Abstract
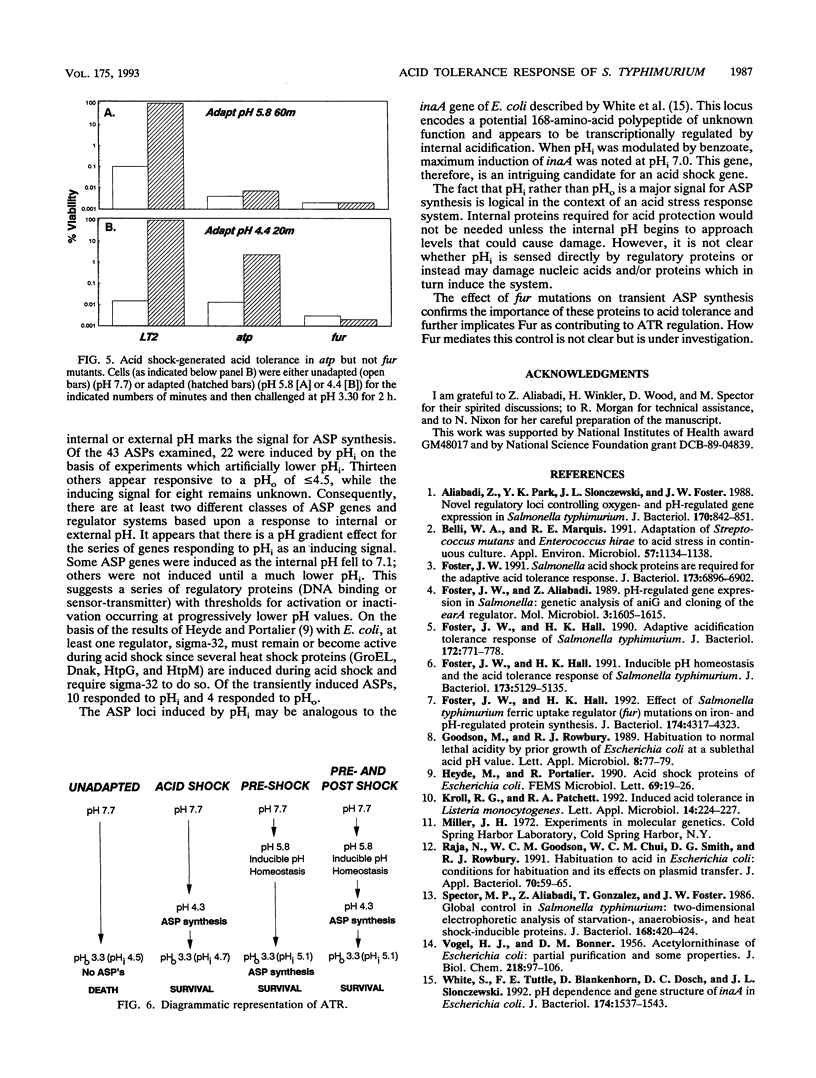
Although Salmonella typhimurium prefers neutral-pH environments, it can adapt to survive conditions of severe low-pH stress (pH 3.3). The process, termed the acid tolerance response (ATR), includes two distinct stages. The first stage, called pre-acid shock, is induced at pH 5.8 and involves the production of an inducible pH homeostasis system functional at external pH values below 4.0. The second stage occurs following an acid shock shift to pH 4.5 or below and is called the post-acid shock stage. During this stage of the ATR, 43 acid shock proteins (ASPs) are synthesized. The present data reveal that several ASPs important for pH 3.3 acid tolerance are only transiently produced. Their disappearance after 30 to 40 min of pH 4.4 acid shock coincides with an inability to survive subsequent pH 3.3 acid challenge. Clearly, an essential feature of inducible acid tolerance is an ability to synthesize these key ASPs. The pre-acid shock stage, with its inducible pH homeostasis system, offers the cell an enhanced ability to synthesize ASPs following rapid shifts to conditions below pH 4.0, an external pH that normally prevents ASP synthesis. The data also address possible signals for ASP synthesis. The inducing signal for 22 ASPs appears to be internal acidification, while external pH serves to induce 13 others. Of the 14 transient ASPs, 10 are induced in response to changes in internal pH. Mutations in the fur (ferric uptake regulator) locus that produce an Atr- acid-sensitive phenotype also eliminate induction of six transiently induced ASPs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Park Y. K., Slonczewski J. L., Foster J. W. Novel regulatory loci controlling oxygen- and pH-regulated gene expression in Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):842–851. doi: 10.1128/jb.170.2.842-851.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli W. A., Marquis R. E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991 Apr;57(4):1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Aliabadi Z. pH-regulated gene expression in Salmonella: genetic analysis of aniG and cloning of the earA regulator. Mol Microbiol. 1989 Nov;3(11):1605–1615. doi: 10.1111/j.1365-2958.1989.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992 Jul;174(13):4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991 Nov;173(21):6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde M., Portalier R. Acid shock proteins of Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):19–26. doi: 10.1016/0378-1097(90)90406-g. [DOI] [PubMed] [Google Scholar]
- Raja N., Goodson M., Chui W. C., Smith D. G., Rowbury R. J. Habituation to acid in Escherichia coli: conditions for habituation and its effects on plasmid transfer. J Appl Bacteriol. 1991 Jan;70(1):59–65. doi: 10.1111/j.1365-2672.1991.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- White S., Tuttle F. E., Blankenhorn D., Dosch D. C., Slonczewski J. L. pH dependence and gene structure of inaA in Escherichia coli. J Bacteriol. 1992 Mar;174(5):1537–1543. doi: 10.1128/jb.174.5.1537-1543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]






