Abstract
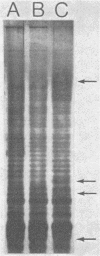
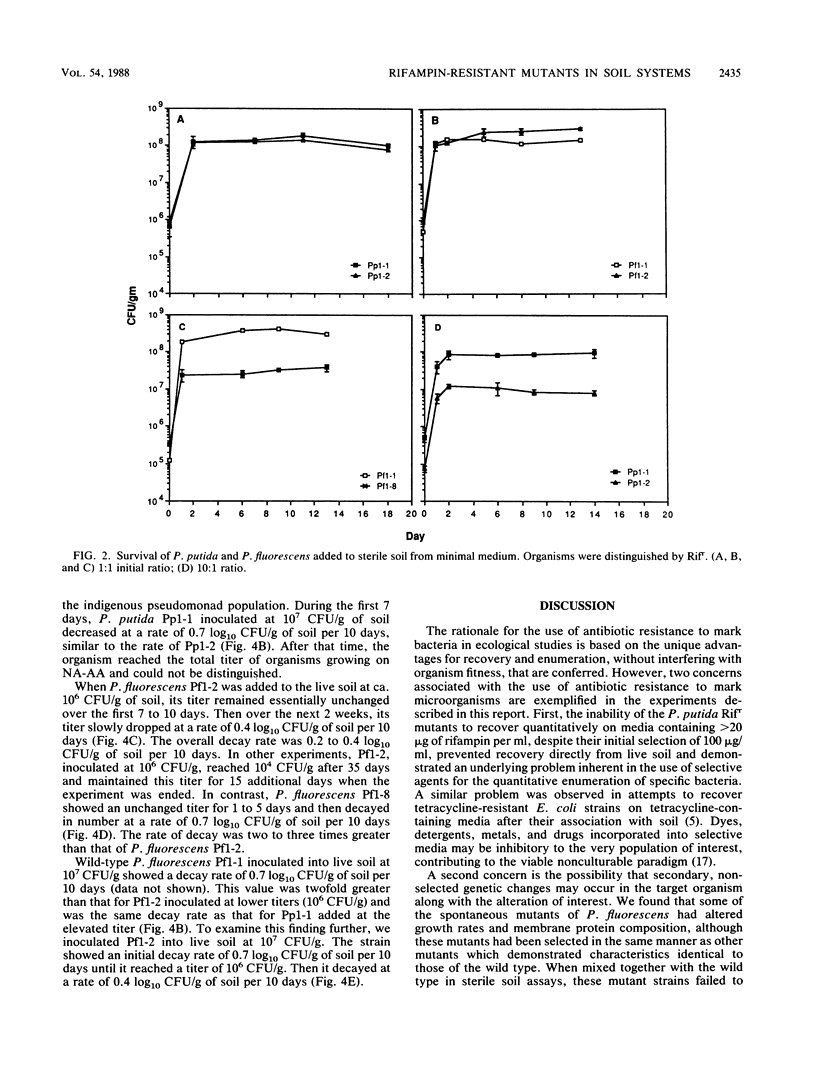
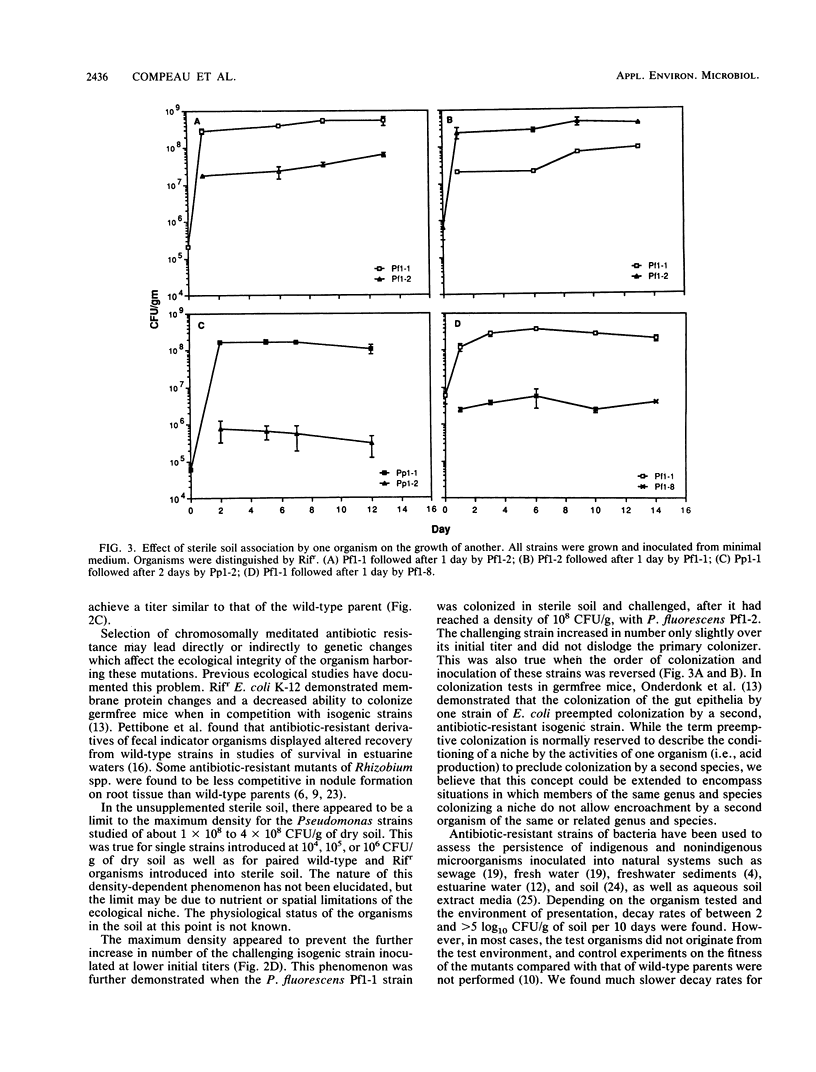
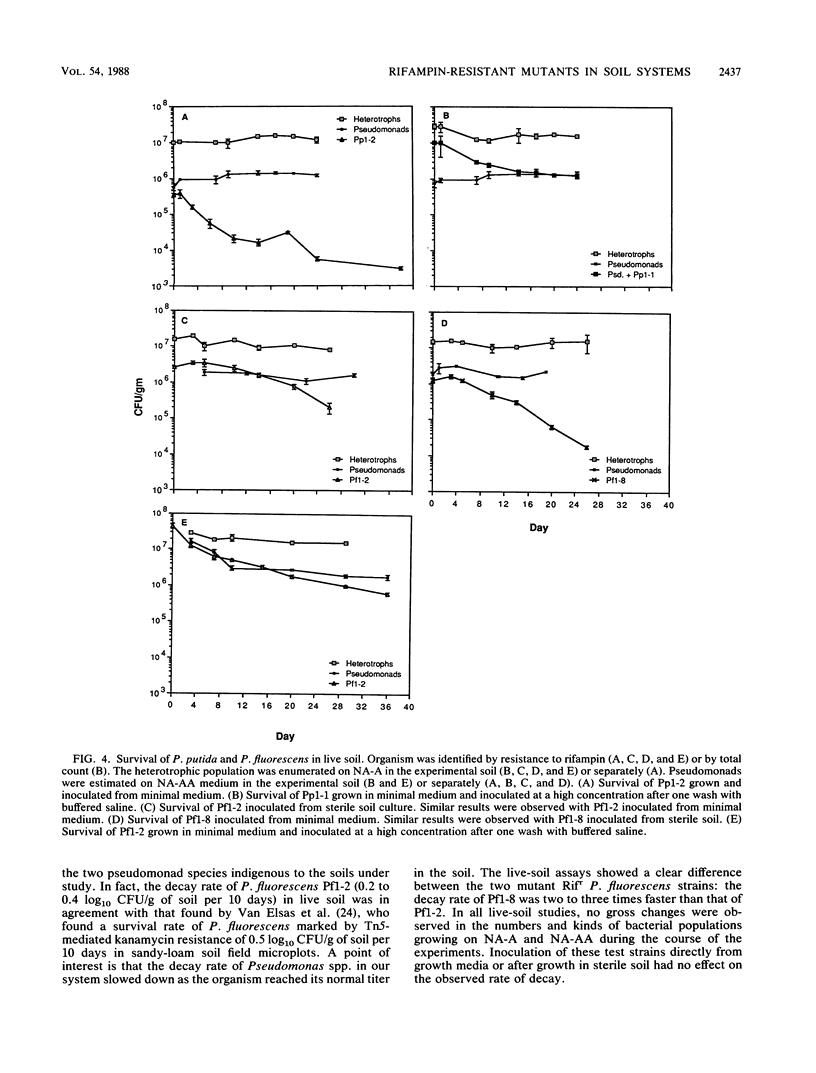
The fate of spontaneous chromosomal rifampin-resistant (Rifr) mutants of Pseudomonas putida and Pseudomonas fluorescens in sterile and live organic soil from which they were isolated was studied. In sterile native-soil assays, a Rifr mutant of P. putida showed no decrease in competitive fitness when compared with the wild-type parent. However, mutants of P. fluorescens were of two general categories. Group 1 showed no difference from the wild type in terms of growth rate, competitive fitness, and membrane protein composition. Group 2 showed a slower growth rate in both minimal and enriched media and an altered membrane protein profile. These mutants also demonstrated decreased competitive fitness compared with the wild-type strain. In live soil, the Rifr P. putida strain persisted throughout the 38-day test period with a decay rate of 0.7 log10 CFU/g of soil per 10 days. A group 1 Rifr P. fluorescens mutant maintained its inoculated titer for 7 to 10 days and then decayed at a rate of 0.2 to 0.4 log10 CFU/g of soil per 10 days. A group 2 Rifr P. fluorescens mutant remained at its titer for 1 to 5 days before decaying at a two- to threefold-faster rate. These findings indicate that rifampin resistance may not be an innocuous mutation in some pseudomonads and that marked strains should be compared with wild-type parents before being used as monitors of parental strain survival. Colonization of sterile soil with either the wild-type or mutant strain precluded normal colonization of the second added strain.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bromfield E. S., Lewis D. M., Barran L. R. Cryptic plasmid and rifampin resistance in Rhizobium meliloti influencing nodulation competitiveness. J Bacteriol. 1985 Oct;164(1):410–413. doi: 10.1128/jb.164.1.410-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. A., Jr, Gunnison D., Lanza G. R. Survival of pathogenic bacteria in various freshwater sediments. Appl Environ Microbiol. 1987 Apr;53(4):633–638. doi: 10.1128/aem.53.4.633-638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang L. N., Sinclair J. L., Mallory L. M., Alexander M. Fate in model ecosystems of microbial species of potential use in genetic engineering. Appl Environ Microbiol. 1982 Sep;44(3):708–714. doi: 10.1128/aem.44.3.708-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A., Marshall B., Cisneros R., Levy S. B. Competition between congenic Escherichia coli K-12 strains in vivo. Infect Immun. 1981 Apr;32(1):74–79. doi: 10.1128/iai.32.1.74-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Pankhurst C. E. Symbiotic effectiveness of antibiotic-resistant mutants of fast- and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol. 1977 Aug;23(8):1026–1033. doi: 10.1139/m77-152. [DOI] [PubMed] [Google Scholar]
- Pettibone G. W., Sullivan S. A., Shiaris M. P. Comparative survival of antibiotic-resistant and -sensitive fecal indicator bacteria in estuarine water. Appl Environ Microbiol. 1987 Jun;53(6):1241–1245. doi: 10.1128/aem.53.6.1241-1245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. L., Alexander M. Role of resistance to starvation in bacterial survival in sewage and lake water. Appl Environ Microbiol. 1984 Aug;48(2):410–415. doi: 10.1128/aem.48.2.410-415.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Temple K. L., Camper A. K., McFeters G. A. Survival of two enterobacteria in feces buried in soil under field conditions. Appl Environ Microbiol. 1980 Oct;40(4):794–797. doi: 10.1128/aem.40.4.794-797.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]