Abstract
Background and Purpose:
Effects of locally administered agonists and antagonists for cannabinoid CB1 and CB2 receptors on mechanical and thermal hypersensitivity were compared after the establishment of chronic inflammation.
Experimental approach:
Carrageenan was administered unilaterally to the rat hindpaw on day 1. Prophylactic efficacy of locally administered CB1- and CB2-selective agonists −arachidonyl-2-chloroethylamide (ACEA) and (R,S)-(2-iodo-5-nitro-phenyl)-[l-(l-methyl-piperidin-2-ylmethyl)-lH-ubdik-3-yl]-methanone ((R,S)-AM1241), respectively− on mechanical and thermal hypersensitivity were compared on day 2. Pharmacological specificity was evaluated using locally administered CB1 and CB2-selective antagonists −N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamidehydrochloride (SR141716A) and N-[(1S)-endo-1,3,3-trimethyl bicycle [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR144528), respectively.
Key Results:
Administration of either ACEA or AM1241 to the inflamed but not noninflamed paw suppressed the maintenance of carrageenan-evoked mechanical hyperalgesia and tactile allodynia and attenuated thermal hyperalgesia. The ACEA-induced suppression of mechanical and thermal hypersensitivity was blocked by local injection of SR141716A but not SR144528. AM1241 suppressed mechanical hypersensitivity with the reverse pharmacological specificity. The AM1241-induced suppression of thermal hyperalgesia was blocked by SR144528 and to a lesser extent by SR14176A. Co-administration of ACEA with AM1241 in the inflamed paw increased the magnitude but not the duration of thermal antihyperalgesia compared to intraplantar administration of either agonist alone.
Conclusions and Implications:
Cannabinoids act locally through distinct CB1 and CB2 mechanisms to suppress mechanical hypersensitivity after the establishment of chronic inflammation, at doses that produced modest changes in thermal hyperalgesia. Additive antihyperalgesic effects were observed following prophylactic co-administration of the CB1- and CB2-selective agonists. Our results suggest that peripheral cannabinoid antihyperalgesic actions may be exploited for treatment of inflammatory pain states.
Keywords: carrageenan, endocannabinoid, peripheral inflammation, allodynia, hyperalgesia
Introduction
Activation of cannabinoid CB1 and CB2 receptor subtypes suppresses pain behaviour resulting from tissue injury and inflammation. Intraplantar (i.pl.) administration of exogenous anandamide suppresses the development and maintenance of carrageenan-evoked thermal hyperalgesia through a CB1 mechanism (Richardson et al., 1998). Whereas CB1 is expressed primarily in the central nervous system (CNS) (Matsuda et al., 1990; Munro et al., 1993; Zimmer et al., 1999), CB2 is expressed predominantly, but not exclusively (Van Sickle et al., 2005; Beltramo et al., 2006), outside the CNS (Munro et al., 1993; Zimmer et al., 1999; Buckley et al., 2000) and is most prevalent in cells of the immune system (Lynn and Herkenham, 1994). The relative anatomical segregation of CB1 and CB2 receptors is consistent with the failure of systemically administered CB2 agonists to induce centrally mediated effects such as catalepsy, hypoactivity and hypothermia (Hanus et al., 1999; Malan et al., 2001). These observations raise the possibility that CB2-selective agonists may represent useful therapeutic agents that lack the CNS side effects that accompany activation of CB1 receptors.
The development of subtype-selective cannabinoid agonists and antagonists has provided the pharmacological tools required to assess the role of CB1 and CB2 receptors in inflammatory nociception. The CB1-selective agonist arachidonyl-2-chloroethylamide (ACEA) exhibits over 1400-fold preferential affinity for CB1 over CB2 (Hillard et al., 1999). The CB2-selective agonist AM1241 exhibits a 340-fold selectivity for CB2 over CB1 (Goutopoulos and Makriyannis, 2002). The CB1 antagonist/inverse agonist SR141716A binds to cannabinoid receptors in rat brain with high affinity and displays low affinity for rat spleen or cloned human CB2 receptors (Rinaldi-Carmona et al., 1994; Showalter et al., 1996). By contrast, the CB2 antagonist SR144528 shows high affinity for rat spleen and cloned human CB2 receptors but has minimal affinity for rat brain or cloned human CB1 receptors (Rinaldi-Carmona et al., 1994).
I.pl. and systemic administration of AM1241 induces a CB2-mediated antinociception in naive rats (Malan et al., 2001). Activation of CB2 receptors also suppresses the development of inflammation-evoked mechanical and thermal hyperalgesia (Clayton et al., 2002; Nackley et al., 2003a; Quartilho et al., 2003; Hohmann et al., 2004) and Fos protein expression, a marker of neuronal activity (Nackley et al., 2003a). Systemic administration of the CB2 agonist JWH-133 similarly suppresses inflammation-evoked decreases in weight bearing and peripheral oedema (Elmes et al., 2005).
CB1 is synthesized neuronally in cells of the dorsal root ganglia (Hohmann and Herkenham, 1999a, 1999b; Ahluwalia et al., 2000; Bridges et al., 2003) and may be transported to peripheral terminals (Hohmann and Herkenham, 1999a) to contribute to peripherally mediated antihyperalgesic effects. However, the mechanisms underlying antihyperalgesic effects of CB2 agonists remain poorly understood. CB2 receptors were initially detected in cultures of neonatal dorsal root ganglion cells using fluorescence-activated cell sorting analyses (Ross et al., 2001). Consistent with this receptor distribution, electrophysiological studies indicate that C-fibre-mediated responses and windup are suppressed in spinal wide dynamic range neurons through activation of either CB1 (Drew et al., 2000; Kelly and Chapman, 2001) or CB2 (Nackley et al., 2004) receptor mechanisms. CB2 agonists also suppress capsaicin-evoked release of calcitonin gene-related peptide in rat spinal cord in vitro (Beltramo et al., 2006), suggesting a possible neuronal mechanism of action. Furthermore, CB2 receptor protein has been identified in microglial cultures of neonatal rat spinal cord (Beltramo et al., 2006), suggesting the existence of additional non-neuronal substrates capable of mediating the observed antihyperalgesic actions. Activation of CB2 receptors on non-neuronal cells has been postulated to suppress the release of inflammatory mediators that excite nociceptors (Mazzari et al., 1996). Furthermore, activation of CB2 receptors on skin keratinocytes stimulates production of β-endorphin to induce antinociception through activation of μ-opioid receptors (Ibrahim et al., 2005). Finally, expression of CB2 is markedly upregulated in the dorsal root ganglia and spinal cord following sciatic nerve injury (Zhang et al., 2003; Wotherspoon et al., 2005; Beltramo et al., 2006), whereas expression levels remain near the threshold for detection in naive animals. Thus, several distinct mechanisms may contribute to antihyperalgesic actions of CB2 agonists. The recent localization of CB2 receptors and mRNA within the central nervous system, including the spinal cord (Beltramo et al., 2006), brainstem and cortex (Van Sickle et al., 2005), suggest that elevated levels of endocannabinoids may engage central CB2 receptors to alter neuronal physiology. These observations have raised questions about the sites of action of systemically administered CB2 agonists and antagonists in modulating hyperalgesia.
Although the ability of peripheral cannabinoid mechanisms to modulate the development of inflammatory nociception is well established, less is known about peripheral cannabinoid antihyperalgesic mechanisms after the establishment of chronic inflammation. I.pl. administration of a high dose of carrageenan (6 mg 150 μl−1 saline) induces a long-lasting thermal hyperalgesia (stable over at least four days post-carrageenan) without affecting normal behaviour in the rat (Iadarola et al., 1988). This sustained hyperalgesia is associated with marked elevation of dynorphin mRNA and dynorphin A 1–8 protein in the spinal cord (Iadarola et al., 1988), suggestive of regulatory changes induced by chronic inflammation at the level of the spinal dorsal horn.
The present studies were conducted to evaluate the effects of activating either CB1 or CB2 receptors at the site of inflammation on the maintenance of carrageenan-evoked inflammatory nociception. We compared the efficacy of locally administered CB1- and CB2-selective agonists and antagonists on inflammation-induced hypersensitivity to different modalities of cutaneous stimulation (mechanical, thermal) under identical conditions. Specifically, we examined whether locally administered cannabinoids retain the in vivo pharmacological specificity suggested by their in vitro binding affinities by using site-specific rather than systemic injections of cannabinoid antagonists. Finally, we tested the hypothesis that CB1- and CB2-selective agonists would act synergistically to suppress the maintenance of carrageenan-evoked inflammatory nociception.
Methods
Animals
Two hundred and six male Sprague-Dawley rats (300–350 g; Harlan, Indianapolis, IN, USA) were used in these experiments. All procedures were approved by the University of Georgia Animal Care and Use Committee and followed the guidelines for the treatment of animals of the International Association for the Study of Pain (Zimmermann, 1983).
General experimental methods
Withdrawal responses to thermal and mechanical stimulation of the paw were evaluated in separate groups of rats. Thermal paw withdrawal latencies were measured in duplicate. Baseline responses to thermal and mechanical stimulation were established on day 1. Rats subsequently received a unilateral i.pl. injection (150 μl) of 6% carrageenan in the mid-plantar surface of the right hind paw. Saline (150 μl) was administered to the contralateral (noninflamed) hind paw. On day 2, ∼16 h post-carrageenan injection, thermal and mechanical hyperalgesia was assessed before initiation of pharmacological manipulations. One hour following hyperalgesia assessment, i.pl. injections of drug or vehicle were performed bilaterally (50 μl). Responsiveness to thermal and mechanical stimulation of the paw was reassessed in duplicate at 20, 50, 80 and 120 min post-drug manipulation. The investigator was blind to the experimental conditions in all studies.
Assessment of tactile allodynia and mechanical hyperalgesia
Tactile allodynia was assessed using the up-down method (Chaplan et al., 1994). To determine the paw withdrawal threshold to punctuate stimuli, a series of nine calibrated filaments (with bending forces of 0.28, 0.38, 0.57, 2.4, 3.9, 5.4, 8.6, 12.1 and 23.3; Stoelting) with approximately equal logarithmic spacing between stimuli (mean±s.e.m.; 0.201±0.06 units) were applied to each hind paw in successive order, whether ascending or descending. Filaments were positioned in contact with the hindpaw for a duration of 5 s or until a withdrawal response occurred. Testing was initiated with the middle hair of the series (3.9 g). In the absence of a paw withdrawal response, an incrementally stronger filament was presented. In the event of a paw withdrawal, an incrementally weaker filament was presented. After the initial response threshold was crossed, this procedure was repeated four times in order to obtain a total of six responses in the immediate vicinity of the threshold. The pattern of withdrawals (X) and absence of withdrawal (O) was noted together with the terminal filament used in the series of six responses. The 50% g threshold was interpolated using the formula: 50% g threshold=(10[Xf+kδ])/10 000 where Xf=is the value (in log units) of the final von Frey hair used; k=is the tabular value of pattern of positive (X) and negative (O) responses, as described previously (Chaplan et al., 1994) and δ=is the mean difference (in log units) between stimuli.
Immediately following determination of the response threshold, a von Frey monofilament (12.02 g) was presented to the hind paw 10 times for a duration of 1 s with an interstimulus interval of approximately 1 s. Only immediate, robust withdrawal responses from the stimulus were recorded as positive responses. The frequency of paw withdrawal to punctuate mechanical stimulation was assessed in the inflamed (ipsilateral) and noninflamed (contralateral) paws. Mechanical hyperalgesia was defined as an increase in the percentage (%) frequency (i.e. [no. of paw withdrawals/10] × 100) of paw withdrawal evoked by stimulation with the von Frey monofilaments.
Assessment of thermal hyperalgesia
Thermal hyperalgesia was assessed in separate groups of rats using the radiant heat method (Hargreaves et al., 1988) and a commercially available plantar stimulation unit (IITC model 33; Woodland Hills, CA, USA). Rats were placed in plastic cages and positioned on an elevated glass platform. Rats were allowed to habituate for 15 min before testing. Radiant heat was presented to the midplantar region of the hind paw through the floor of the glass platform. Paw withdrawal latencies were determined in duplicate. Stimulation to the paw was terminated upon paw withdrawal or after 25 s, to prevent tissue damage, if the rat failed to withdraw its paw from the radiant heat source.
Assessment of site of action
On day 2, following assessment of carrageenan-evoked sensitization to mechanical or thermal stimulation, separate groups of rats (n=5–12/group) received local (50 μl i.pl.) injections of ACEA (33 μg kg−1) AM1241 (33 μg kg−1), or vehicle in the ipsilateral (inflamed) paw. A separate group of rats received the same dose of ACEA or AM1241 in the contralateral (noninflamed) paw. In all groups, vehicle was administered to the opposite paw. The dose of AM1241 was selected based upon its efficacy in suppressing C-fibre-mediated responses and windup (Nackley et al., 2004) as well as carrageenan and capsaicin-evoked mechanical and thermal hyperalgesia (Nackley et al., 2003a; Hohmann et al., 2004) following pre-emptive administration in our previous work. Mechanical and thermal stimulation of the paw was performed as described above.
Assessment of pharmacological specificity
On day 2, separate groups of rats received i.pl. injections of SR141716A (33 μg kg−1), SR144528 (33 μg kg−1), ACEA (33 μg kg−1) co-administered with SR141716A (33 μg kg−1), ACEA (33 μg kg−1) co-administered with SR144528 (33 μg kg−1), AM1241 (33 μg kg−1) co-administered with SR141716A (33 μg kg−1) or AM1241 (33 μg kg−1) co-administered with SR144528 (33 μg kg−1). Responsiveness to von Frey monofilaments or thermal stimulation was reassessed at 20, 50, 80 and 120 min post-drug injections as described above.
Assessment of putative synergistic effects
To evaluate putative synergistic effects of ACEA and AM1241, separate groups of rats (n=6/group) received i.pl. (50 μl) injections of either ACEA (33 μg kg−1), AM1241 (33 μg kg−1), ACEA (33 μg kg−1) co-administered with AM1241 (33 μg kg−1) or dimethylsulphoxide (DMSO) 16 h following administration of carrageenan. Vehicle was administered to the opposite paw. Rats were evaluated for thermal hyperalgesia as described above.
Drugs and chemicals
Lambda carrageenan was obtained from Sigma Aldrich (St Louis, MO, USA). (R,S)-AM1241 ((R,S)-(2-iodo-5-nitro-phenyl)-[l-(l-methyl-piperidin-2-ylmethyl)-lH-ubdik-3-yl]-methanone), a potent CB2 selective agonist, was custom synthesized (by AMZ and AM). SR141716A (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamidehydrochloride) and SR144528 (N-[(1S)- endo-1,3,3-trimethyl bicycle [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were provided by NIDA. ACEA (arachidonyl-2-chloroethylamide) was obtained from Tocris (Ellisville, MO, USA). Carrageenan (6%) was dissolved in saline and administered in a volume of 150 μl. Drugs were dissolved in DMSO for local (50 μl) administration.
Statistical analysis
Behavioural data were analysed parametrically using analysis of variance (ANOVA) for repeated measures and analysis of covariance (ANCOVA), as appropriate. Mechanical thresholds within each group were analysed by one-way nonparametric repeated measures ANOVA (the Friedman test). The non-parametric Kruskal–Wallis ANOVA by ranks was subsequently used to assess group differences in carrageenan-evoked paw withdrawal thresholds at time points characterized by maximal carrageenan-evoked allodynia. Post hoc comparisons for parametric and nonparametric ANOVA were performed using Fisher's protected least significant difference (PLSD) and Dunn's multiple comparison post hoc tests, respectively. P<0.05 was considered to be statistically significant.
Results
General effects of inflammation
In all studies, responses to mechanical and thermal stimulation did not differ between groups or between paws before administration of carrageenan. Carrageenan lowered the withdrawal threshold (Friedman statistic, Fr⩾12.89, P<0.01 in all studies) and increased the frequency of paw withdrawal to punctuate mechanical stimulation (P<0.01 for all comparisons) and decreased the latency of paw withdrawal to thermal stimulation (P<0.0002). No group differences in responses to mechanical or thermal stimulation were observed in the non-inflamed (contralateral) paw either before or after the establishment of inflammation.
Assessment of tactile allodynia after local administration of ACEA or AM1241
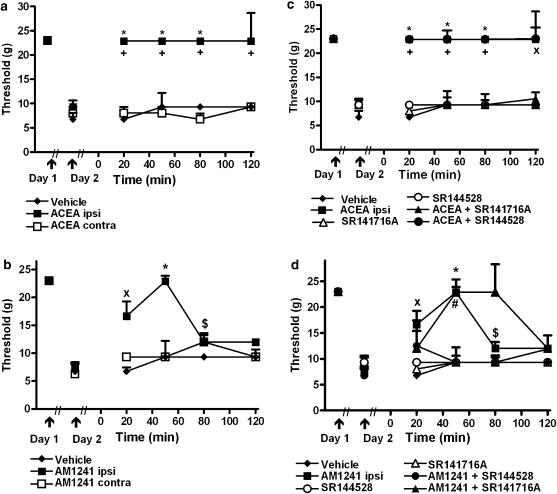
In separate studies, ACEA (Fr=40.69, P<0.0001; Figure 1a) and AM1241 (Fr=27.121, P<0.0001; Figure 1b) increased mechanical withdrawal thresholds in the ipsilateral (inflamed) paw relative to the post-carrageenan threshold (P<0.01). ACEA (Kruskal–Wallis statistic, KW=11.44, P<0.003) and AM1241 (KW=8.93, P<0.02) also raised mechanical withdrawal thresholds relative to vehicle (P<0.05). Ipsilateral hindpaw injections of either ACEA (KW=11.44, P<0.003) or AM1241 (KW=10.997, P<0.004) increased mechanical withdrawal thresholds relative to groups receiving the same dose in the contralateral (noninflamed) paw (P<0.05 for each comparison), consistent with a local site of action. Antihyperalgesic efficacy persisted throughout the observation interval for ACEA and over 80 min post-injection for AM1241. Antihyperalgesia was maximal at 50 min post-injection of AM1241 (Figure 1a and b, respectively).
Figure 1.
Prophylactic administration of the CB1-selective agonist ACEA and the CB2-selective agonist AM1241 suppressed carrageenan-evoked tactile allodynia. (a) ACEA (33 μg kg−1 i.pl.) and (b) AM1241 (33 μg kg−1 i.pl.) raise the threshold for paw withdrawal to punctuate mechanical stimulation following intraplantar administration in the ipsilateral but not the contralateral paw after the establishment of inflammation. (c and d) The CB1 antagonist SR141716A (33 μg kg−1 i.pl.) and CB2 antagonist SR144528 (33 μg kg−1 i.pl.) blocked the antiallodynic effect induced by ACEA and AM1241, respectively. Data (median±semi-interquartile range (SIQR; vertical lines)) are shown for the carrageenan-injected paw only. *P<0.05 different from post-carrageenan threshold (non-parametric one way repeated measures ANOVA (Friedman test) and Dunn's multiple comparison post hoc test). +P<0.05 different from all groups except ACEA+SR144528, xP<0.05 different from vehicle, #P<0.05 different from all groups except AM1241+SR141716A,$P<0.05 different from vehicle and AM1241+SR144528 (Kruskal–Wallis nonparametric ANOVA and Dunn's multiple comparison post hoc test). n=5–12 per group.
The antiallodynic effect of ACEA (KW=33.07, P<0.0001) was blocked by the CB1 (P<0.01) but not by the CB2 antagonist (Figure 1c). In contrast, the CB2 but not the CB1 antagonist blocked the anti-allodynic effects of AM1241 (KW=24.48, P<0.002; P<0.05 for all comparisons). AM1241 administered alone or together with SR141716A increased mechanical withdrawal thresholds (at 50 min post-drug) relative to the post carrageenan threshold (Fr⩾20.31, P<0.001; P<0.05 for all comparisons; Figure 1d). In contrast, mechanical withdrawal thresholds did not differ from post-carrageenan levels in groups receiving vehicle, either antagonist administered alone or AM1241 co-administered with SR144528 (Figure 1d). Neither antagonist altered mechanical withdrawal thresholds relative to vehicle when administered alone.
Assessment of mechanical hyperalgesia after local administration of ACEA or AM1241
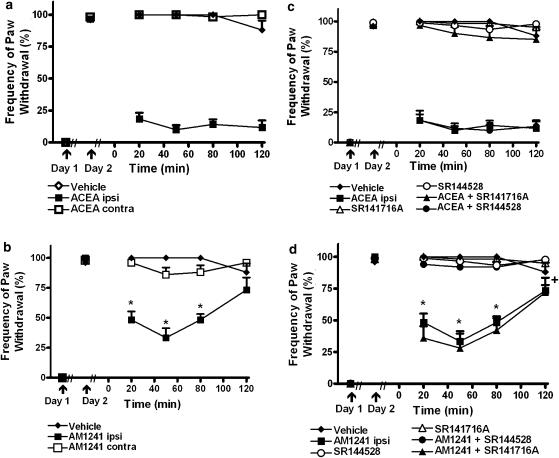
Ipsilateral hindpaw administration of either ACEA (F2,20=226.17,. P<0.0002; P<0.0002 for all comparisons; Figure 2a) or AM1241 (F2,13=36.12, P<0.0002; P<0.0002 for all comparisons; Figure 2b) suppressed the maintenance of carrageenan-evoked mechanical hyperalgesia. Mechanical hyperalgesia did not differ in groups receiving vehicle or either agonist in the noninflamed contralateral hindpaw (Figure 2a and b).
Figure 2.
Prophylactic administration of the CB1-selective agonist ACEA and the CB2-selective agonist AM1241 suppressed carrageenan-evoked mechanical hyperalgesia. (a) ACEA (33 μg kg−1 i.pl.) and (b) AM1241 (33 μg kg−1 i.pl.) administered in the ipsilateral but not the contralateral paw reversed carrageenan-evoked mechanical hyperalgesia. (c) SR141716A (33 μg kg−1 i.pl.) and (d) SR144528 (33 μg kg−1 i.pl.) block the antihyperalgesic effects induced by ACEA and AM1241, respectively. *P<0.05 different from all groups except AM1241+SR141716A. +P<0.05 different from all groups except vehicle and AM1241+SR141716A, (ANOVA, Fisher's PLSD post hoc test). Data are expressed as mean±s.e.m. (vertical lines). n=5–12 per group.
AM1241 induced a time-dependent suppression of mechanical hyperalgesia (F6,39=5.664, P<0.003; P<0.0002 for all comparisons; Figure 2b). Administration of AM1241 to the inflamed paw suppressed established mechanical hyperalgesia relative to groups receiving vehicle or the same dose in the noninflamed paw over an 80 min interval post-drug (Figure 2b).
The ACEA-induced suppression of mechanical hyperalgesia was blocked by the CB1 but not the CB2 antagonist (F5,38=181.20, P<0.0002; P<0.0002 for all comparisons Figure 2c). In contrast, the AM1241-induced suppression of mechanical hyperalgesia was blocked by the CB2 but not the CB1 antagonist (F5,30=45.75, P<0.0002; P<0.0002 for all comparisons; Figure 2d).
Assessment of thermal hyperalgesia after local administration of ACEA or AM1241
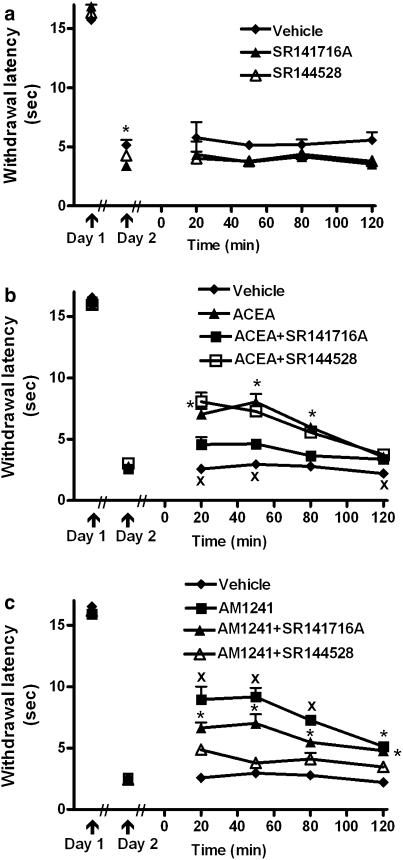
Both ACEA (F2,18=7.75, P<0.004) and AM1241 (F2, 24= 16.24, P<0.0002) administered alone suppressed established thermal hyperalgesia following local administration in the ipsilateral but not the contralateral hind paw (Figure 3a and b respectively). A modest but reliable difference in carrageenan-evoked thermal hyperalgesia was also observed before vehicle or SR141716 administration (F2,15=4.010, P<0.04; P<0.03 Figure 4a). However, ANCOVA confirmed that locally administered CB1 and CB2 antagonists did not alter thermal withdrawal latencies relative to vehicle (Figure 4a).
Figure 3.
Prophylactic administration of the CB1-selective agonist ACEA and the CB2-selective agonist AM1241 suppressed carrageenan-evoked thermal hyperalgesia. (a) ACEA (33 μg kg−1 i.pl.) and (b) AM1241 (33 μg kg−1 i.pl.) suppressed established thermal hyperalgesia following intraplantar administration in the ipsilateral (inflamed) but not contralateral paw (ANOVA, Fisher's, PLSD post hoc test). Data are expressed as mean±s.e.m. (vertical lines). n=6–12 per group.
Figure 4.
Pharmacological specificity of the suppression of thermal hyperalgesia induced by the CB1-selective agonist ACEA and the CB2-selective agonist AM1241. (a) I.pl. administration of SR141716A (33 μg kg−1 i.pl.) and SR144528 (33 μg kg−1 i.pl.) to the site of inflammation did not alter established thermal hyperalgesia (ANCOVA). A modest difference in carrageenan-evoked thermal hyperalgesia was detected before drug or vehicle administration (P<0.01; ANOVA, Fisher's, PLSD post hoc test). (b) Intraplantar administration of SR141716A but not SR144528 blocked the ACEA-induced suppression of thermal hyperalgesia. xP<0.05, different from all other groups, *P<0.05, ACEA different from vehicle and ACEA+SR141716A, (ANOVA, Fisher's PLSD post hoc test), xP<0.05, different from all other groups. (c) Intraplantar administration of SR144528 blocked the suppression of thermal hyperalgesia induced by AM1241. Local administration of SR141716A partially attenuated the AM1241-induced suppression of thermal hyperalgesia. xP<0.05, different from all other groups, *P<0.05, different from vehicle and AM1241+SR144528, (ANOVA, Fisher's PLSD post hoc test). Data are expressed as mean±s.e.m. (vertical lines). Drug dose for all conditions, 33 μg kg−1 i.pl. n=6 per group.
The ACEA-induced attenuation of thermal hyperalgesia was blocked by the CB1 antagonist SR141716A but not by the CB2 antagonist SR144528 (F3,20=55.46, P<0.0002; P<0.0004 for all comparisons, Figure 4b). The AM1241-induced suppression of thermal hyperalgesia was blocked by the CB2 antagonist SR144528 but was also partially blocked by the CB1 antagonist SR141716A (F3,20=45.87, P<0.0002; P<0.01 for all comparisons, Figure 4c). A time-dependent blockade of the antihyperalgesic effects of ACEA (F9,60=6.089, P<0.0002) and AM1241 (F9,60=4.387, P<0.005) were observed following antagonist coadministration (Figure 4b and c).
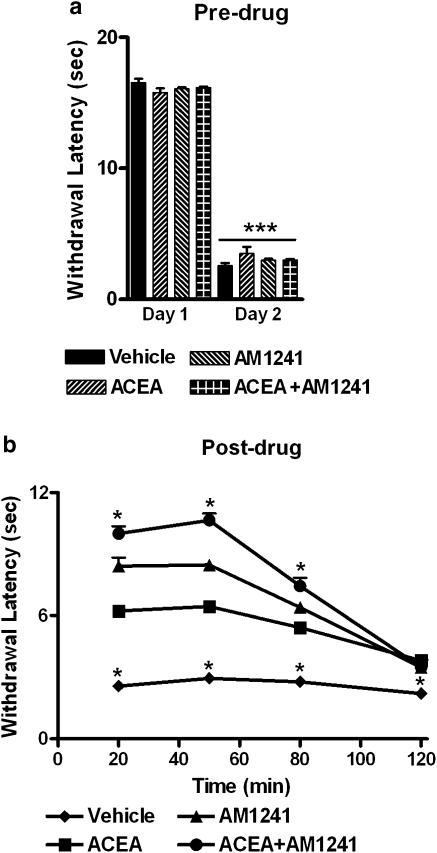
Assessment of putative synergistic effects after local administration of ACEA and AM1241
The antihyperalgesic effect induced by coadministration of the CB1- and CB2-selective agonists was greater than that induced by either agonist administered alone (F3,20=253.593, P<0.0002; P<0.0002 for all comparisons Figure 5a and b). Co-administration of AM1241 with ACEA induced a time-dependent suppression of thermal hyperalgesia relative to vehicle over 80 min post-injection (F9, 60= 27.705, P<0.0002; P<0.02 for all comparisons; Figure 5b). Coadministration of ACEA with AM1241 increased the magnitude without altering the duration of the antihyperalgesic effect, relative to groups receiving either agonist alone. In this study, AM1241 induced a greater suppression of thermal hyperalgesia relative to groups receiving vehicle or ACEA alone (Figure 5b).
Figure 5.
Suppression of thermal hyperalgesia following co-administration of the CB1-selective agonist ACEA and the CB2-selective agonist AM1241. (a) I.pl. administration of carrageenan induced thermal hyperalgesia on day 2. ***P<0.001 different from pre-carrageenan day 1 withdrawal latencies. (b) Antihyperalgesic efficacy following intraplantar co-administration of ACEA (33 μg kg−1 i.pl.) with AM1241 (33 μg kg−1 i.pl.) was greater than that observed following local administration of either agonist alone. *P<0.05, different from all other groups, (ANOVA, Fisher's PLSD post hoc test). Data are expressed as mean±s.e.m. (vertical lines). n=6 per group.
Discussion
Cannabinoids suppress inflammatory nociception through effects at CB1 and CB2 receptors (Hanus et al., 1999; Clayton et al., 2002; Malan et al., 2002; Nackley et al., 2003b; Elmes et al., 2004; Hohmann and Walker, 2004). Support for peripheral antihyperalgesic action is largely derived from the ability of site-specific administration of agonists to suppress inflammatory nociception (typically to thermal stimulation) following administration in the ipsilateral but not the contralateral paw. However, most studies have evaluated pharmacological specificity using systemic rather than local administration of agonists and antagonists. Because concentrations of locally administered agonists in peripheral paw tissue may exceed physiologically relevant concentrations, it is unclear whether antihyperalgesic doses exhibit identical pharmacological profiles to those observed following systemic administration. We therefore compared the effects of locally administered CB1-selective and CB2-selective agonists and antagonists on the maintenance of carrageenan-evoked hyperalgesia and allodynia under identical conditions. To evaluate the clinical relevance of peripheral cannabinoid pharmacotherapies for pain better we: (1) evaluated prophylactic efficacy following more sustained inflammation (i.e. using a dose of carrageenan that produces stable hyperalgesia over several days), (2) examined the efficacy of both CB1- and CB2-selective agonists in suppressing mechanical as well as thermal hypersensitivity under identical conditions, (3) confirmed that locally administered antagonists were indeed capable of blocking agonist actions through subtype-specific mechanisms and (4) evaluated the presence of possible synergistic effects following coadministration of a CB1- and CB2-selective agonist.
Antihyperalgesic efficacy following sustained inflammation
In our study, either the CB1-selective agonist ACEA or the CB2-selective agonist AM1241, administered alone, suppressed the maintenance of carrageenan-evoked tactile allodynia and mechanical and thermal hyperalgesia through a local site of action. Ipsilateral but not contralateral hindpaw administration of either cannabinoid agonist suppressed inflammatory nociception. Dose–response analyses are required to verify the suggested increase in potency of cannabinoid agonists following chronic inflammation.
Differential suppressions of mechanical and thermal hypersensitivity
Locally administered CB1- and CB2-selective agonists induced qualitatively similar suppressions of allodynia and hyperalgesia. A profound suppression of mechanical hyperalgesia and allodynia was observed following local administration of either ACEA or AM1241 into the inflamed paw. The ACEA-induced suppression of mechanical hyperalgesia and allodynia outlasted that induced by AM1241; this observation probably reflects metabolism of AM1241 limiting the duration of action of the CB2 agonist. The same agonist doses induced only a partial suppression of thermal hyperalgesia, suggesting that antihyperalgesic efficacy may depend in part upon stimulus modality or the parameters of thermal stimulation employed (Yeomans and Proudfit, 1996; Yeomans et al., 1996). The DMSO vehicle was unlikely to alter sensory thresholds to alter the pattern of results obtained; paw withdrawal latencies and thresholds observed following local injections of vehicle did not differ from those observed after the establishment of carrageenan inflammation before DMSO administration (see also Malan et al., 2001). Importantly, intraplantar injections of vehicle did not prevent detection of antihyperalgesic and antiallodynic efficacy of locally administered CB1- and CB2-selective agonists in the present study.
Pharmacological specificity
Following sustained inflammation, local prophylactic administration of either agonist alone suppressed tactile allodynia and mechanical hyperalgesia with the expected pharmacological specificity. However, antihyperalgesic efficacy and pharmacological specificity for the CB2-selective agonist was less robust in tests of thermal compared to mechanical hypersensitivity. As predicted, local administration of the CB2- but not the CB1-selective antagonist blocked the suppressive effects of AM1241 on tactile allodynia and mechanical hyperalgesia. Moreover, the antihyperalgesic effects of ACEA were blocked by antagonists with the reverse pharmacological specificity. Although the CB2 antagonist SR144528 completely blocked the AM1241-induced suppression of thermal hyperalgesia, this effect was also partially blocked by the CB1 antagonist SR141716A. In contrast, the same dose of the CB1 antagonist largely eliminated the antihyperalgesic effect of ACEA, which was not blocked by the CB2 antagonist. It is possible that changes in endocannabinoid tone are present following chronic but not acute inflammatory treatment and contribute to the partial CB1-mediated blockade of the AM1241-induced suppression of thermal hyperalgesia. However, the local doses of the CB1 and CB2 antagonists employed here did not enhance hyperalgesia or allodynia in our study. Thus, our results suggest that a cannabinoid mechanism does not tonically modulate nociceptive thresholds in the present chronic inflammation model, although floor effects could also contribute to a failure to detect increases in hyperalgesia following local antagonist administration. More work is necessary to determine whether inflammation alters levels of endocannabinoids, the activity of enzymes catalysing endocannabinoid hydrolysis or the expression of CB1 and CB2 receptors. Alternatively, AM1241-induced mobilization of β-endorphin could produce downstream regulatory changes that are also modulated by a CB1 mechanism.
AM1241 suppresses the development of thermal and mechanical hyperalgesia and allodynia in models of inflammatory nociception (Quartilho et al., 2003; Nackley et al., 2003a; Hohmann et al., 2004). Systemically administered CB2 but not CB1 antagonists, administered pre-emptively, blocked the suppressive effects of AM1241 on behavioural hypersensitivity to both mechanical and thermal stimulation in our previous work (Nackley et al., 2003a; Hohmann et al., 2004). Quartilho et al. (2003) showed that systemic and local application of AM1241 also reverses carrageenan-evoked thermal hyperalgesia; these effects were blocked by a CB2- but not a CB1-selective antagonist. Our study, using a higher concentration of carrageenan, a longer duration of inflammation and lower agonist doses compared the duration, pharmacological specificity and antihyperalgesic efficacy of a CB2-selective agonist (AM1241) with a CB1-selective agonist (ACEA) in tests of mechanical and thermal stimulation under identical conditions. Our results thus verified that each locally administered antagonist was capable of blocking the effects of each respective subtype-selective agonist under the conditions of testing. Our results are consistent with the recent observation that AM1241 suppresses thermal nociception in CB2+/+ mice, but not in CB2−/− mice (Ibrahim et al., 2006). Our data are also consistent with a recent electrophysiological study which confirms that local administration of a CB1-selective agonist (ACEA) (Kelly et al., 2003) or CB2-selective agonist (JWH-133) (Elmes et al., 2004) suppresses mechanically evoked responses in wide dynamic range neurons.
Primary afferents become sensitized under inflammatory conditions (Treede et al., 1992; Koltzenburg, 2000). Peripheral sensitization of mechanoheat nociceptors contributes to primary hyperalgesia to heat at the site of tissue injury (Treede et al., 1992). In contrast, mechanical hyperalgesia may involve recruitment of low threshold mechanoreceptors under conditions that do not normally induce pain (Treede et al., 1992; Koltzenburg, 2000). Local administration of either ACEA or AM1241 at the site of inflammation may suppress antihyperalgesic efficacy by reducing primary afferent sensitization, effects consistent with the observation that cannabinoids suppress capsaicin-evoked calcitonin gene-related peptide (CGRP) release (Richardson et al., 1998; Beltramo et al., 2006). Carrageenan also enhances C-fibre-mediated responses and windup in spinal dorsal horn neurons, effects that enhance spinal neuronal excitability (Woolf et al., 1994; Hedo et al., 1999; Nackley et al., 2004). These effects are also modulated by both CB1 (Drew et al., 2000; Kelly et al., 2003) and CB2-specific mechanisms (Nackley et al., 2004).
Peripheral inflammation can induce phenotypic changes in dorsal root ganglion cells that could contribute to the ability of cannabinoids to suppress mechanical hypersensitivity preferentially. Most notably, myelinated fibres, known to express CB1 (Bridges et al., 2003) become sensitized following chronic inflammation and express characteristics of nociceptors, including the expression of pronociceptive peptides such as CGRP (Neumann et al., 1996). Mechanically sensitive primary afferents also become sensitized in zones of secondary hyperalgesia and exhibit enhanced spontaneous activity (Serra et al., 2004). Localization of cannabinoid receptors to such fibres could contribute to the preferential suppression of sensitization to mechanical vs thermal stimulation, in the absence of regulatory changes in expression of CB1 (Hohmann, 2002). Neuroanatomical studies are required to study the changes in expression of CB1 and/or CB2 that could potentially accompany the behavioural phenotype observed following sustained inflammation.
Peripheral CB2 mechanisms
The mechanism through which activation of CB2 receptors inhibit nociceptive processing in the periphery is not completely understood. Local or systemic administration of AM1241 suppresses C-fibre responses and windup in spinal WDR neurons via a CB2-sensitive mechanism in the absence and presence of inflammation (Nackley et al., 2004). AM1241 may also produce antinociception by indirectly stimulating peripheral release of β-endorphin, an endogenous opioid, from keratinocytes in skin (Ibrahim et al., 2005). More work is necessary to determine whether AM1241 similarly stimulates local release of β-endorphin after the establishment of chronic inflammation to modulate nociceptive thresholds. It is noteworthy that CB2 mRNA is also induced in the spinal cord in pathological pain states coincident with the appearance of activated microglia (Zhang et al., 2003; Beltramo et al., 2006). Such observations collectively suggest that both neuronal and nonneuronal substrates may mediate the suppressive effects of systemically administered CB2-selective agonists on neuronal sensitization in persistent pain states. These mechanisms may also contribute to the more pronounced effects of cannabinoid agonists in inflamed compared to noninflamed tissue. However, in the present study all agonists and antagonists were administered locally to the site of injury; therefore central CB2 receptors (Van Sickle et al., 2005; Beltramo et al., 2006) could not mediate the antihyperalgesic effects of AM1241 observed here. The possible contribution of central CB2 receptors to the antihyperalgesic effects of systemically administered cannabinoids remains to be determined.
Additive antihyperalgesic effects following local co-administration of CB1 and CB2 agonists
In our study, coadministration of AM1241 with ACEA suppressed established carrageenan-evoked thermal hyperalgesia. These studies were conducted using thermal stimulation only so that ceiling effects would not prevent detection of synergistic antihyperalgesic effects. Our results suggest that coadministration of the CB2 and CB1 agonists induced additive rather than synergistic effects at the doses tested, as the duration of antihyperalgesic actions was not reliably prolonged compared to administration of either agonist alone. In the same study, local administration of AM1241 induced a greater suppression of established thermal hyperalgesia compared to ACEA, further highlighting the therapeutic potential of CB2-selective agonists.
In summary, our results demonstrate that selective activation of CB1 or CB2 receptors in the inflamed paw is sufficient to suppress tactile allodynia and mechanical hyperalgesia. This suppression is observed under conditions in which only a partial suppression of thermal hyperalgesia was observed. Collectively, our data suggest that peripheral cannabinoid analgesic mechanisms may be exploited to suppress the tactile hypersensitivity observed in chronic inflammatory pain states.
Acknowledgments
Supported by DA14265, DA14022 (AGH) and DA9158, DA3801 (AM).
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- CNS
central nervous system
- Fr
Friedman statistic
- i.pl.
intraplantar
- KW
Kruskal–Wallis statistic
Conflict of interest
One of the authors declares a conflict of interest. AM serves as a consultant for MAK Scientific.
References
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Harris J, Millns PJ, Kendall DA, Chapman V. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2000;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Elmes SJR, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, et al. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Goutopoulos A, Makriyannis A. From cannabis to cannabinergics: new therapeutic opportunities. Pharmacol Ther. 2002;95:103–117. doi: 10.1016/s0163-7258(02)00250-4. [DOI] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hedo G, Laird JM, Lopez-Garcia JA. Time-course of spinal sensitization following carrageenan-induced inflammation in the young rat: a comparative electrophysiological and behavioural study in vitro and in vivo. Neuroscience. 1999;92:309–318. doi: 10.1016/s0306-4522(98)00734-9. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999a;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999b;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Walker JM.Endocannabinoids and related fatty acid derivatives in pain modulation: Behavioral, neurophysiological and neuroanatomical perspectives on cannabinoid antinociception Cannabinoids 2004Kluwer Academic Publishers: The Netherlands; In: DiMarzo V (ed) [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Kelly S, Chapman V. Selective cannabinoid CB1 receptor activation inhibits spinal nociceptive transmission in vivo. J Neurophysiol. 2001;86:3061–3064. doi: 10.1152/jn.2001.86.6.3061. [DOI] [PubMed] [Google Scholar]
- Kelly S, Jhaveri MD, Sagar DR, Kendall DA, Chapman V. Activation of peripheral cannabinoid CB1 receptors inhibits mechanically evoked responses of spinal neurons in noninflamed rats and rats with hindpaw inflammation. Eur J Neurosci. 2003;18:2239–2243. doi: 10.1046/j.1460-9568.2003.02957.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M. Neural mechanisms of cutaneous nociceptive pain. Clin J Pain. 2000;16:S131–S138. doi: 10.1097/00002508-200009001-00004. [DOI] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F. Inhibition of pain responses by activation of CB2 cannabinoid receptors. Chem Phys Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A. N-(2-hydroxyethyl) hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur J Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003a;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Suplita RL, II, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003b;117:659–670. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, et al. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–2781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Sivilotti LG. Sensitization of high mechanothreshold superficial dorsal horn and flexor motor neurones following chemosensitive primary afferent activation. Pain. 1994;58:141–155. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]