0.054); correcting using TNM stage was not possible due to missing data. Correcting for stage in ovarian cancer was not possible due to missing data. For prostate cancer, correcting for T stage produced no difference (log rank = 0.03, df = 1, P = 0.864). Similar trends were apparent when correcting survival data for stage when comparing all urgent referrals (guideline and letter) with other routes (data not presented but available from authors).
Stage at diagnosis
Findings for stage of cancer at diagnosis are presented in Table 3 (Supplementary Table 1).
Table 3.
Stage of cancer at diagnosis by referral source.
| Diagnosed through other routes | |||||||
|---|---|---|---|---|---|---|---|
| Urgent guideline referrals | Total | Urgent GP letter | Non-urgent GP letter | Inter-specialty referral | A&E referral | Unknown | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Lung (TNM stage) | |||||||
| Stage IA | 1 (6) | 10 (14) | 1 (50) | 2 (33) | 7 (16) | 0 (0) | 0 (0) |
| Stage IB | 3 (17) | 15 (21) | 0 (0) | 1 (17) | 13 (29) | 0 (0) | 1 (6) |
| Stage IIB | 0 (0) | 16 (22) | 0 (0) | 1 (17) | 13 (29) | 0 (0) | 1 (6) |
| Stage IIIA | 5 (28) | 15 (21) | 1 (50) | 0 (0) | 8 (18) | 1 (50) | 5 (29) |
| Stage IIIB | 4 (22) | 3 (4) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 2 (12) |
| Stage IV | 5 (28) | 13 (18) | 0 (0) | 1 (17) | 3 (7) | 1 (50) | 8 (47) |
| Total | 18 | 72 | 2 | 6 | 45 | 2 | 17 |
| Colorectal (Duke's stage) | |||||||
| A | 4 (12) | 23 (18) | 2 (6) | 13 (32) | 4 (14) | 2 (15) | 2 (17) |
| B | 14 (42) | 48 (37) | 18 (50) | 11 (27) | 9 (32) | 4 (31) | 6 (50) |
| C | 15 (46) | 56 (43) | 16 (44) | 17 (42) | 13 (46) | 6 (46) | 4 (33) |
| D | 0 (0) | 3 (2) | 0 (0) | 0 (0) | 2 (7) | 1 (8) | 0 (0) |
| Total | 33 | 130 | 36 | 41 | 28 | 13 | 12 |
| Prostate (Gleason score) | |||||||
| Moderate (5–7) | 33 (94) | 77 (91) | 5 (63) | 54 (95) | 8 (89) | 7 (88) | 3 (100) |
| High (8–10) | 2 (6) | 8 (9) | 3 (37) | 3 (5) | 1 (11) | 1 (12) | 0 (0) |
| Total | 35 | 85 | 8 | 57 | 9 | 8 | 3 |
| Ovarian | |||||||
| I | 1 (13) | 7 (19) | 0 (0) | 4 (21) | 2 (20) | 0 (0) | 1 (14) |
| II | 1 (13) | 3 (8) | 0 (0) | 2 (11) | 1 (10) | 0 (0) | 0 (0) |
| III | 3 (38) | 21 (57) | 0 (0) | 9 (47) | 5 (50) | 1 (100) | 6 (86) |
| IV | 3 (38) | 6 (16) | 0 (0) | 4 (21) | 2 (20) | 0 (0) | 0 (0) |
| Total | 8 | 37 | 0 | 19 | 10 | 1 | 7 |
TNM = tumor, node, metastasis.
Lung cancer
There was a difference between urgent guideline referrals and patients diagnosed through other routes and TNM stage of cancer at diagnosis (χ2 = 11.97, df = 5, P = 0.035). Urgent guideline referrals had more advanced stage at diagnosis. For patients diagnosed through other routes, although there were smaller numbers in the subgroups, trends were seen between referral source. GP-referred patients were diagnosed at an earlier stage, A&E referrals at a later stage, and inter-specialty referrals spread between the stage categories. There was a difference (χ2 = 8.52, df = 1, P = 0.004) with regard to the extent of cancer. A higher proportion of urgent guideline referrals (85%) had extensive disease compared with patients diagnosed through other routes (54%). Comparison of all urgent referrals (guideline and letter) with other routes also showed differences for both TNM stage (χ2 = 11.79, df = 5, P = 0.038), and extensive versus limited stage (χ2 = 11.06, df = 1, P = 0.001).
Colorectal cancer
There was no difference between urgent guideline referrals and patients diagnosed through other routes and TNM stage (χ2 = 1.81, df = 4, P = 0.770) or Duke's stage (χ2 = 1.50, df = 3, P = 0.683). Comparison of all urgent referrals (guideline and letter) with other routes also showed a difference for Duke's stage (χ2 = 8.91, df = 3, P = 0.030), but not for TNM stage (χ2 = 3.67, df = 4, P = 0.453).
Prostate cancer
There was no difference between urgent guideline referrals and patients diagnosed through other routes and T stage (χ2 = 1.79, df = 3, P = 0.616) or Gleason score (χ2 = 0.44, df = 1, P = 0.505). Comparison of all urgent referrals (guideline and letter) with other routes also showed no difference for T stage (χ2 = 1.51, df = 3, P = 0.680), or Gleason score (χ2 = 0.84, df = 1, P = 0.361).
Ovarian cancer
There was no difference between urgent guideline referrals and patients diagnosed through other routes and stage (χ2 = 2.24, df = 3, P = 0.524). Comparison of all urgent referrals (guideline and letter) with other routes also showed no difference (χ2 = 2.24, df = 3, P = 0.510).
Delays in diagnosis
Referral delay was defined as being seen within 2 weeks (Table 4 [Supplementary Table 2]).
Table 4.
Referral and secondary care delays by referral source.
| Diagnosed through other routes | |||||||
|---|---|---|---|---|---|---|---|
| Urgent guideline referrals | Totala | Urgent letter | Non-urgent letter | Inter-specialty referral | A&E | Unknown | |
| Lung | |||||||
| Referral delays | |||||||
| Median delay in days, (IQR) | 10 6–13 | 10 4–17 | 9 3–18 | 12 4–28 | n/a | n/a | n/a |
| Seen within 2 weeks, % | 89 | 57 | 60 | 55 | n/a | n/a | n/a |
| Secondary care delays | |||||||
| Median delay in days, (IQR) | 18 8–36 | 15 4–28 | 14 4–25 | 19 14–35 | 14 4–28 | 28 4–42 | 14 8–23 |
| Colorectal | |||||||
| Referral delays | |||||||
| Median delay in days, (IQR) | 12 9–13 | 8 0–22 | 2 0–13 | 23 17–35 | n/a | n/a | n/a |
| Seen within 2 weeks, % | 86 | 49 | 81 | 14 | n/a | n/a | n/a |
| Secondary care delays | |||||||
| Median delay in days, (IQR) | 30 14–61 | 21 5–64 | 14 3–42 | 67 21–165 | 8 0–25 | 15 9–35 | 50 12–95 |
| Prostate | |||||||
| Referral delays | |||||||
| Median delay in days, (IQR) | 13 8–15 | 40 13–58 | 30 13–41 | 48 28–71 | n/a | n/a | n/a |
| Seen within 2 weeks, % | 68 | 16 | 25 | 15 | n/a | n/a | n/a |
| Secondary care delays | |||||||
| Median delay in days, (IQR) | 87 (61–140) | 103 (71–72) | 99 (51–121) | 118 (77–155) | 70 (11–146) | 116 (66–188) | n/a |
| Ovarian | |||||||
| Referral delays | |||||||
| Median delay in days, (IQR) | 12 8–15 | 23 11–40 | 14 3–21 | 31 21–57 | n/a | n/a | n/a |
| Seen within 2 weeks, % | 75 | 26 | 58 | 9 | n/a | n/a | n/a |
| Secondary care delays | |||||||
| Median delay in days, (IQR) | 31 4–60 | 45 20–82 | 47 17–76 | 69 25–107 | 37 15–49 | 18 16–18 | 26 14–42 |
Totals calculated from data available and therefore exclude the non-applicables (for example, secondary delay cannot be calculated from secondary care referrals). IQR = interquartile range.
Lung cancer
Eighty-nine per cent of urgent guideline referrals were seen within 2 weeks, compared with 57% of non-urgent GP referrals (χ2(1) = 0.99, P<0.001). For the non-urgent GP referrals, there was no difference between whether or not the GP letter was marked urgent (60%), or not (55%).
Colorectal cancer
Eighty-six per cent of urgent guideline referrals were seen within 2 weeks, compared with 49% of non-urgent GP referrals (χ2(1) df = 1.00, P<0.001). For the non-urgent GP referrals, there was a difference (χ2(1) = 1.00, P<0.001) between whether or not the GP letter was marked urgent (81%), or not (14%).
Prostate cancer
Sixty-eight per cent of urgent guideline referrals were seen within 2 weeks, compared with 16% for the non-urgent GP referrals. For the non-urgent GP referrals, there was no difference whether the GP letter was marked urgent (25%), or not (15%).
Ovarian cancer
Seventy-five per cent of urgent guideline referrals were seen within 2 weeks, compared with 26% of non-urgent GP referrals (χ2(1) = 0.99, P<0.001). For the non-urgent GP referrals, there was a difference (χ2(1) = 0.96, P = 0.001) between whether or not the GP letter was marked urgent (58%), or not (9%).
Analysis of data of all urgent referrals (guideline and letter) was not undertaken because the 2-week wait initiative is specific to guideline referrals.
Secondary care delay
Colorectal cancer
There was a difference (F(5) df = 8.32, P<0.001) in secondary care delay between referral source. For GP referrals, patients who were referred via a letter marked urgent had the shortest delay in diagnosis, followed by the urgent guideline referrals, and those referred by non-urgent letter. Interspeciality referrals had the shortest delay. There was considerable variation between these groups with the mean delay being five times longer for non-urgent GP letters compared with inter-specialty referrals. A difference was still found when all urgent referrals (guideline and letter) were compared with other routes.
Lung cancer
There was no difference in secondary care delay between referral source. Delays were similar for all the referral route categories. No difference was found when all urgent referrals (guideline and letter) were compared with other routes.
Prostate cancer
There was no difference in secondary care delay between referral source. Delays were similar for all the referral route categories. No difference was found when all urgent referrals (guideline and letter) were compared with other routes.
Ovarian cancer
There was a difference (F(5) = 2.94, P = 0.021) in secondary care delay between referral source. For GP referrals, urgent guideline referrals had the shortest delay, followed by urgent GP letter referrals, and those referred by non-urgent letter. Patients who arrived via A&E had the shortest delay. Delays were 2.5 times longer for non-urgent GP letters compared with inter-specialty referrals. No difference was found when all urgent referrals (guideline and letter) were compared with other routes.
DISCUSSION
Summary of main findings
This is the first comprehensive study to show some evidence for differences in outcomes for lung cancer between urgent guideline referrals (and all referrals marked as urgent) and those diagnosed through other routes. No difference was found for colorectal, prostate or ovarian cancer. While the aim of the urgent referral guidance is not necessarily to lead to earlier stage diagnosis or to lead to better survival, these findings are of importance, and have implications for further revisions of the guidance.
Comparison with existing literature
Weak evidence for differences in diagnostic stage was identified for lung cancer, and showed the apparent paradox that urgent guideline referrals had later stage diagnosis compared with patients diagnosed through other routes. Weak evidence was also found to suggest that more advanced stage colorectal cancer was found among all urgent referrals, whatever the mechanism (that is, guideline or urgent letter referrals), suggesting that GPs identify the more advanced cancers more easily. These findings, to a degree, were unexpected, but can be explained by the fact that aggressive tumours may lead to the rapid progression of symptoms leading to earlier presentation and fulfilment of urgent referral criteria; such patients are also likely to have disease that is less amenable to life prolonging treatments. This so-called ‘waiting-time’ paradox has been demonstrated in other cancers.10,11 The guidance therefore seems to be best at identifying patients with more advanced lung cancer with, possibly, least to gain in terms of survival benefit from earlier diagnosis. For patients diagnosed through other routes, GP referrals tended to be diagnosed at an earlier stage, and A&E referrals at a later stage. Differences in survival were only apparent for lung cancer, with patients diagnosed through urgent GP referrals having poorer survival; this can be explained by differences in diagnostic stage. The finding that for the three other cancers no difference in stage or survival was demonstrated is important. Lung cancer may be different from the other cancers because of its more poorly differentiated symptom presentation and extent of disease at diagnosis.12,13
With regard to delay, the majority of urgent GP referrals in this study were seen within the 2 weeks. Subsequent improvements in hospitals' performance have ensured that, nationally, this figure is now 99–100%. ‘Non-urgent’ GP referral letters marked ‘urgent’ were dealt with faster than those that were not, demonstrating that while many intended urgent referrals were not made on the designated fast-track forms, they were ‘triaged’ by secondary care and often managed as such. Secondary care delays were less for inter-specialty referrals, presumably because patients were in hospital already and avoided delays in outpatient diagnostics, and for urgent referrals because of pressures on hospitals to achieve diagnostic targets. However, in the overall cancer diagnostic journey, reductions in secondary care delays are likely to have minimal impact on clinical outcomes.11
For all cancers, there may be differences in psychological outcomes for patients, their families, and indeed their GPs between urgent and nonurgent referrals. While there is some evidence that waiting for a diagnosis is associated with increasing psychological distress,14 patients who are referred urgently also experience considerable distress while waiting to be seen.15
Strengths and limitations of the study
The main strengths of this study are its originality, its large sample of cancers diagnosed over a 2-year period, and its comprehensive approach to data collection. Limitations include the extent of missing data from missing notes and from absent entries in the notes; this was unavoidable, and there was no way of undertaking sensitivity analyses to try to determine the representativeness of the records used. However, the potential for systematic biases between the retrieved and missing records was minimal. The time duration between diagnosis and data collection may have resulted in a death bias, although significant efforts were made to extract data from the notes of the deceased wherever possible. The study analysed observational data collected retrospectively; it is difficult to reach firm conclusions as to the nature of the associations found as a result. Only a prospective, ideally experimental, design could have overcome this. However, this was impossible given the nonevidence based way in which the guidance was developed and implemented.4 A gold-standard randomised controlled trial would now be unethical.
Implications for future research and clinical practice
These findings will inform further revisions of the urgent cancer referral guidance, and begin to question the value of the guidance. While no adverse effect on diagnostic stage or survival has been shown for patients with colorectal, prostate or ovarian cancer not referred as an urgent referral, compliance with the guidance by GPs is strongly encouraged as these still represent the best evidence on recognition of suspicious symptoms currently available. The fact that, at present, this guidance is identifying more advanced lung cancer, highlights the need for a better evidence base of the clinical epidemiology of presenting symptoms. While this body of evidence is growing,16,17 there is still more work to be done. Even when this work is closer to completion, it is unclear whether the guidance will result in better clinical outcomes. Referring GPs should be aware that patients with lung cancer diagnosed through an urgent referral may have more advanced disease at diagnosis and poorer survival as a result. This work needs replicating in other centres with the revised version of the guidance,18 and for other cancers. Work is needed to determine the psychological effects of fast-track referral and guidance; optimal strategies for more widespread implementation of the guidance; and how consultations between patients with suspicious symptoms and their doctors are facilitated.
Supplementary Material
Acknowledgments
Thanks go to staff at the Bradford Hospitals NHS Trust medical records library, who helped us to locate the notes of patients in the study. We would also like to thank Kath Nuttall, Gillian Hollingsworth and Helena Berry from the Bradford Royal Infirmary, who helped set up the project in the early stages, for supplying the initial patient data, and feedback on the findings throughout the data collection. We are also grateful to all the members of the advisory group for their invaluable comments: Chris Bradley, Gillian Hollingsworth, Kath Nuttall, Andrew O'Shaughnessy, Joan Stone and John Sullivan.
Supplementary information
Additional information accompanies this paper at: http://www.rcgp.org.uk/bjgp-suppinfo
Funding body
The study was funded by Bradford PCTs (no specific reference number); the research team was independent of the funders
Ethics committee
Research ethics approval was received from Bradford Local Research Ethics Committee (03/02/297)
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Berrino F, Gatta G, Sant M, Capocaccia R. The EUROCARE study of survival of cancer patients in Europe: aims, current status, strengths and weaknesses. Eur J Cancer. 2001;37(6):673–677. doi: 10.1016/s0959-8049(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health. Referral guidelines for suspected cancer. London: Department of Health; 2000. [Google Scholar]
- 3.Department of Health. The NHS Cancer Plan. London: Department of Health; 2000. [Google Scholar]
- 4.Jones R, Rubin G, Hungin P. Is the two week rule for cancer referrals working? BMJ. 2001;322(7302):1555–1556. doi: 10.1136/bmj.322.7302.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allgar VL, Neal RD, Pascoe SW. Grading referrals to specialist breast units. BMJ. 2002;325(7360):392. [PMC free article] [PubMed] [Google Scholar]
- 6.Allgar VL, Neal RD, Ali N, et al. Urgent general practitioner referrals for suspected lung, colorectal, prostate and ovarian cancer. Br J Gen Pract. 2006;56(526):355–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Jiwa M, Hamilton W. Referral of suspected colorectal cancer: have guidelines made a difference? Br J Gen Pract. 2004;54(505):608–610. [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh S, Bruce C, Bennington S, Ravi S. The fourteen day rule and colorectal cancer. Ann R Coll Surg Engl. 2002;84(6):386–388. doi: 10.1308/003588402760978166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flashman K, O'Leary DP, Senapati A, Thompson MR. The Department of Health's ‘two week standard’ for bowel cancer: is it working? Gut. 2004;53(3):387–391. doi: 10.1136/gut.2003.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis NR, Le Jeune I, Baldwin DR. Under utilisation of the 2-week wait initiative for lung cancer by primary care and its effect on the urgent referral pathway. Br J Cancer. 2005;93(8):905–908. doi: 10.1038/sj.bjc.6602798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92(11):1959–1970. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sainsbury R, Johnston C, Haward R. Effect on survival of delays in referral of patients with breast cancer symptoms: a retrospective analysis. Lancet. 1999;353(9159):1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 13.Crawford SC, Davis JA, Siddiqui NA, et al. The waiting time paradox: population based retrospective study of treatment delay and survival; in women with endometrial cancer in Scotland. BMJ. 2002;325(7357):196. doi: 10.1136/bmj.325.7357.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risberg T, Sorbye SW, Norum J, Wist EA. Diagnostic delay causes more psychological distress in female than male cancer patients. Anticancer Res. 1996;16(2):995–999. [PubMed] [Google Scholar]
- 15.Cornford CS, Harley J, Oswald N. The ‘2-week rule’ for suspected breast carcinoma: a qualitative study of the views of patients and professionals. Br J Gen Pract. 2004;54(505):584–588. [PMC free article] [PubMed] [Google Scholar]
- 16.Cornford CS, Harley J, Oswald N. The ‘2-week rule’ for suspected breast carcinoma: a qualitative study of the views of patients and professionals. Br J Gen Pract. 2004;54(505):584–588. [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton W, Peters T, Sharp D, Round A. What are the clinical features of lung cancer before the diagnosis is made? A population-based case-control study. Thorax. 2005;60(12):1059–1065. doi: 10.1136/thx.2005.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NICE. Referral guidelines for suspected cancer. London: NICE; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.