Abstract
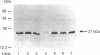
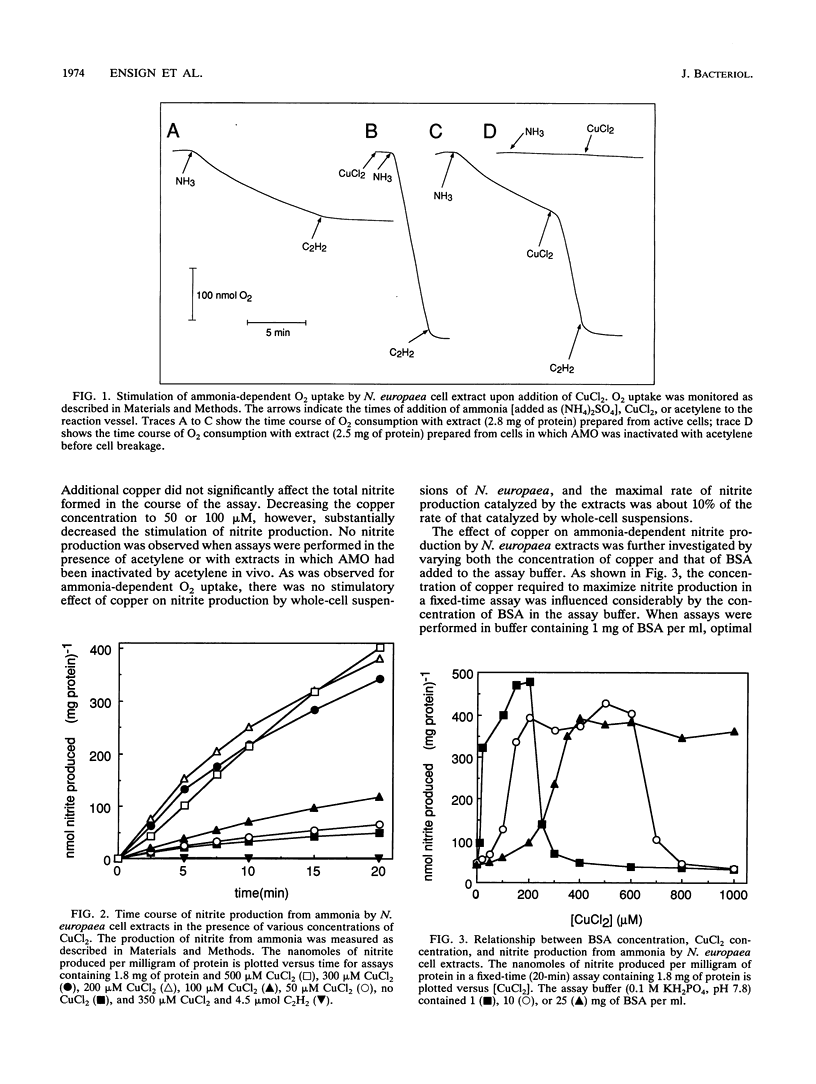
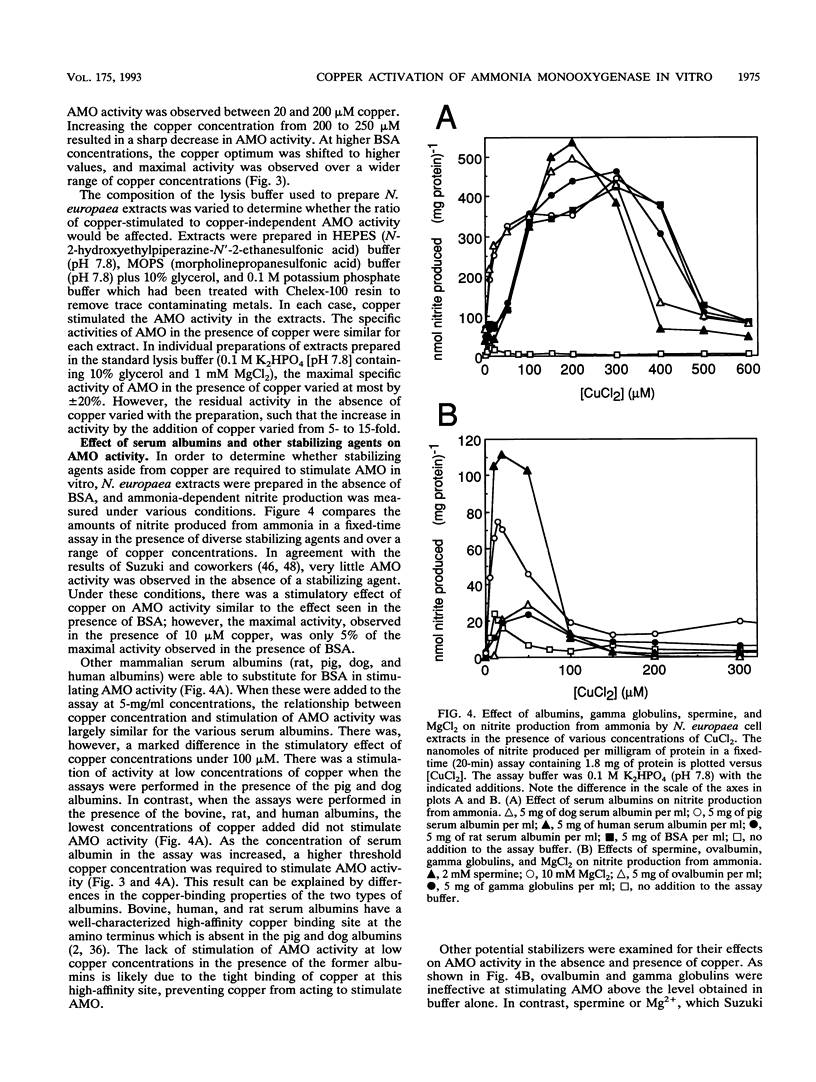
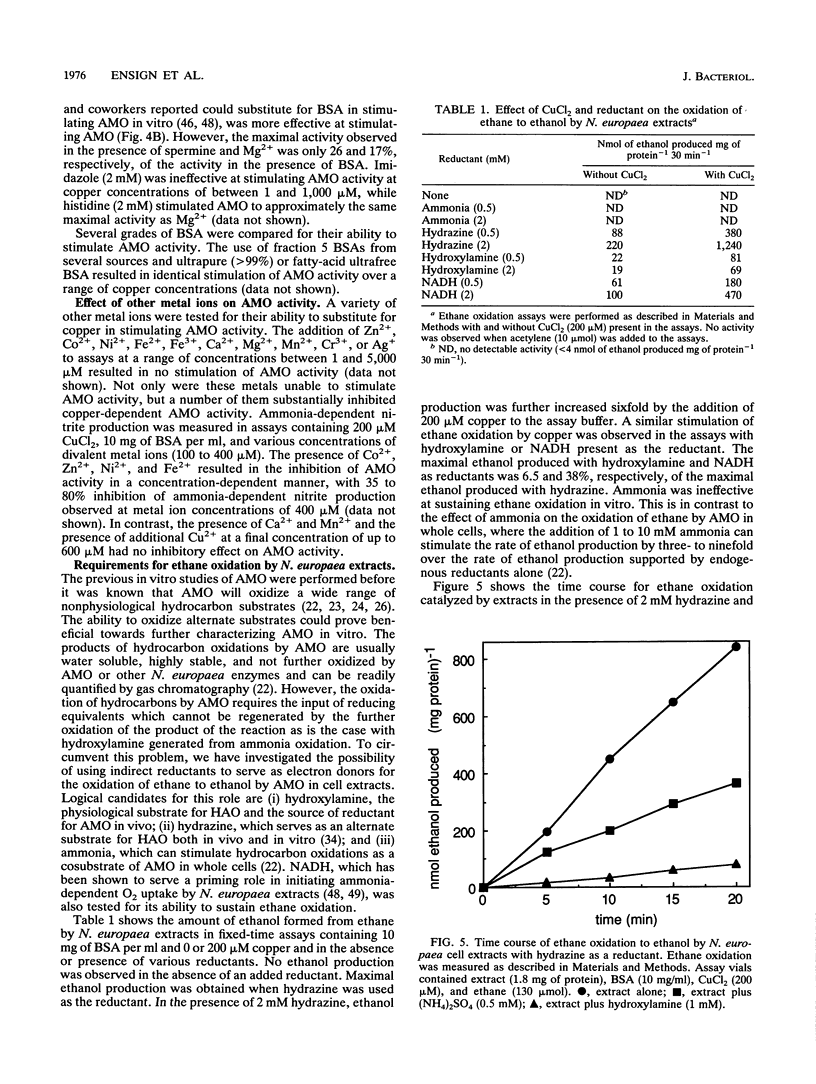
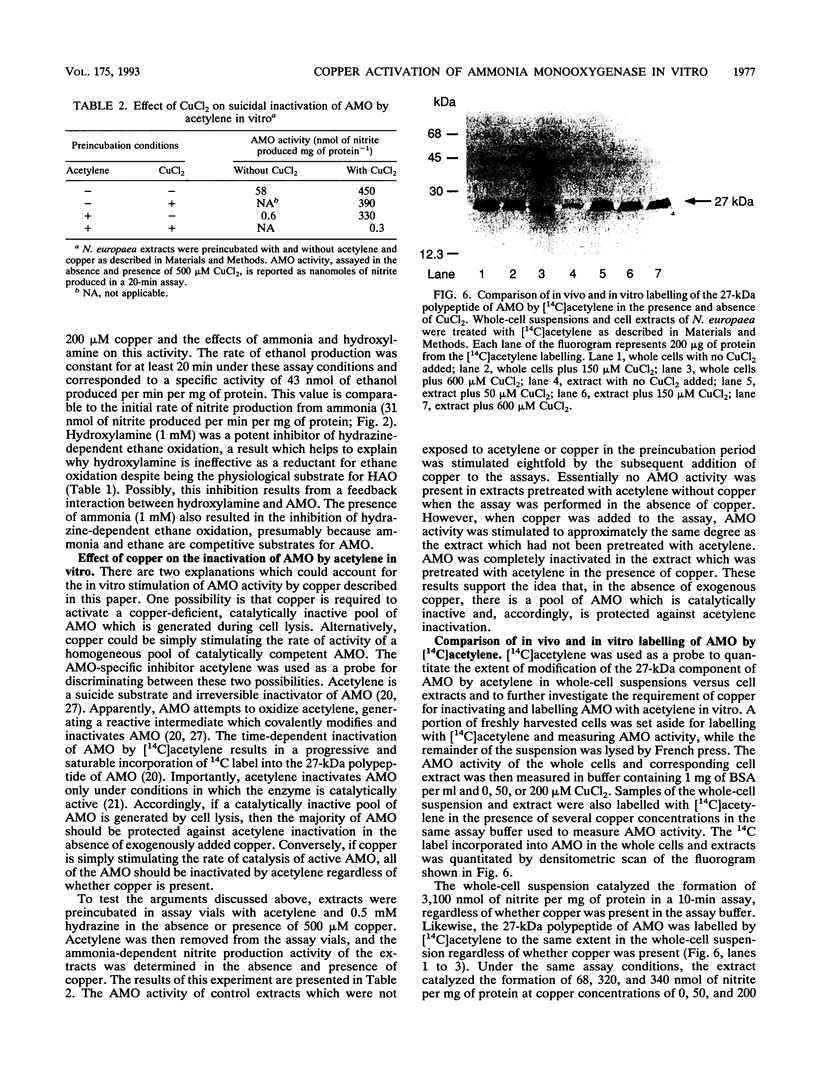
The effect of copper on the in vivo and in vitro activity of ammonia monooxygenase (AMO) from the nitrifying bacterium Nitrosomonas europaea was investigated. The addition of CuCl2 to cell extracts resulted in 5- to 15-fold stimulation of ammonia-dependent O2 consumption, ammonia-dependent nitrite production, and hydrazine-dependent ethane oxidation. AMO activity was further stimulated in vitro by the presence of stabilizing agents, including serum albumins, spermine, or MgCl2. In contrast, the addition of CuCl2 and stabilizing agents to whole-cell suspensions did not result in any stimulation of AMO activity. The use of the AMO-specific suicide substrate acetylene revealed two populations of AMO in cell extracts. The low, copper-independent (residual) AMO activity was completely inactivated by acetylene in the absence of exogenously added copper. In contrast, the copper-dependent (activable) AMO activity was protected against acetylene inactivation in the absence of copper. However, in the presence of copper both populations of AMO were inactivated by acetylene. [14C]acetylene labelling of the 27-kDa polypeptide of AMO revealed the same extent of label incorporation in both whole cells and optimally copper-stimulated cell extracts. In the absence of copper, the label incorporation in cell extracts was proportional to the level of residual AMO activity. Other metal ions tested, including Zn2+, Co2+, Ni2+, Fe2+, Fe3+, Ca2+, Mg2+, Mn2+, Cr3+, and Ag+, were ineffective at stimulating AMO activity or facilitating the incorporation of 14C label from [14C]acetylene into the 27-kDa polypeptide. On the basis of these results, we propose that loss of AMO activity upon lysis of N. europaea results from the loss of copper from AMO, generating a catalytically inactive, yet stable and activable, form of the enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. Bacterial oxidation of methane and methanol. Adv Microb Physiol. 1986;27:113–210. doi: 10.1016/s0065-2911(08)60305-7. [DOI] [PubMed] [Google Scholar]
- Appleton D. W., Sarkar B. The absence of specific copper (II)-binding site in dog albumin. A comparative study of human and dog albumins. J Biol Chem. 1971 Aug 25;246(16):5040–5046. [PubMed] [Google Scholar]
- Arciero D. M., Balny C., Hooper A. B. Spectroscopic and rapid kinetic studies of reduction of cytochrome c554 by hydroxylamine oxidoreductase from Nitrosomonas europaea. Biochemistry. 1991 Dec 3;30(48):11466–11472. doi: 10.1021/bi00112a014. [DOI] [PubMed] [Google Scholar]
- Arciero D. M., Collins M. J., Haladjian J., Bianco P., Hooper A. B. Resolution of the four hemes of cytochrome c554 from Nitrosomonas europaea by redox potentiometry and optical spectroscopy. Biochemistry. 1991 Dec 3;30(48):11459–11465. doi: 10.1021/bi00112a013. [DOI] [PubMed] [Google Scholar]
- Arciero D., Vannelli T., Logan M., Hooper A. B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989 Mar 15;159(2):640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Froland W. A., Dege J. E., Lipscomb J. D. Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph. J Biol Chem. 1989 Jun 15;264(17):10023–10033. [PubMed] [Google Scholar]
- Fox B. G., Surerus K. K., Münck E., Lipscomb J. D. Evidence for a mu-oxo-bridged binuclear iron cluster in the hydroxylase component of methane monooxygenase. Mössbauer and EPR studies. J Biol Chem. 1988 Aug 5;263(22):10553–10556. [PubMed] [Google Scholar]
- Green J., Dalton H. Protein B of soluble methane monooxygenase from Methylococcus capsulatus (Bath). A novel regulatory protein of enzyme activity. J Biol Chem. 1985 Dec 15;260(29):15795–15801. [PubMed] [Google Scholar]
- HOFMAN R., LEES H. The biochemistry of the nitrifying organisms. II. The free-energy efficiency of Nitrosomonas. Biochem J. 1952 Sep;52(1):140–142. doi: 10.1042/bj0520140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Photoinactivation of ammonia oxidation in Nitrosomonas. J Bacteriol. 1974 Sep;119(3):899–906. doi: 10.1128/jb.119.3.899-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992 Jan 25;267(3):1534–1545. [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. Quantification and removal of some contaminating gases from acetylene used to study gas-utilizing enzymes and microorganisms. Appl Environ Microbiol. 1987 Feb;53(2):298–303. doi: 10.1128/aem.53.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. The small-scale production of [U-14C]acetylene from Ba14CO3: application to labeling of ammonia monooxygenase in autotrophic nitrifying bacteria. Anal Biochem. 1990 Nov 1;190(2):348–353. doi: 10.1016/0003-2697(90)90206-o. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Kim C. Y., Arp D. J. Inhibition of ammonia monooxygenase in Nitrosomonas europaea by carbon disulfide. J Bacteriol. 1990 Sep;172(9):4775–4782. doi: 10.1128/jb.172.9.4775-4782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. D., Morita R. Y. Methane Oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983 Feb;45(2):401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES H. The biochemistry of the nitrifying organisms. I. The ammonia oxidizing systems of Nitrosomonas. Biochem J. 1952 Sep;52(1):134–139. doi: 10.1042/bj0520134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin: recent progress in the understanding of its structure and biosynthesis. Clin Chem. 1977 Jan;23(1):5–12. [PubMed] [Google Scholar]
- Rasche M. E., Hicks R. E., Hyman M. R., Arp D. J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990 Sep;172(9):5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears J. H., Wood P. M. Spectroscopic evidence for a photosensitive oxygenated state of ammonia mono-oxygenase. Biochem J. 1985 Mar 1;226(2):499–507. doi: 10.1042/bj2260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. D., Dalton H. Solubilisation of methane monooxygenase from Methylococcus capsulatus (Bath). Eur J Biochem. 1989 Jul 1;182(3):667–671. doi: 10.1111/j.1432-1033.1989.tb14877.x. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Effect of metal-binding and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol. 1977 Jul 26;114(1):71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C. A partial resolution and reconstitution of the ammonia-oxidizing system of Nitrosomonas europaea: role of cytochrome c554. Can J Biochem. 1981 Jul;59(7):484–488. doi: 10.1139/o81-067. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C. Cell-free ammonia oxidation by Nitrosomonas europaea extracts: effects of polyamines, Mg2+ and albumin. Biochem Biophys Res Commun. 1970 Jun 5;39(5):950–955. doi: 10.1016/0006-291x(70)90416-x. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U. Competitive inhibition of ammonia oxidation in Nitrosomonas europaea by methane, carbon monoxide or methanol. FEBS Lett. 1976 Dec 15;72(1):117–120. doi: 10.1016/0014-5793(76)80825-3. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U., Tsang D. C. Cell-free ammonia-oxidizing system of Nitrosomonas europaea: general conditions and properties. Can J Biochem. 1981 Jul;59(7):477–483. doi: 10.1139/o81-066. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C. Oxidation of ammonia by spheroplasts of Nitrosomonas europaea. J Bacteriol. 1969 Sep;99(3):897–898. doi: 10.1128/jb.99.3.897-898.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang D. C., Suzuki I. Cytochrome c554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem. 1982 Nov;60(11):1018–1024. doi: 10.1139/o82-131. [DOI] [PubMed] [Google Scholar]
- Vannelli T., Logan M., Arciero D. M., Hooper A. B. Degradation of halogenated aliphatic compounds by the ammonia- oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990 Apr;56(4):1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland M. P., Dalton H. Purification and characterization of component A of the methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem. 1984 Jan 10;259(1):53–59. [PubMed] [Google Scholar]