Abstract
The extremely halophilic archaebacterium Haloferax mediterranei produces an exocellular polymeric substance that gives the colonies a typical mucous character and is responsible for the appearance of a superficial layer in unshaken liquid medium. This exocellular polymeric substance can be obtained from the supernatant of shaken liquid cultures by cold ethanol precipitation, and yields as high as 3 mg/ml have been detected. The substance was produced under all the conditions tested and with all substrates assayed, although higher yields were obtained with sugars, particularly glucose, as carbon and energy source. The total exocellular polymeric substance produced was proportional to the total biomass. The polymer is a heteropolysaccharide containing mannose as the major component. Glucose, galactose, and another unidentified sugar were also present, as well as amino sugars, uronic acids, and a considerable amount of sulfate, which accounts for the acidic nature of the polymer. The infrared spectrum and specific assays showed the absence of acyl groups. The rheological properties of polymer solutions were studied, showing a pseudoplastic behavior and a high apparent viscosity at relatively low concentrations. Viscosity was remarkably resistant to extremes of pH, temperature, or salinity. These characteristics make this polymer interesting for enhanced oil recovery and other applications for which a very resistant thickening agent is required.
Full text
PDF


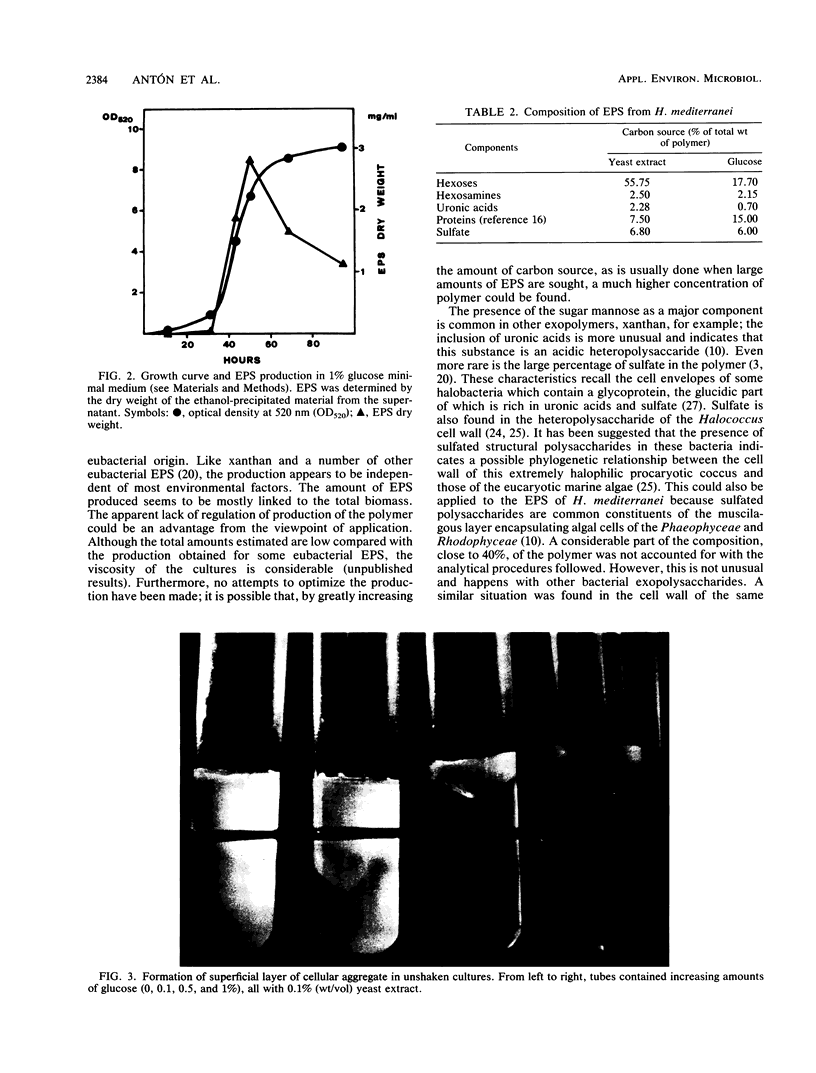
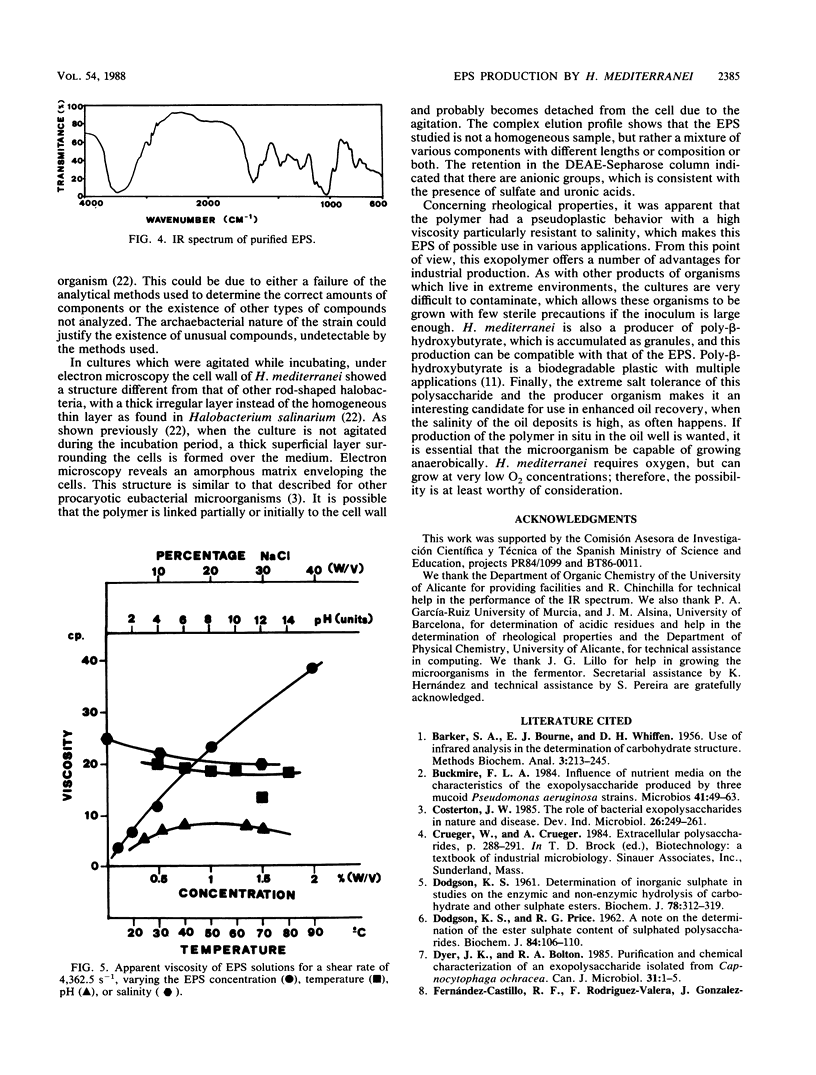

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER S. A., BOURNE E. J., WHIFFEN D. H. Use of infrared analysis in the determination of carbohydrate structure. Methods Biochem Anal. 1956;3:213–245. doi: 10.1002/9780470110195.ch7. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L. Influence of nutrient media on the characteristics of the exopolysaccharide produced by three mucoid Pseudomonas aeruginosa strains. Microbios. 1984;41(163):49–63. [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer J. K., Bolton R. W. Purification and chemical characterization of an exopolysaccharide isolated from Capnocytophaga ochracea. Can J Microbiol. 1985 Jan;31(1):1–5. doi: 10.1139/m85-001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Castillo R., Rodriguez-Valera F., Gonzalez-Ramos J., Ruiz-Berraquero F. Accumulation of Poly (beta-Hydroxybutyrate) by Halobacteria. Appl Environ Microbiol. 1986 Jan;51(1):214–216. doi: 10.1128/aem.51.1.214-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Improved method of hexosamine determination. Anal Biochem. 1971 Dec;44(2):628–635. doi: 10.1016/0003-2697(71)90252-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pfiffner S. M., McInerney M. J., Jenneman G. E., Knapp R. M. Isolation of halotolerant, thermotolerant, facultative polymer-producing bacteria and characterization of the exopolymer. Appl Environ Microbiol. 1986 Jun;51(6):1224–1229. doi: 10.1128/aem.51.6.1224-1229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber J., Schleifer K. H. Halococcus morrhuae: a sulfated heteropolysaccharide as the structural component of the bacterial cell wall. Arch Microbiol. 1975 Oct 27;105(2):173–177. doi: 10.1007/BF00447133. [DOI] [PubMed] [Google Scholar]
- Wieland F., Dompert W., Bernhardt G., Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980 Oct 20;120(1):110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]