Abstract
Primary spermatocytes are the male germ cells before meiosis I. To examine whether these 4n diploid cells are genetically competent to fertilize oocytes and support full embryo development, we introduced the nuclei of pachytene/diplotene spermatocytes into oocytes that were arrested in prophase I (germinal vesicle stage), metaphase I, or metaphase II (Met II). Both the paternal and maternal chromosomes then were allowed to undergo meiosis synchronously until Met II. In the first and second groups, the paternal and maternal chromosomes had intermingled to form a large Met II plate, which was then transferred into a fresh enucleated Met II oocyte. In the third group, the paternal Met II chromosomes were obtained by transferring spermatocyte nuclei into Met II oocytes twice. After activation of the Met II oocytes that were produced, those microfertilized at metaphase I showed the best developmental ability in vitro, and three of these embryos developed into full-term offspring after embryo transfer. Two pups (one male and one female) were proven to be fertile. This finding provides direct evidence that the nuclei of male germ cells acquire the ability to fertilize oocytes before the first meiotic division.
The recent advent of microfertilization techniques has enabled us to use immature male germ cells (spermatogenic cells) as substitute gametes. Normal offspring have been born after microfertilization with round spermatids in the mouse (1, 2), rabbit (3), and human (4), and with secondary spermatocytes in the mouse (5). Genetically, the fertilization of mature oocytes with spermatids or secondary spermatocytes is understandable, because both cell types are in the haploid state like mature spermatozoa (spermatids are 1n haploid and secondary spermatocytes are 2n haploid). A much more interesting question is whether the nucleus of primary spermatocytes, 4n diploid premeiotic spermatogenic cells, can participate in normal fertilization and support full embryonic development. In a previous study, we demonstrated that the chromosomes of primary spermatocytes undergo two meiotic divisions after incorporation into maturing oocytes shortly before or after germinal vesicle breakdown (6). Although some embryos developed to the blastocyst stage, none implanted after embryo transfer. This may be because of asynchrony of the spermatocyte and oocyte chromosomes, because the oocytes entered anaphase I before the spermatocyte chromosomes had fully condensed to form the metaphase configuration. Microfertilization with spermatogenic cells is a kind of nuclear transfer, so the cell-cycle stages of donor spermatogenic cells and recipient oocytes should be synchronized precisely. Because primary spermatocytes are in the G2 cell-cycle phase before the first meiotic division, the nuclei of primary spermatocytes should be introduced into the oocytes in either the G2 or M phase. In this study, the nuclei of primary spermatocytes were transferred into germinal vesicle (GV, G2 phase), prometaphase I to metaphase I (Met I), or metaphase II (Met II) oocytes. The oocytes were kept arrested in each stage for at least 2 hr after transfer of the spermatocyte nuclei to achieve full synchronization between the spermatocyte and oocyte chromosomes.
MATERIALS AND METHODS
Animals.
Female and male hybrid F1 (C57BL/6 × DBA/2 and C57BL/6 × C3H) mice, 2–4 months old, were used in this study. They were kept in an air-conditioned room (23°C, 50% relative humidity) under 14-hr light/10-hr dark light cycles.
Collection of Primary Spermatocytes.
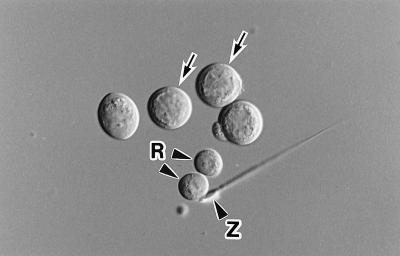
Spermatogenic cells were collected from the testes by a mechanical method reported previously (7) and suspended in Dulbecco’s PBS. Cells were treated with 0.1–0.2 mg/ml pronase E for 5 min at 30°C to increase the fusion efficiency (7). The primary spermatocytes used in this study were in the pachytene to diplotene stages of the meiotic prophase. They were the largest of spermatogenic cells (18–20 μm in diameter) and had a distinct nuclear membrane; i.e., they had not yet entered the first meiotic division (Fig. 1).
Figure 1.
Isolated mouse primary spermatocytes (arrows). Only those with a distinct nuclear membrane were used in this study. R, round spermatid; Z, spermatozoon.
Collection of Oocytes.
Fully grown (GV) oocytes were obtained from the large antral follicles of ovaries from females injected 48 hr previously with 7.5 units of pregnant mare serum gonadotropin (PMSG). GV oocytes were placed in MEM-α (GIBCO catalog no. 12000) supplemented with 0.4 mg BSA (fraction V, Sigma). Mature oocytes at Met II were collected from the oviducts of females superovulated with PMSG and human chorionic gonadotropin (hCG) (7.5 units of each given 48–52 hr apart). They were placed in CZB medium (8) and freed of cumulus cells with hyaluronidase.
Microfertilization with Primary Spermatocytes.
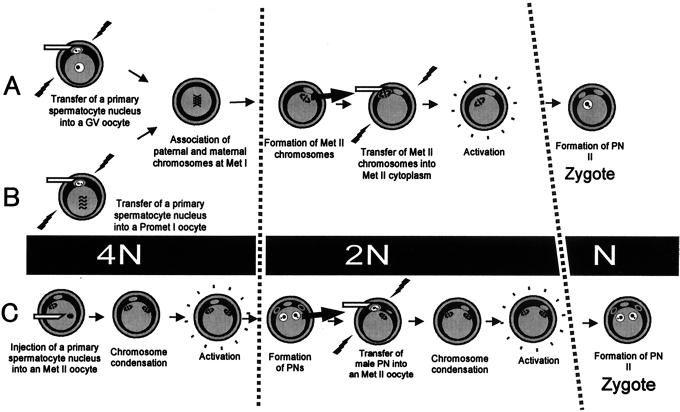
Microfertilization was undertaken by either electrofusion (6) or intracytoplasmic injection (5) (Fig. 2). When GV oocytes were used for microfertilization (Method A in Fig. 2), they were transferred to MEM-α containing 100 μM dibutyryl cAMP (dbcAMP) and freed of cumulus cells by repeated pipetting. Spermatocyte nuclei were introduced into the oocytes by electrofusion as reported previously (6), except that only oocytes arrested at the GV stage were used. By applying an electric pulse (3,750–4,000 V/cm, 20 μsec) with pre- and postpulse AC in fusion medium (300 mM mannitol, 0.1 mM MgSO4, and 0.5 mg/ml polyvinyl alcohol), 20–40% of the oocyte–spermatocyte pairs fuse, depending on the experiment (6). Oocytes that fused with spermatocytes were further cultured in MEM-α containing dbcAMP for 2 hr. After washing in fresh MEM-α medium, oocytes were cultured for 14–17 hr, until they entered the Met II stage. When Met I oocytes were used for microfertilization (Method B), GV oocytes collected from the ovaries were cultured in MEM-α medium for 4 hr (until prometaphase I). After removing the cumulus cells by pipetting, the oocytes were placed into MEM-α containing 7.5 μM cytochalasin B (CCB). After electrofusion with primary spermatocytes as described above, the oocytes were cultured in CCB-containing medium for 2 hr to arrest them in Met I (9). Then they were washed and cultured in MEM-α for 12–16 hr until they reached the Met II stage. To achieve better embryo development, Met II chromosomes (karyoplasts) from the oocytes in Methods A and B were fused with enucleated fresh Met II oocytes by an electric pulse (6). Met II karyoplasts were inserted into the perivitelline space of enucleated Met II oocytes with a piezo-driven micromanipulator (2). The oocytes were then activated by CZB medium containing Sr2+ (10) and cultured in fresh CZB medium. In Method C, microinjection of primary spermatocytes into Met II oocytes and production of pronuclei/polar bodies was accomplished by using the method reported previously for secondary spermatocytes (5). The spermatocyte-derived pronucleus and polar body were each fused with an intact Met II oocyte in a fashion similar to the Met II karyoplast transfer. After premature condensation of the spermatocyte-derived pronuclei and polar bodies, the oocytes were activated and cultured in CZB medium as in Methods A and B.
Figure 2.
Methods of producing diploid embryos by using primary spermatocytes as male gametes. “N” indicates the number of chromatid sets from primary spermatocytes. The cell-cycle stages of primary spermatocytes and oocytes were synchronized at the GV (Method A), Met I (Method B), or Met II (Method C) stages. Spermatocyte chromosomes (4N) undergo two meiotic divisions in oocytes and participate in the formation of a haploid (N) male pronucleus. PN, pronucleus.
Embryo Transfer.
Embryos reaching the morulae/blastocyst stage 96 hr after activation were transferred into the uteri of day 3 pseudopregnant ICR females (day 1 was the day after mating with vasectomized males). Forty-eight hours after activation, 4- to 8-cell embryos were transferred into the oviducts of day 1 pseudopregnant females. The uteri of pseudopregnant females were examined for fetuses and implantation sites on day 19 or 20. Live pups were raised by lactating foster mothers.
Chromosomal Analysis.
Meiosis I is distinct from mitosis and meiosis II in that sister chromatids remain associated with each other. To see whether the spermatocyte chromosomes undergo meiosis I within the oocytes, fertilized Met II oocytes from each method group were examined cytogenetically by using a previously described method (6). The chromosomes of oocytes prepared by Methods B and C were removed before microfertilization, so that only the spermatocyte chromosomes were observed. In Method A, the oocyte chromosomes were left intact, because enucleation of GV oocytes often causes maturational arrest at anaphase I.
Statistical Analysis.
The data were analyzed by using Fisher’s exact probability test.
RESULTS
The primary spermatocyte nuclei introduced into GV oocytes (Method A) showed no changes in their chromatin structure as long as the oocytes stayed in the GV stage (Fig. 3). In contrast, those introduced into prometaphase I oocytes (Method B) started to condense soon after electrofusion, and condensation was completed within 1 hr (Fig. 4). In Methods A and B, the paternal and maternal chromosomes intermingled to form a large Met II plate during oocyte maturation (Fig. 4). About 80% of the oocytes reached the Met II stage. The majority of the remaining oocytes were arrested in anaphase I. In Method C, about 60% of the oocytes survived the injection procedure (Fig. 5). The majority (>75%) of the injected oocytes formed two pronuclei and two polar bodies after oocyte activation (Fig. 5). Most of the remaining oocytes had three pronuclei and one polar body. The larger pronucleus and polar body were presumably derived from spermatocyte chromosomes. Most (>95%) of the presumptive spermatocyte-derived pronuclei and polar bodies were successfully transferred into Met II oocytes by electrofusion (Fig. 5).
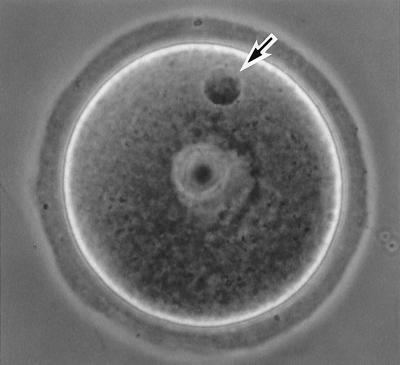
Figure 3.
A GV-arrested oocyte fused with a primary spermatocyte (Method A) 4 hr after electrofusion. The spermatocyte nucleus shows its original chromatin configuration (arrow).
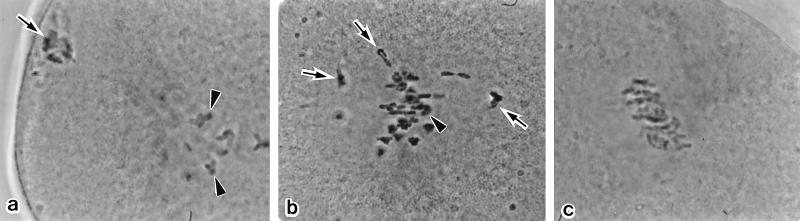
Figure 4.
The configuration of primary spermatocyte chromosomes in maturing oocytes (Method B). (a) Thirty minutes after electrofusion. The spermatocyte chromosomes have started to condense (arrow). The oocyte chromosomes are at prometaphase I (arrowheads). (b) One hour after electrofusion. The fully condensed spermatocyte chromosomes (arrows) are mingling with the oocyte chromosomes, which already have formed the Met I configuration (arrowheads, slightly out of focus). (c) Two hours after electrofusion. Both the spermatocyte and oocyte chromosomes are aligned on the Met I spindle of the oocyte, which has been arrested by cytochalasin treatment. Thus, the spermatocyte and oocyte chromosomes are successfully synchronized.
Figure 5.
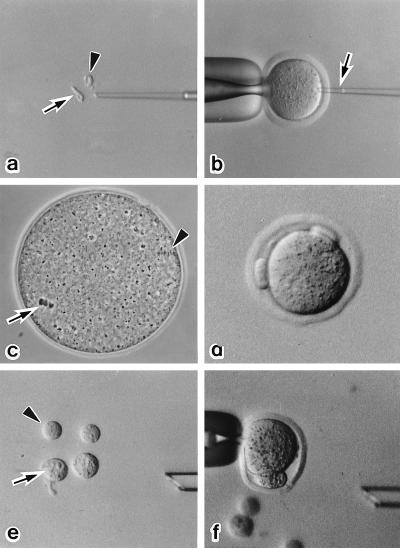
Serial nuclear transfer of a spermatocyte nucleus (Method C). (a) The nucleus (arrow) and cytoplasm (arrowhead) of primary spermatocytes are isolated after drawing in and out of the injection pipette. (b) The nucleus of a primary spermatocyte is injected into a Met II oocyte (arrow). (c) The spermatocyte chromosomes form a metaphase plate (arrow) within 2 hr. The arrowhead indicates the Met II chromosomes of the oocyte. (d) After oocyte activation, two polar bodies are extruded. (e) The larger pronucleus (arrow) and polar body (arrowhead) are used for the second nuclear transfer. (f) A karyoplast, pronucleus or polar body, is inserted into the perivitelline space of another Met II oocytes, and a fusion pulse is applied.
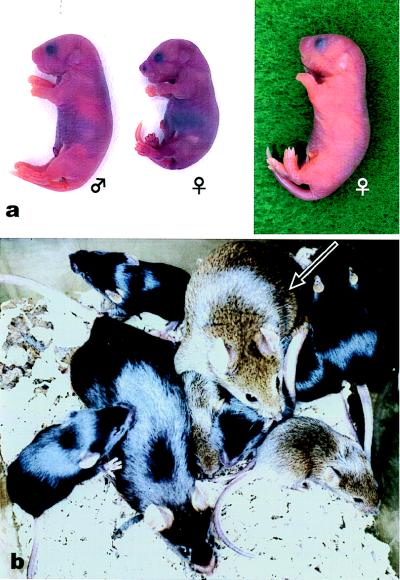
At least some of the activated Met II oocytes containing spermatocyte chromosomes developed to the morula/blastocyst stage (Table 1). Early postimplantation development was achieved in Methods A and B, although none of the oocytes developed to term after uterine transfer. Because embryos in Method B showed the best developmental ability both in vitro and in vivo, we transferred these 4- to 8-cell embryos (day 3) into the oviducts of day 1 recipients. Embryos retarded by in vitro manipulation are known to recover their viability after a few days of delayed implantation (11). Most embryos (98%, 90/92) developed to the 4- to 8-cell stage after culture for 48 hr. The implantation rate after uterine transfer was improved from 9 to 39%, and all the recipient females became pregnant (Table 2). Two recipients gave birth to a total of three pups, one male and two females, with the respective body weight of 1.4, 1.0, and 2.0 g (Table 2). All had black eyes and a pigmented coat (Fig. 6a). Their mothers were hybrid (C57BL/6 × C3H/HeJ) F1 and their fathers were hybrid (C57BL/6 × DBA/2) F1 with the respective genotypes AaBBCC and aaBbCC. Therefore, the phenotype of their pups should be ABC (agouti) or aBC (black). The recipient ICR females (albino, AABBcc) had never been exposed to pigmented males, so the offspring must have been derived from the oocytes fused with primary spermatocytes. The smaller female pup died soon after birth, although no gross abnormality was found. The other pups grew normally and were proven to be fertile (Fig. 6b).
Table 1.
Development of embryos after microfertilization with primary spermatocytes
| Method | Stage of oocytes | No. of embryos cultured | No. (%) of embryos that developed to
|
||||
|---|---|---|---|---|---|---|---|
| 1 cell | 2 cell | 4/8 cell | Morula/blastocyst | Postimplantation* | |||
| A | GV | 103 | 4 (4) | 19 (18) | 39 (38) | 41 (40)† | 1/41 (2) |
| B | Met I | 122 | 2 (2) | 1 (1) | 32 (26) | 87 (71)‡ | 8/87 (9)† |
| C | Met II | 247 | 45 (18) | 34 (14) | 98 (40) | 70 (28)† | 0/70 (0)‡ |
Embryos were cultured for 96 hr after artificial activation.
Morulae and blastocysts were transferred into pseudopregnant females.
Values within the same columns with different superscripts differ (P < 0.05).
Table 2.
Development after oviductal transfer of oocytes fertilized with primary spermatocytes (Method B)
| No. of embryos transferred | No. (%) of embryos implanted | No. (%) of term offspring | Day of examination* |
|---|---|---|---|
| 15 | 4 (27) | 0 (0) | 19 |
| 15 | 5 (33) | — | 11 |
| 22 | 8 (36) | 2 (9) | 19 |
| 23 | 3 (13) | 0 (0) | 20 |
| 15 | 9 (60) | 1 (7) | 20 |
| Total 90 | 29 (39) | 3/75† (4) |
Day of recipients’ pregnancy.
Exclusive of the second recipient, which was examined at mid-gestation.
Figure 6.
Pups born after microfertilization with primary spermatocytes. (a) Just after birth. The smallest pup (female, Center) died soon after birth, although no gross abnormality was found. (b) The male pup grew normally and developed into a fertile adult (arrow).
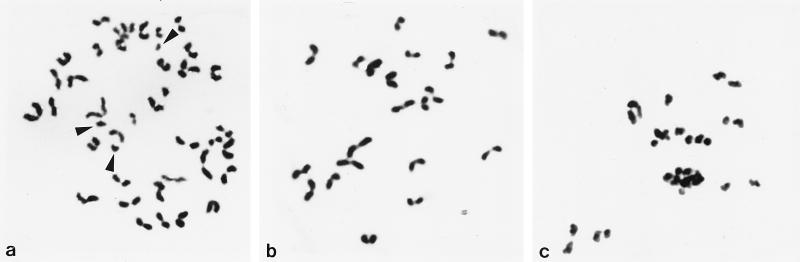
Chromosome analyses of Met II oocytes revealed partial premature separation of sister chromatids during meiosis I in all the oocytes from Methods A (10 oocytes) and C (12 oocytes), and in 8 of 15 oocytes from Method B (Fig. 7).
Figure 7.
Chromosome preparation made at Met II. (a) Method A. Some chromatids that separated prematurely show single “I” shapes (arrowheads) whereas normal sister chromatid pairs show characteristic “V” shapes. Oocyte chromosomes are also included in the preparation. (b) Method B. Normal Met II chromosomes (n = 20) derived from a primary spermatocyte. (c) Method C. The spermatocyte chromosomes are very damaged with premature chromatid separation.
DISCUSSION
This study demonstrated that mouse male germ cells become genetically competent to fertilize oocytes before they enter meiosis I. At least a part of this genetic competence to fertilize oocytes is brought about by primary genomic imprinting (gametic imprinting). It marks imprinted alleles so that parental distinction is possible and normal embryo development is ensured. Although the molecular mechanism regulating gametic imprinting is not yet fully understood, heritable epigenetic modifications such as DNA methylation are thought to occur at imprinted loci. In oocytes, investigations of the DNA methylation status of individual imprinted genes have revealed that the specific DNA methylation pattern is established during oogenesis at early prophase I (for a review, see ref. 12). In contrast, in male germ cells changes in the methylation pattern during gametogenesis have not been detected because of the difficulty in isolating specific spermatogenic cell types. The present study provides direct evidence that gametic imprinting in male germ cells is completed before the end of prophase I, as occurs in the oocytes.
Because the pachytene/diplotene spermatocytes we used were in the mid- to late prophase of meiosis I, one may wonder whether earlier prophase I spermatocytes, namely, the leptotene and zygotene spermatocytes, can also be used for microfertilization. However, the chromosomes of leptotene/zygotene spermatocytes are not ready to enter Met I, as demonstrated after treatment with the phosphatase inhibitor okadaic acid (13). This is probably because the synapses between homologous chromosomes in leptotene/zygotene spermatocytes are immature (13). Thus, the pachytene/diplotene spermatocytes used in this study likely will be the youngest spermatogenic cells that can be used for microfertilization to produce normal diploid embryos.
Very recently, the birth of two mouse pups after microfertilization with primary spermatocytes was reported (14). In that study, diploid embryos were constructed by injecting spermatocyte nuclei into Met II oocytes twice, as in Method C in this study. However, one offspring died shortly after birth and the other at 3 weeks. The birth rate was 0.7% (2/258). The authors attributed the poor embryonic and neonatal development to some technical problems. We suppose that, at least in part, the reason is related to the fact that sister chromatid pairs of primary spermatocytes segregate prematurely within oocytes undergoing meiosis II. In this study, microfertilization at Met I (Method B) produced two pups that grew normally. Unfortunately, a third pup, a female, died soon after birth. This pup might have been saved if the recipient had been examined at day 20, as in the second delivery. We found that the primary spermatocyte chromosomes normally segregated only when they were introduced into Met I oocytes, and that embryos thus obtained developed to the morula/blastocyst stage more efficiently than embryos obtained by other methods. Taken together, it is likely that Met I-arrested oocytes are the best recipients for the nuclei of primary spermatocytes, at least in the mouse. However, the birth rate (4%, 3/75) after embryo transfer is still low when compared with that after spermatid injection, which ranges between 10 and 30% (2, 15). Further studies will be needed to know whether the poor full-term development is caused by some technical problems or by a limited spermatocyte population that has acquired the ability to fertilize oocytes.
Spermatogenic arrest is one of the major causes of male-factor infertility. Spermatogenesis may arrest at any stage, but most frequently arrests in the primary spermatocyte stage (16). A cytogenetic investigation of testicular biopsies from infertile men revealed that about 50% of the patients with spermatogenic arrest at the primary spermatocyte level showed normal synaptonemal complexes and bivalent chromosomes (17). Because there are no effective methods for culturing spermatogenic cells in vitro, our microfertilization technique using primary spermatocytes and maturing oocytes may provide the last opportunity for treating most infertile patients with spermatogenic arrest. However, we first must determine how safe this technique is for immediate and successive generations with an adequate number of animal experiments.
Acknowledgments
This study was supported by grants from the Ministry of Education, Science, Culture, and Sports of Japan, the Ministry of Health and Welfare of Japan, and the Japan Health Sciences Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: Met, metaphase.
References
- 1.Ogura A, Matsuda J, Yanagimachi R. Proc Natl Acad Sci USA. 1994;91:7460–7462. doi: 10.1073/pnas.91.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura Y, Yanagimachi R. Development. 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- 3.Sofikitis N V, Miyagawa I, Agapitos E, Pasyianos P, Toda T, Hellstrom W J G, Kawamura H. J Assist Reprod Genet. 1994;11:335–341. doi: 10.1007/BF02214138. [DOI] [PubMed] [Google Scholar]
- 4.Tesarik J, Mendoza C, Testart J. N Engl J Med. 1995;333:525. doi: 10.1056/NEJM199508243330819. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, Yanagimachi R. Biol Reprod. 1995;53:855–862. doi: 10.1095/biolreprod53.4.855. [DOI] [PubMed] [Google Scholar]
- 6.Ogura A, Wakayama T, Suzuki O, Shin T-Y, Matsuda J, Kobayashi Y. Zygote. 1997;5:177–182. doi: 10.1017/s096719940000383x. [DOI] [PubMed] [Google Scholar]
- 7.Ogura A, Yanagimachi R, Usui N. Zygote. 1993;1:1–8. doi: 10.1017/s0967199400001234. [DOI] [PubMed] [Google Scholar]
- 8.Chatot C L, Ziomek C A, Bavister B D, Lewis J L, Torres I. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 9.Wassarman P M, Josefowicz W J, Letourneau G E. J Cell Sci. 1976;22:531–545. doi: 10.1242/jcs.22.3.531. [DOI] [PubMed] [Google Scholar]
- 10.Bos-Mikich A, Swann K, Whittingham D G. Mol Reprod Dev. 1995;41:84–90. doi: 10.1002/mrd.1080410113. [DOI] [PubMed] [Google Scholar]
- 11.Evsikov S V, Solomko A P. J Exp Zool. 1996;276:201–208. doi: 10.1002/(SICI)1097-010X(19961015)276:3<201::AID-JEZ4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Latham K E, McGrath J, Solter D. Int Rev Cytol. 1995;160:53–98. doi: 10.1016/s0074-7696(08)61553-3. [DOI] [PubMed] [Google Scholar]
- 13.Handel M A. Theriogenology. 1998;49:423–430. doi: 10.1016/s0093-691x(97)00414-7. [DOI] [PubMed] [Google Scholar]
- 14.Sasagawa I, Kuretake S, Eppig J J, Yanagimachi R. Biol Reprod. 1998;58:248–254. doi: 10.1095/biolreprod58.1.248. [DOI] [PubMed] [Google Scholar]
- 15.Ogura A, Yamamoto Y, Suzuki O, Takano K, Wakayama T, Mochida K, Kimura H. Theriogenology. 1996;45:1141–1149. doi: 10.1016/0093-691x(96)00070-2. [DOI] [PubMed] [Google Scholar]
- 16.Martin-du Pan R C, Campana A. Fertil Steril. 1993;60:937–946. doi: 10.1016/s0015-0282(16)56388-2. [DOI] [PubMed] [Google Scholar]
- 17.Lange R, Krause W, Engel W. Hum Reprod. 1997;12:2154–2158. doi: 10.1093/humrep/12.10.2154. [DOI] [PubMed] [Google Scholar]