Abstract
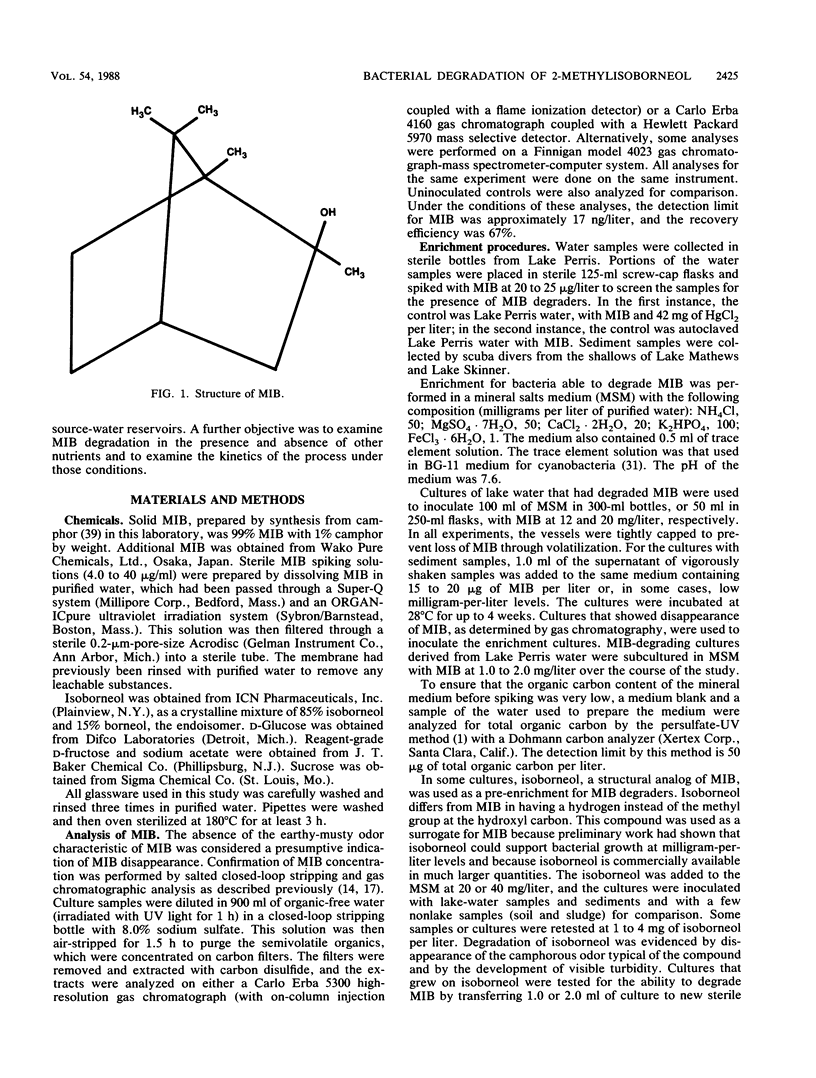
2-Methylisoborneol (MIB) is a musty- or muddy-smelling compound which occurs in some natural waters and which is difficult to remove by conventional water treatment methods. Bacterial degradation of MIB was examined in batch culture experiments. Cultures able to metabolize MIB were enriched in a mineral salts medium supplemented with milligram-per-liter levels of the compound and were inoculated with water and sediment samples from reservoirs where MIB is seasonally produced. Bacteria from degrading cultures were isolated on R2A agar and identified as predominantly Pseudomonas spp. Degradation occurred only in cultures consisting of three or more different bacteria. MIB supported growth as the sole added carbon source at 1 to 6.7 mg/liter. MIB was also degraded at microgram-per-liter levels in sterile filtered lake water inoculated with washed bacteria and in synthetic medium supplemented with various sugars or acetate. Complete degradation of MIB took from 5 days to more than 2 weeks. Enrichment with isoborneol, a structural analog of MIB, failed as a preenrichment for MIB degraders. Isoborneol at 20 to 40 mg/liter readily supported bacterial growth, whereas MIB at 12 to 20 mg/liter took months to degrade. The relative recalcitrance of MIB compared with isoborneol may be a result of the additional methyl group in MIB.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard D. L., Wagner R. E., Brennan M. J., Haberl M. L., Brown J. F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilon C., Beckmann W., Hellwig M., Knackmuss H. J. Enrichment and isolation of naphthalenesulfonic Acid-utilizing pseudomonads. Appl Environ Microbiol. 1981 Jul;42(1):39–43. doi: 10.1128/aem.42.1.39-43.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. H. The metabolic versatility of pseudomonads. Antonie Van Leeuwenhoek. 1982 May;48(2):105–130. doi: 10.1007/BF00405197. [DOI] [PubMed] [Google Scholar]
- Dagley S. Determinants of biodegradability. Q Rev Biophys. 1978 Nov;11(4):577–602. doi: 10.1017/s0033583500005679. [DOI] [PubMed] [Google Scholar]
- Gerber N. N. A volatile metabolite of actinomycetes, 2-methylisoborneol. J Antibiot (Tokyo) 1969 Oct;22(10):508–509. doi: 10.7164/antibiotics.22.508. [DOI] [PubMed] [Google Scholar]
- Gerber N. N., Lechevalier H. A. Geosmin, an earthly-smelling substance isolated from actinomycetes. Appl Microbiol. 1965 Nov;13(6):935–938. doi: 10.1128/am.13.6.935-938.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D. Genetic aspects of biodegradation by pseudomonads. Experientia. 1983 Nov 15;39(11):1199–1213. doi: 10.1007/BF01990357. [DOI] [PubMed] [Google Scholar]
- Hoover D. G., Borgonovi G. E., Jones S. H., Alexander M. Anomalies in mineralization of low concentrations of organic compounds in lake water and sewage. Appl Environ Microbiol. 1986 Feb;51(2):226–232. doi: 10.1128/aem.51.2.226-232.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre G., Hwang C. J., Krasner S. W., McGuire M. J. Geosmin and 2-methylisoborneol from cyanobacteria in three water supply systems. Appl Environ Microbiol. 1982 Mar;43(3):708–714. doi: 10.1128/aem.43.3.708-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbane J. J., Chatterjee D. K., Karns J. S., Kellogg S. T., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Jul;44(1):72–78. doi: 10.1128/aem.44.1.72-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S., Bigley D. V., Grimes D. J. Distribution of Pseudomonas aeruginosa in a riverine ecosystem. Appl Environ Microbiol. 1983 Jan;45(1):328–332. doi: 10.1128/aem.45.1.328-332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wang Y. S., Subba-Rao R. V., Alexander M. Effect of substrate concentration and organic and inorganic compounds on the occurrence and rate of mineralization and cometabolism. Appl Environ Microbiol. 1984 Jun;47(6):1195–1200. doi: 10.1128/aem.47.6.1195-1200.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N. R., Wolfe R. L., Justice C. A., Olson B. H. The identification of gram-negative, nonfermentative bacteria from water: problems and alternative approaches to identification. Adv Appl Microbiol. 1986;31:293–365. doi: 10.1016/s0065-2164(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Wiggins B. A., Jones S. H., Alexander M. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. Appl Environ Microbiol. 1987 Apr;53(4):791–796. doi: 10.1128/aem.53.4.791-796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


