Abstract
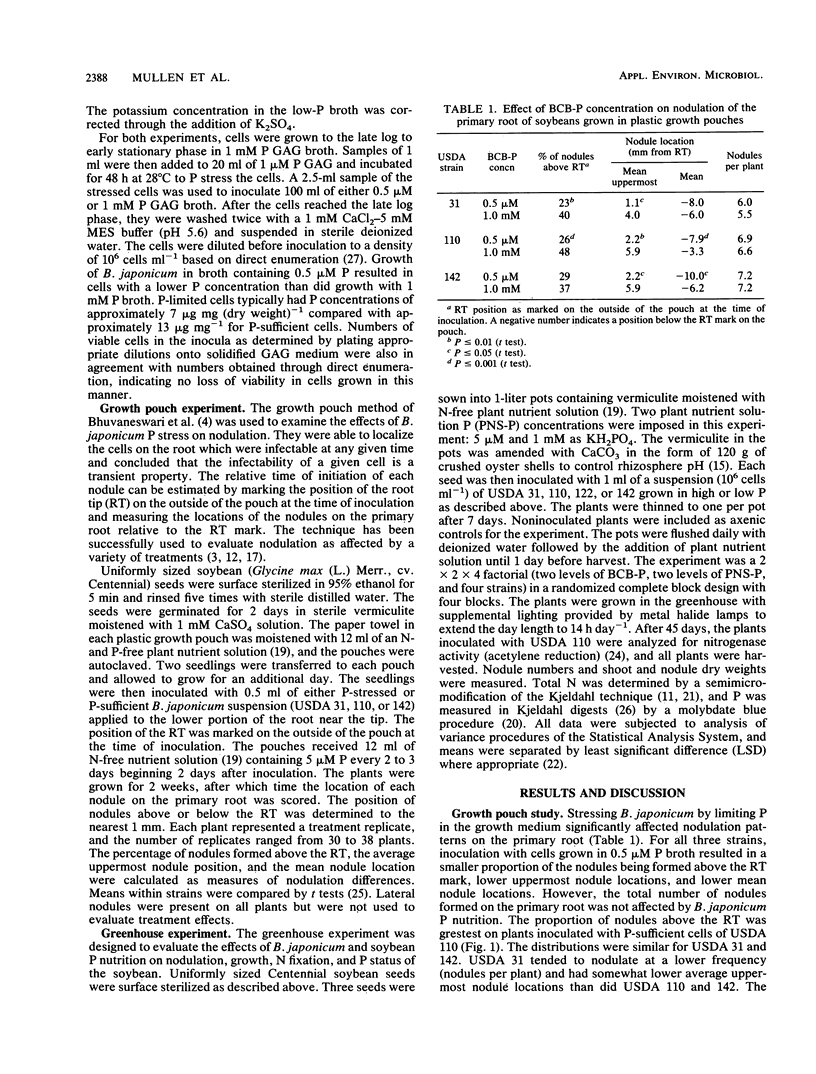
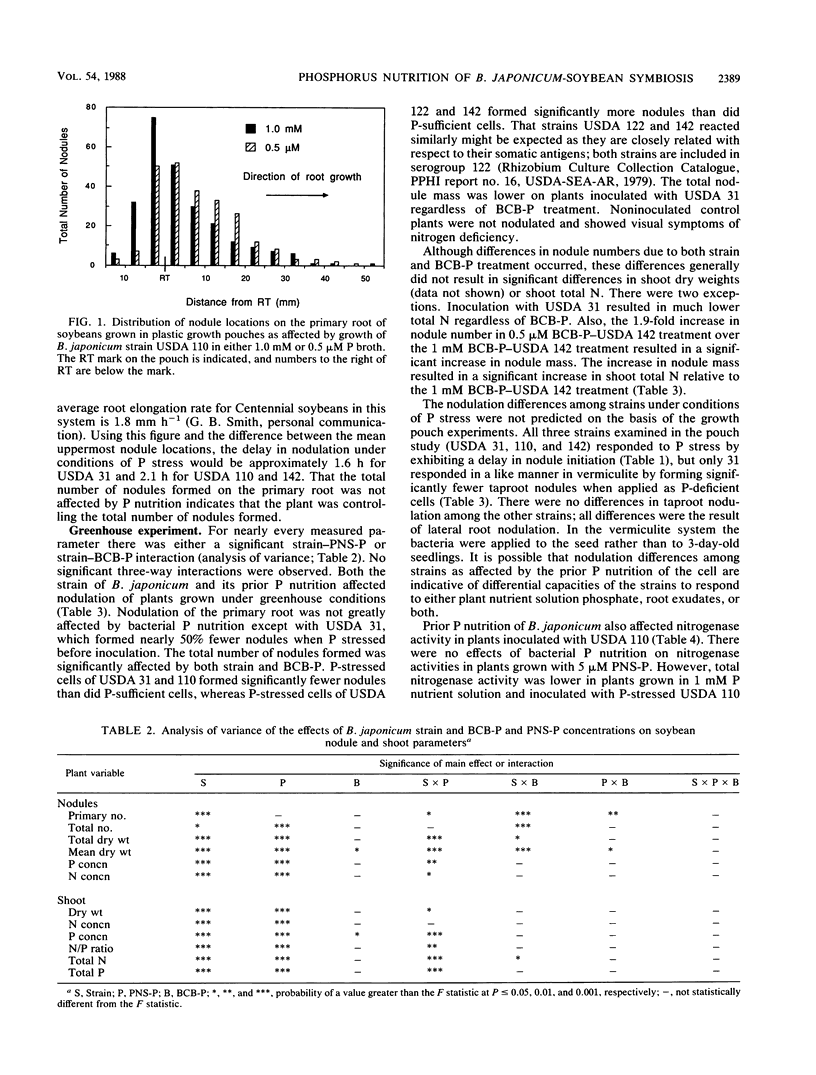
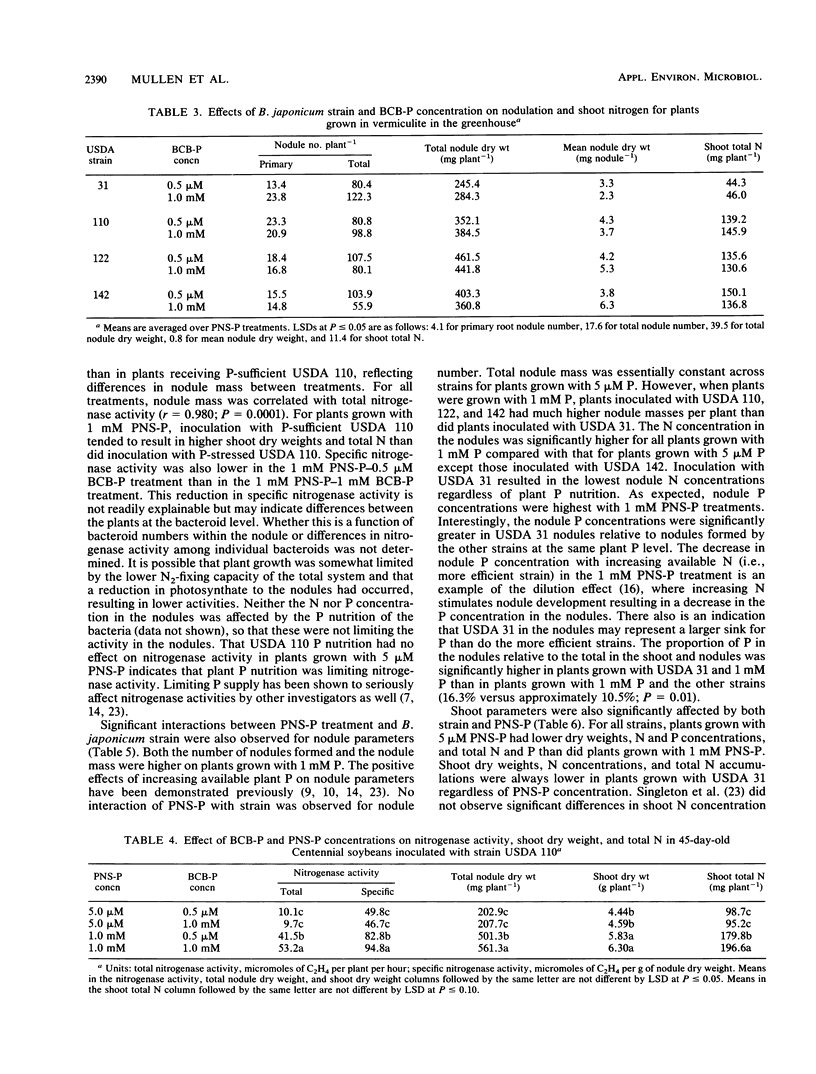
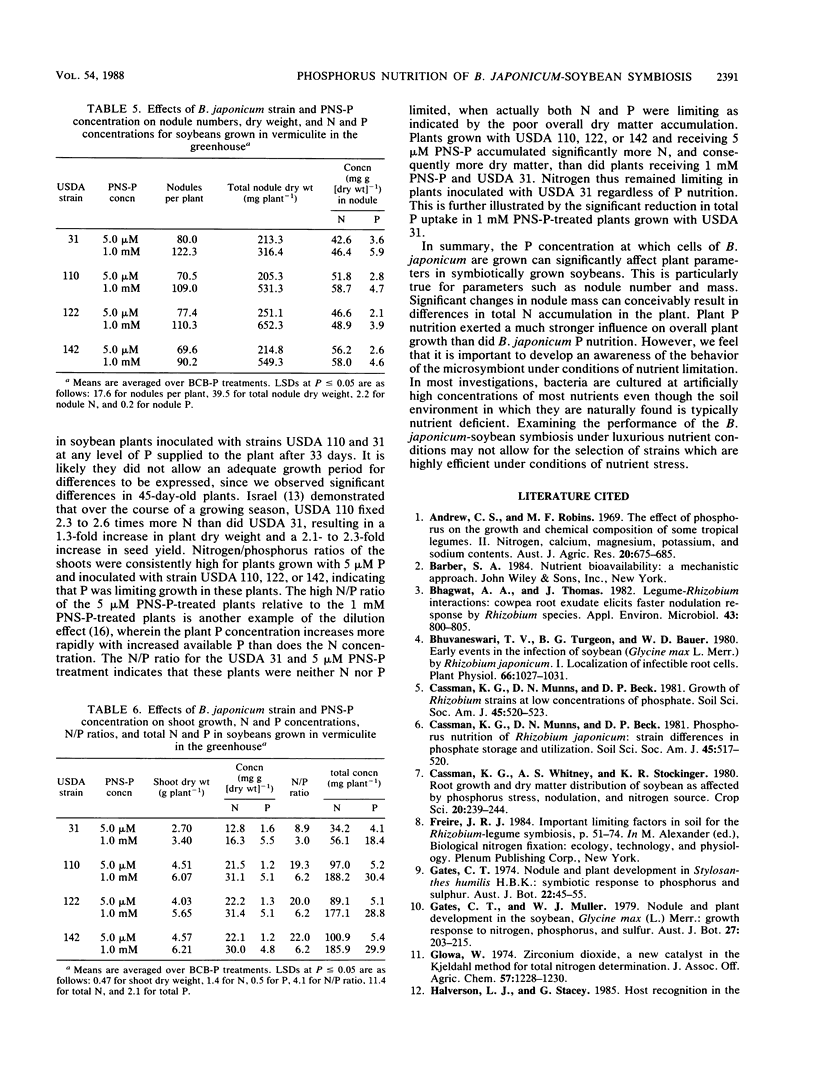
Cells of Bradyrhizobium japonicum were grown in media containing either 1.0 mM or 0.5 μM phosphorus. In growth pouch experiments, infection of the primary root of soybean (Glycine max (L.) Merr.) by B. japonicum USDA 31, 110, and 142 was significantly delayed when P-limited cells were applied to the root. In a greenhouse experiment, B. japonicum USDA 31, 110, 122, and 142 grown with sufficient and limiting P were used to inoculate soybeans which were grown with either 5 μM or 1 mM P nutrient solution. P-limited cells of USDA 31 and 110 formed significantly fewer nodules than did P-sufficient cells, but P-limited cells of USDA 122 and 142 formed more nodules than P-sufficient cells. The increase in nodule number by P-limited cells of USDA 142 resulted in significant increases in both nodule mass and shoot total N. In plants grown with 1 mM P, inoculation with P-limited cells of USDA 110 resulted in lower total and specific nitrogenase activities than did inoculation with P-sufficient cells. Nodule numbers, shoot dry weights, and total N and P were all higher in plants grown with 1 mM P, and plants inoculated with USDA 31 grew poorly relative to plants receiving strains USDA 110, 122, and 142. Although the effects of soybean P nutrition were more obvious than those of B. japonicum P nutrition, we feel that it is important to develop an awareness of the behavior of the bacterial symbiont under conditions of nutrient limitation similar to those found in many soils.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. W. Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol. 1987 Jul;84(3):835–840. doi: 10.1104/pp.84.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. W., Jackson W. A. Ion balance, uptake, and transport processes in n(2)-fixing and nitrate- and urea-dependent soybean plants. Plant Physiol. 1982 Jan;69(1):171–178. doi: 10.1104/pp.69.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F., Quehenberger P., Greger R., Silbernagl S., Stockinger P. Evidence for a bicarbonate leak in the proximal tubule of the rat kidney. Pflugers Arch. 1980 Aug;386(3):239–244. doi: 10.1007/BF00587474. [DOI] [PubMed] [Google Scholar]
- Mathis J. N., Israel D. W., Barbour W. M., Jarvis B. D., Elkan G. H. Analysis of the Symbiotic Performance of Bradyrhizobium japonicum USDA 110 and Its Derivative I-110 and Discovery of a New Mannitol-Utilizing, Nitrogen-Fixing USDA 110 Derivative. Appl Environ Microbiol. 1986 Jul;52(1):75–80. doi: 10.1128/aem.52.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloger C. Symbiotic effectiveness and n(2) fixation in nodulated soybean. Plant Physiol. 1969 Dec;44(12):1666–1668. doi: 10.1104/pp.44.12.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throneberry G. O. Phosphorus and zinc measurements in Kjeldahl digests. Anal Biochem. 1974 Aug;60(2):358–362. doi: 10.1016/0003-2697(74)90242-5. [DOI] [PubMed] [Google Scholar]


