Abstract
Background and purpose:
Neuroactive steroids are potent modulators of GABAA receptors and are thus of interest for their sedative, anxiolytic, anticonvulsant and anaesthetic properties. Cyclodextrins may be useful tools to manipulate neuroactive effects of steroids on GABAA receptors because cyclodextrins form inclusion complexes with at least some steroids that are active at the GABAA receptor, such as (3α,5α)-3-hydroxypregnan-20-one (3α5αP, allopregnanolone).
Experimental approach:
To assess the versatility of cyclodextrins as steroid modulators, we investigated interactions between γ-cyclodextrin and neuroactive steroids of different structural classes.
Key results:
Both a bioassay based on electrophysiological assessment of GABAA receptor function and optical measurements of cellular accumulation of a fluorescent steroid analogue suggest that γ-cyclodextrin sequesters steroids rather than directly influencing GABAA receptor function. Neither a 5β-reduced A/B ring fusion nor a sulphate group at carbon 3 affected the presumed inclusion complex formation between steroid and γ-cyclodextrin. Apparent dissociation constants for interactions between natural steroids and γ-cyclodexrin ranged from 10-60 μM. Although γ-cyclodextrin accommodates a range of natural and synthetic steroids, C11 substitutions reduced inclusion complex formation. Using γ-cyclodextrin to remove steroid not directly bound to GABAA receptors, we found that cellular retention of receptor-unbound steroid rate limits potentiation by 3α- hydroxysteroids but not inhibition by sulphated steroids.
Conclusions and implications:
We conclude that γ-cyclodextrins can be useful, albeit non-specific, tools for terminating the actions of multiple classes of naturally occurring neuroactive steroids.
Keywords: GABAA receptors, inhibitory postsynaptic currents, γ-aminobutyric acid, neurosteroid
Introduction
Neurosteroids are receiving increasing interest as potent endogenous modulators of γ-amino-n-butyric acid type A (GABAA) receptors (Belelli and Lambert, 2005). Agents that antagonize or inhibit the effects of neurosteroids are not generally available as experimental tools. Such tools could be powerful adjuncts to the currently available inhibitors of neurosteroid function, which are largely limited to drugs that inhibit neurosteroidogenesis or neurosteroid metabolism (Concas et al., 1998; Reddy et al., 2001; Belelli and Herd, 2003; Puia et al., 2003). We previously demonstrated a steroid analogue, (3α,5α)-17-phenylandrost-16-en-3-ol, that inhibits the enhancing effects of neurosteroids, but the potency and water solubility of this compound are weak, and effects are relatively selective for certain structural classes of neuroactive steroids (Mennerick et al., 2004). Cyclodextrins are also potential tools. We recently showed that cyclodextrins, cyclic oligosaccharides with a hydrophilic exterior and a lipophilic central cavity can be useful for manipulating the effects of the 5α-reduced steroid, (3α,5α)-3-hydroxypregnan-20-one (3α5αP), at GABAA receptors. The effects of cyclodextrins are presumably by formation of inclusion complexes with 3α5αP (Shu et al., 2004), although evidence for this conclusion is not definitive, especially given recent findings indicating that cyclodextrins may have direct effects on GABAA receptors (Pytel et al., 2006). Although cyclodextrin inclusion complexes can be used to deliver steroids in vivo (Vivian et al., 1997; Reddy and Rogawski, 2000; Ford et al., 2005), on the time scale of brief applications to individual cells, cyclodextrins at relatively low concentrations can potentially be used to complex steroids and terminate their actions (Shu et al., 2004; Akk et al., 2005).
Cyclodextrins are available as α, β and γ forms containing 6, 7 and 8 cyclic sugars, respectively (Wallimann et al., 1997). We showed that α-cyclodextrin does not effectively complex 3α5αP (Shu et al., 2004). Furthermore, β-cyclodextrins may have more nonspecific effects than γ-cyclodextrins (Ohtani et al., 1989). Therefore, we have focused on γ-cyclodextrin, the cyclodextrin with the largest hydrophobic cavity, to accommodate steroids of various structures.
Neurosteroids of differing structural classes have diverse effects on GABAA receptors. A hydroxyl group in the α configuration at C3 and a hydrogen bond acceptor at C17, on the β face of the steroid have been shown to be important for the enhancing effects of steroids at GABAA receptors (Majewska et al., 1986; Callachan et al., 1987). Potentiation also tolerates a trans (5α-reduced) or cis (5β-reduced) A/B ring fusion, despite a large difference in the three-dimensional structure of 5α-reduced vs 5β-reduced steroids (Figure 1a1 and a2). It is not clear whether equally effective inclusion complexes can be formed between cyclodextrins and both of these structural classes of potentiating steroids. The finding that inclusion complexes form between bile steroids that are 5β-reduced, but which contain a carboxylic acid in the side chain present on the steroid, suggest that cyclodextrin/5β-reduced steroid complexes are possible (Redenti et al., 2001).
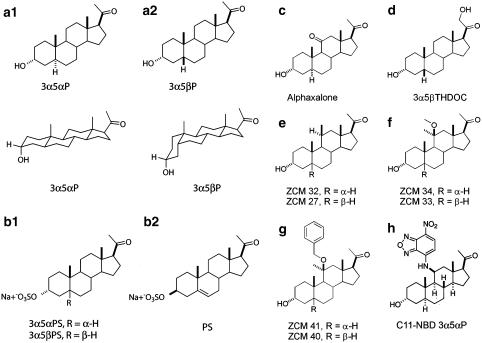
Figure 1.
Structures of types of GABA potentiating and GABA antagonistic neuroactive steroids used in this study. (a) 3α5αP and 3α5βP (allopregnanolone and pregnanolone) are GABAA receptor potentiators despite their structural differences. (b) Sulphated pregnane and pregnene steroids are effective non-competitive GABAA receptor antagonists. (c–h) Other structural modifications tolerated by potentiating steroids. (c) Alphaxalone is a synthetic anaesthetic steroid (Phillipps, 1975; Barker et al., 1987). (d) 3α5βTHDOC is a naturally occurring steroid (Majewska et al., 1986; Mellon et al., 2001). (e–h) Synthetic neuroactive steroids with substitutions at C11 are shown as ZCM 32, ZCM 34 and ZCM 41 (5α-reduced series) and ZCM 27, ZCM 33 and ZCM 40 (5β-reduced series). C11-NBD 3α5αP has a fluorescent substituent at carbon 11.
Another class of neuroactive steroids inhibits GABAA receptors in a use-dependent manner (Eisenman et al., 2003; Reddy, 2003). The steroids most potent at inhibiting these receptors have a sulphate group at C3. This inhibitory effect appears to be tolerant of many structural modifications, including trans (5α-reduced) and cis (5β-reduced) A/B ring fusions (Park-Chung et al., 1999; Wang et al., 2002). In fact, pregnenolone sulphate (PS), which contains a double bond at the A/B ring fusion (Figure 1b2), is also a potent inhibitory steroid. The blocking actions are also tolerant of positioning of the C3 sulphate group in either the α or β configuration. Again, whether cyclodextrins complex with sulphated steroidal GABAA receptor antagonists is unclear, although there is precedent for using β-cyclodextrins to promote PS solubility (Wang et al., 1997).
We sought to test the general ability of cyclodextrins to inhibit neuroactive steroids of different structures. Our results suggest that γ-cyclodextrins are quite tolerant of a variety of structural modifications in potentiating and inhibiting steroids. However, when substitutions are made at C11 to increase the molecular size of a potentiating steroid, potentiation was retained, but cyclodextrin effects were lost. Our data suggest that cyclodextrins can, in principle, be used to reverse the effects of any of the commonly studied endogenous neurosteroids, including the broad classes of potentiating and inhibiting neurosteroids. Using γ-cyclodextrin, we showed that different factors control the offset time of 3α-hydroxysteroid-mediated potentiation compared to sulphated steroid-mediated inhibition of GABAA receptors.
Part of this work has been published previously in abstract form (Shu et al., 2005).
Methods
Test systems used
Stage V–VI oocytes were harvested from sexually mature female Xenopus laevis (Xenopus One, Northland, MI, USA) under 0.1% 3-aminobenzoic acid ethyl ester anaesthesia, according to protocols approved by the Washington University Animal Studies Committee. Oocytes were defolliculated by shaking for 20 min at 37°C in collagenase (2 mg ml−1) dissolved in calcium-free solution containing (in mM): NaCl 96, KCl 2, MgCl2 1 and HEPES 5 at pH 7.4. Capped mRNA, encoding rat GABAA receptor α1, β2 and γ2L subunits was transcribed in vitro using the mMESSAGE mMachine Kit (Ambion, Austin, TX, USA) from linearized pBluescript vectors containing receptor coding regions. Subunit transcripts were injected in equal parts (20–40 ng total RNA) 8–24 h following defolliculation. Oocytes were incubated up to 5 days at 18°C in ND96 medium containing (in mM): NaCl 96, KCl 1, MgCl2 1, CaCl2 2 and HEPES 10 at pH 7.4, supplemented with pyruvate (5 mM), penicillin (100 μ ml−1), streptomycin (100 μg ml−1) and gentamycin (50 μg ml−1).
Hippocampal cultures were prepared from postnatal day 0–3 rat pups as previously described (Mennerick et al., 1995). Hippocampi from isoflurane-anaesthetized rats were removed and sliced 500 μm-thick. The hippocampal slices were digested with 1 mg ml−1 papain in oxygenated Leibovitz L-15 medium, followed by mechanical trituration in modified Eagle's medium containing 5% horse serum, 5% fetal calf serum, 17 mM D-glucose, 400 μM glutamine, 50 μ ml−1 penicillin and 50 μg ml−1 streptomycin. Cells were plated in modified Eagle's medium at a density of 75 cells mm−2 onto 35 mm plastic culture dishes pre-coated with collagen microdroplets sprayed on a layer of 0.15% agarose. The anti-mitotic cytosine arabinoside (10 μM) was added on the third day after plating to halt glial proliferation. Electrophysiology was performed 6–14 days following plating.
Measurements made
Two-electrode voltage-clamp experiments were performed with a Warner OC725 amplifier 2–5 days following RNA injection. The extracellular recording solution was ND96 medium with no supplements. Intracellular recording pipettes were filled with 3 M KCl and had open tip resistances of ∼1 MΩ. Drugs were applied from a common tip via a gravity-driven multibarrel delivery system. Unless indicated otherwise, drugs were co-applied with no pre-application period. Cells were voltage-clamped at −70 mV for all experiments, and the peak current (for potentiated responses) or the current at the end of 20–30 s drug applications (for inhibition of responses) was measured for quantification of current amplitudes. Vertical arrows in the figures give an indication of measurements used for summary quantification.
Whole-cell, patch-clamp recordings were made from hippocampal neurons using pipettes filled with 140 mM caesium methanesulphonate (responses to exogenous GABA) or with 140 mM potassium chloride (autaptic responses). Other components of the pipette solution included (in mM): NaCl 4, EGTA 5, CaCl2 0.5, HEPES 10, pH 7.25. The bath solution for recording included (in mM) NaCl 140, KCl 4, CaCl2 2, MgCl2 1, HEPES 10, D-2-amino-5-phosphonovalerate 0.05. For autaptic responses, solitary neurons devoid of other synaptic input were stimulated with a 1.5-ms depolarizing pulse to 0 mV to trigger an axonal spike and resultant recurrent transmitter release. Drug applications were made with a multibarrel pipette. EC50 for GABA in both oocytes and hippocampal neurons is approximately 10 μM (Eisenman et al., 2003; Shu et al., 2004). Imaging of fluorescent steroid was performed using a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI, USA) mounted on a TE2000 Nikon microscope. Fluorescence was excited with a metal halide light source through a 480-nm excitation filter. Emission was collected at 535 nm. All experiments in oocytes and cultured hippocampal neurons were performed at 25°C.
To estimate solution exchange times during whole-cell recordings from hippocampal neurons, we examined the τ for perfusing a low chloride concentration (74.5 mM, 50% of normal concentration) during the steady-state response to 2 μM GABA. We found that the exchange τ was 254.7±30.2 ms, well below the τ of drug offset for experiments depicted in Figure 8. Thus solution exchange time was not limiting in these experiments.
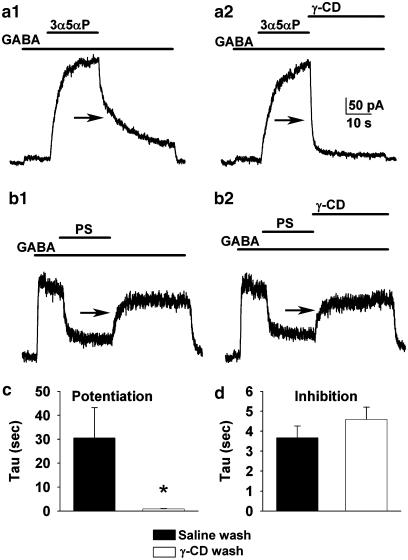
Figure 8.
Cyclodextrins demonstrate different factors governing time course of potentiation vs inhibition by steroids. (a1) 3α5αP (0.3 μM) potentiated the response to 0.5 μM GABA. Removal of the steroid produced a slow relaxation (arrow) back towards the original GABA response when GABA alone was applied. (a2) When γ-cyclodextrin (0.5 mM) was included in the GABA solution upon steroid removal, the relaxation (arrow) was accelerated. (b1 and 2) When PS (5 μM) was used to inhibit the response to 2 μM GABA, γ-cyclodextrin (0.5 mM) had no effect on the recovery time course (arrows) of PS offset. (c and d) Summary of the effect of γ-cyclodextrin on the offset τ for potentiation (c, n=11) and inhibition (d, n=5) from experiments like those shown in (a) and (b), respectively. Asterisk indicates P<0.05. In (d), there was no significant difference between the offset τ for inhibition (paired t-test).
Experimental design
Design of individual experiments is described in the Results. Wherever possible, each cell served as its own control, and experimental conditions and drug applications were interleaved to negate any time-dependent changes in cell responsiveness.
Data analysis and statistical procedures
Data acquisition and analysis were performed with pCLAMP 9.0 software (Molecular Devices, Union City, CA, USA). Data plotting and curve fitting were performed with Sigma Plot 8.0 software (SPSS Science, Chicago, IL, USA). Fits to concentration response relationships were achieved using a least-squares minimization to the Hill equation:
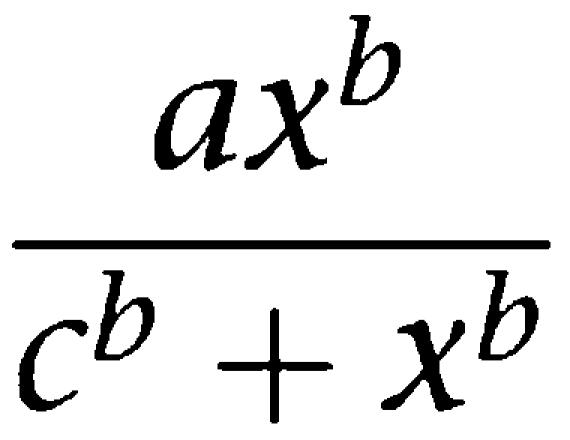 |
where a is the maximum potentiation, b is the Hill coefficient and c is the EC50 concentration of steroid. Fits of time constants to current decays (Figure 8) were performed using a variable metric least-squares minimization algorithm (Clampfit 9.0). Data are presented in the text and figures as mean±s.e. Statistical differences were determined using Student's two-tailed t-test.
Calculations of the affinity of steroid for the γ-cyclodextrin buffer were made with the equation (Keq=[steroid][cyclodextrin]/[complex]), where Keq is the dissociation constant. The concentration of free steroid ([steroid]) was estimated from EC50 values (or IC50 values in the case of sulphated steroids) in the presence and absence of γ-cyclodextrin. A one-to-one binding stoichiometry was assumed for the calculations.
Drugs, chemicals and other materials
Steroids without ZCM designations were from Sigma (St Louis, MO, USA). Steroids with ZCM designations and C11-NBD 3α5αP were prepared by us using multistep synthetic procedures and had spectroscopic properties (infrared spectroscopy, and both proton and carbon nuclear magnetic resonance spectroscopy) consistent with the structures given. These compounds were determined to be pure by thin layer chromatography and elemental analysis. Full details of their synthesis will be reported elsewhere. Steroids were prepared as stock solutions in dimethylsulphoxide (DMSO). Final DMSO concentration was always below 0.13%, and solutions were matched for DMSO concentration.
Results
Steroid structures
Figure 1 shows the major structural classes of steroids used and evaluated in the present work. The most obvious structural differences between naturally occurring neuroactive steroids is the stereochemistry at the A/B ring fusion that produces 5α- and 5β-reduced steroids (Figure 1a1 and a2). The different ring fusion stereochemistry produces two major diastereomer classes, both with potentiating activity at GABAA receptors.
Figure 1b shows two GABA antagonist steroids with the characteristic sulphate substitute at C3. An example of a sulphated steroid with trans (5α-reduced) A/B ring fusion is shown in Figure 1b1 and the commonly used PS, with an unsaturated A/B ring fusion is shown in Figure 1b2. GABA antagonism is also tolerant of a cis (5β-reduced) A/B ring fusion (Park-Chung et al., 1999; Wang et al., 2002). Several members of all these classes exhibit similar potency for GABAA receptor antagonism (Park-Chung et al., 1999; Wang et al., 2002).
Figure 1c–h shows other potentiating steroids with various structural modifications at C11 and C21. Figure 1c and d shows the anaesthetic alphaxalone and the naturally occurring steroid (3α,5β)-3,21-dihydroxypregnan-20-one (3α5βTHDOC). Figure 1e and f depict our synthetic steroids containing modifications at C11. Compared to 3α5αP, ZCM 32 has an additional methyl group as an axial β substitute at C11. ZCM 34 has a methyl group as an equatorial α substitute and a methoxy group as an axial β substitute at C11. Finally, in the 5α-series, ZCM 41 has a methyl group as an equatorial α substitute and a benzyloxy group as an axial β substitute at C11 (Figure 1g). C11-NBD 3α5αP has a fluorescent 7-nitro-2,1,3-benzoxadiazol-4-amino (NBD) group as a β sustitute at C11 (Figure 1h), and can serve as a marker of cellular accumulation (Akk et al., 2005). ZCM 27, ZCM 33 and ZCM 40 are the designations assigned to the 5β-reduced diastereomers of ZCM 32, ZCM 34 and ZCM 41, respectively. Overall, Figure 1 demonstrates a broad structural diversity of steroid analogues with GABAA receptor activity. We set out to test the breadth of cyclodextrin effects against these various classes of neuroactive steroids.
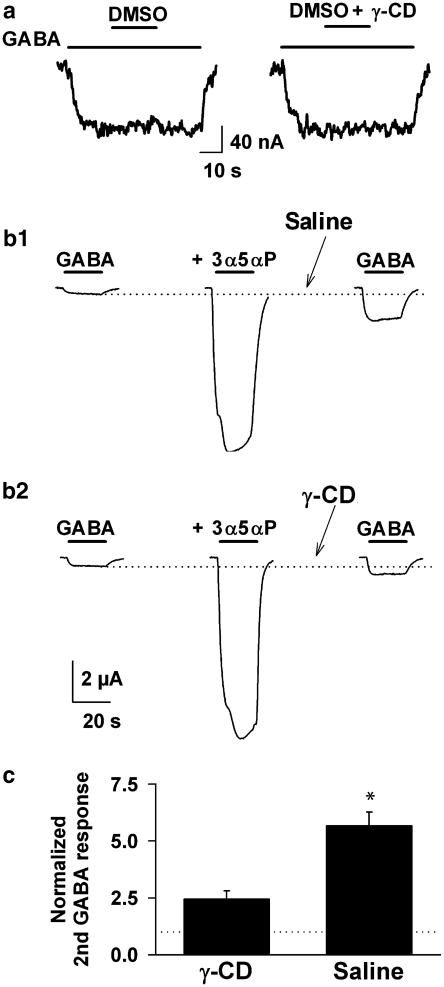
Initially, we tested some potential confounding effects of cyclodextrins on GABAA receptors. Cyclodextrins have been shown to complex DMSO, a solvent used in our steroid-containing solutions (Aree and Chaichit, 2002). If DMSO has direct effects on GABAA receptors this could confound our results. Furthermore, it was recently suggested that β-cyclodextrins may directly potentiate GABAA receptor function (Pytel et al., 2006). This effect would invalidate the use of GABAA receptor-based bioassays to assess cyclodextrin effects. To determine whether cyclodextrin, DMSO, or the combination of cyclodextrin plus DMSO affected GABAA receptor function in our primary assay, we examined currents generated in voltage-clamped Xenopus oocytes expressing α1β2γ2L receptor subunit combinations. We found that γ-cyclodextrin at the highest concentrations used in our studies had no effect on GABA-gated responses (Figure 2a). Furthermore, DMSO at 0.2%, higher than the highest working concentration we used in any experiment (0.13%), had no effect on GABA responses. γ-Cyclodextrin alone (0.5 mM) had no effect on GABA-gated currents in oocytes (0.6±6% change, n=6) or in cultured hippocampal neurons (1±4% change in steady-state current generated by 0.5 μM GABA, n=11). Furthermore, in oocytes the combination of DMSO and γ-cyclodextrin had no significant effect on GABA responses (Figure 2a).
Figure 2.
The effects of γ-cyclodextrin are induced through steroid sequestration. (a) The formation of a complex between γ-cyclodextrin and DMSO cannot explain γ-cyclodextrin effects. Neither DMSO alone nor co-application with γ-cyclodextrin affected the GABA (2 μM) responses of Xenopus oocytes expressing rat α1β2γ2L subunit combinations. (b) Cyclodextrin wash in the absence of DMSO and GABA increased recovery rate for GABA responses. (b1) Response to 2 μM GABA, and the same concentration of GABA co-applied with 10 μM 3α5αP are shown in the two left traces. The co-application trace was followed with a wash for 60 s in saline, then a re-challenge with GABA alone. Note that the response to GABA was still potentiated above the original GABA responses (dotted line). (b2) The same oocyte was re-challenged following the eventual return of the GABA response to baseline. In this case, the wash after drug application included 30 s of 0.5 mM γ-cyclodextrin followed by 30 s of plain saline. The response had been restored to much nearer the original GABA response. (c) Summary of results from six oocytes subjected to the protocol shown in (b). Wash with cyclodextrin significantly speeded recovery toward the original GABA response (* indicates P<0.05, paired t-test). The dotted line at a y-value of 1.0 indicates the original (first) GABA response, to which the other responses were normalized.
Steroids, particularly at high concentrations, can be difficult to wash out (Figure 2b1). If a primary effect of cyclodextrin is to sequester steroids, then we expect that cyclodextrin may speed washout of steroid effects. In this case, cyclodextrin is applied in the absence of GABA or DMSO solvent, so direct effects on GABAA receptor function or on solvent actions are circumvented. We tested cyclodextrin sequestration of steroid using oocytes expressing GABAA receptor subunits. We assessed baseline responses to 2 μM GABA, then responses to 2 μM GABA, co-applied with 10 μM 3α5αP. As expected, 3α5αP dramatically enhanced the GABA response. We then washed the ooycte with either a 60-s wash of saline alone or with 0.5 mM γ-cyclodextrin for 30 s, followed by saline alone for an additional 30 s. GABA responses were then re-assessed in the absence of steroid and γ-cyclodextrin. The cyclodextrin wash clearly accelerated recovery of the original, non-potentiated GABA response amplitude (Figure 2b and c). These results are consistent with steroid-sequestering actions of cyclodextrin.
To provide another assay, completely independent of GABAA receptor function, we examined a fluorescently tagged version of 3α5αP (Figure 1h) in imaging experiments. This steroid has a NBD group attached at carbon 11 of the steroid. We previously found that this compound potentiates GABA responses, but with reduced potency compared with the parent 3α5αP (Akk et al., 2005). Consistent with sequestration by γ-cyclodextrin (but in contrast to the lack of effect of cyclodextrin on GABAA receptor function), we found that γ-cyclodextrin (0.5 mM) significantly reduced the potentiation generated by 2 μM C11-NBD 3α5αP (Figure 3a1). This inhibitory effect on potentiation was more marked against the parent natural steroid 3α5αP (Figure 3a2 and b), suggesting that substitutions at C11 may hinder inclusion complex formation (see also Figure 7). Nevertheless, C11-NBD 3α5αP was sufficiently sensitive to cycodextrin to allow us to perform imaging experiments on the fluorescent steroid in the absence and presence of γ-cyclodextrin.
Figure 3.
Direct imaging of a fluorescent steroid analogue suggests sequestration by γ-cyclodextrin explains reduced GABA responses. (a) Comparison of the effect of γ-cyclodextrin on equimolar concentrations of C11-NBD 3α5αP and 3α5αP. (a1) An oocyte responded to GABA (2 μM) and co-application of GABA and 2 μM C11-NBD 3α5αP. γ-Cyclodextrin (0.5 mM) partially reversed the effect of the tagged steroid. (a2) By contrast, 3α5αP (2 μM) more dramatically potentiated the GABA response, but γ-cyclodextrin more fully reversed the potentiation. (b) Summary of the protocol in (a) performed on four oocytes. Potentiation refers to the fold increase in GABA response, with 1.0 representing a doubling. The inhibition of 3α5αP potentiation was significantly larger than inhibition of C11-NBD 3α5αP potentiation (P< 0.05). (c) Imaging of C11-NBD 3α5αP applied to neurons in culture in the absence (two left panels, two right panels) and presence (two middle panels) of 0.5 mM γ-cyclodextrin. The reduction in fluorescence of the cells during co-application of steroid and cyclodextrin was consistent with sequestration of steroid by cyclodextrin. Images were taken after a 5 min pre-equilibration with steroid (left two panels), 15 s after addition of γ-cyclodextrin to the steroid-containing solution (γ-CD; middle panels) and 15 s after re-applying steroid alone. Interval between successive panels in the same experimental condition was 5 s.
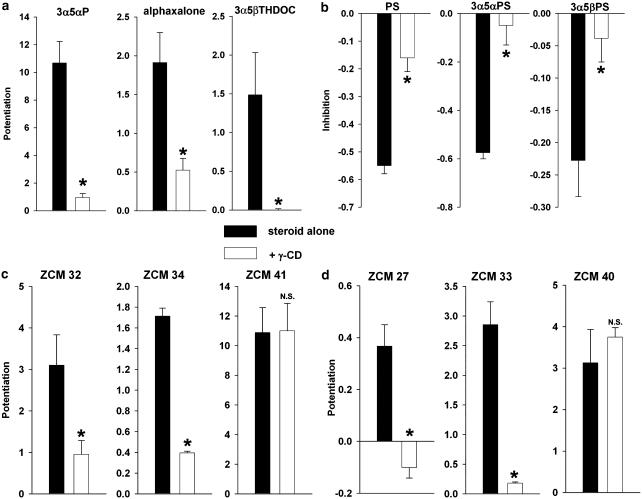
Figure 7.
Cyclodextrin effects on neuroactive steroid potentiation and inhibition. (a) Neuroactive steroid potentiators were used to modulate responses to 2 μM GABA, 3α5αP at 0.5 μM, alphaxalone at 0.5 μM and 3α5βTHDOC at 0.5 μM. Co-application with 0.5 mM γ-cyclodextrin (γ-CD) significantly blocked potentiation (n=4–13 for each experiment, asterisks indicate P<0.05 compared to steroid alone). (b) Neuroactive steroid inhibitors were used to antagonize responses to 30 μM GABA, PS at 1 μM, (3α,5α)-3-hydroxypregnan-20-one sulphate (3α5αPS) at 1 μM, and 1 μM (3α,5β)-3-hydroxypregnan-20-one sulphate (3α5βPS). Co-application with 0.5 mM γ-cyclodextrin significantly blocked inhibition. (c and d) The interaction of synthetic 5α- and 5β-reduced neuroactive steroids and cyclodextrins. (c) Synthetic 5α-reduced neuroactive steroids with small to large substitutions at C11 (ZCM 32<ZCM 34<ZCM 41) were applied at 0.25 μM with 2 μM GABA. Co-application with 0.5 mM γ-cyclodextrin blocked potentiation in steroids with the smaller substitution at C11 but failed to block potentiation of ZCM 41, which contains a large aromatic substitution at C11. (d) Synthetic 5β-reduced neuroactive steroids in the same series (ZCM 27<ZCM 33<ZCM 40). Cyclodextrin blocked potentiation of the smaller steroids but not that of the larger steroids. The mean potentiation was apparently reduced below zero in the case of ZCM 27, but this appeared to be due to nonspecific small rundown of the GABA response in several cells, and the depression below zero was not statistically significant (P>0.05). In (b, c and d) n=8, asterisks indicate P<0.05 compared with steroid alone, NS indicates not significant.
We reasoned that if γ-cyclodextrin works by inclusion complex formation, then it should reduce cellular fluorescence when co-applied with C11-NBD 3α5αP. We used cultured hippocampal neurons for these studies because of their amenability to imaging and to rapid solution exchange. Figure 3c shows that C11-NBD 3α5αP accumulated in neurons and that simultaneously applied γ-cyclodextrin reduced this accumulation. Because γ-cyclodextrin is membrane impermeant but the neuronal fluorescence is largely intracellular, these data suggest that the intracellularly accumulated steroid is in relatively rapid equilibrium with the plasma membrane, which in turn is directly accessible to γ-cyclodextrin. Taken together, the results of Figures 2 and 3 confirm the validity of our GABAA receptor-based bioassay for cyclodextrin-steroid complex formation. The results suggest that interactions between steroid and γ-cyclodextrin arise directly from the ability of cyclodextrin to complex and sequester steroid. It is unlikely that the effect of γ-cyclodextrin is mediated by a direct action on the receptor or due to unanticipated interactions with DMSO.
5β-Reduced neuroactive steroids and cyclodextrins
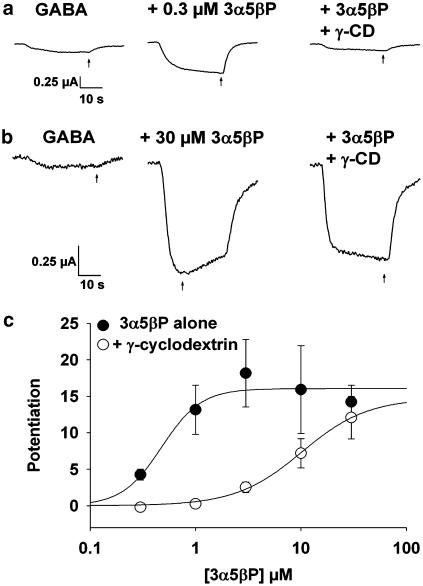
Figures 2 and 3 suggest that γ-cyclodextrin effectively complexes 3α5αP, consistent with our previous results (Shu et al., 2004). To test whether the structural change induced by a 5β reduction at C5 of the steroid affects the ability of cyclodextrins to complex neurosteroids, we performed experiments with 3α5βP and γ-cyclodextrin in Xenopus oocytes for comparison with our previous results. We challenged oocytes expressing the α1β2γ2L subunit combination with GABA (2 μM) solutions containing potentiating steroid alone (0.3–30 μM) or potentiating steroid plus 0.5 mM γ-cyclodextrin, pre-mixed in the perfusion solution. Figure 4a shows a response in which 0.3 μM 3α5βP increased the GABA response 3.9-fold, and 0.5 mM γ-cyclodextrin fully reversed the potentiation (to 94% of the original GABA response). By contrast, in the example of Figure 4b, 30 μM 3α5βP increased the GABA response 8.5-fold, which fell only slightly to 7.1-fold in the presence of γ-cyclodextrin. In some oocytes, particularly at low steroid concentration, we observed two phases (fast and slow) of potentiation elicited by co-application of steroid and GABA. We noticed no differential effect of cyclodextrin on these two phases of potentiation.
Figure 4.
Cyclodextrin binds 5β-reduced potentiating steroids. (a) The effect of γ-cyclodextrin co-applied with a low concentration of 3α5βP (0.3 μM). (b) The effect of γ-cyclodextrin at a saturating concentration of 30 μM 3α5βP. (c) The concentration–response curve of 3α5βP was obtained in the presence and absence of 0.5 mM γ-cyclodextrin. The lines represent fits of the Hill equation to the averaged data points shown in the figure. Parameters from fits to the Hill equation were maximum response: 16.0-fold (baseline) vs 14.7 fold (cyclodextrin), EC50: 0.5 μM (baseline) vs 10.1 μM (cyclodextrin), Hill co-efficient: 2.3 (baseline) vs 1.4 (cyclodextrin); n=5 oocytes. In statistics performed on individual oocytes (see Results), the difference in Hill coefficient was not statistically significant.
To quantify the apparent binding affinity between 3α5βP and γ-cyclodextrin, we created concentration–response curves for 3α5βP in the absence and presence of 0.5 mM γ-cyclodextrin (Figure 4c). The presence of cyclodextrin shifted the 3α5βP EC50 from 0.5±0.4 μM (absence of γ-cyclodextrin) to 9.5±0.7 μM (presence of 0.5 mM γ-cyclodextrin, P<0.01, paired t-test, n=5) with similar predicted maximum potentiation (16.1±3.3, baseline vs 13.6±3.4, cyclodextrin) and no significant effect of cyclodextrin on the Hill slope (P>0.3, paired t-test, n=5). The EC50 shift was slightly larger than the shift in 3α5αP potentiation that we obtained previously, where the EC50 shifted from 1.1 to 9.2 μM (Shu et al., 2004). The shifts in EC50 for these two steroids, assuming a one-to-one binding stoichiometry (see Methods), predict affinities for cyclodextrin of 27.3 μM (3α5βP) and 66.8 μM (3α5αP). We conclude that inclusion complexes readily form between 5β-reduced steroids and γ-cyclodextrin and that γ-cyclodextrin is a similar blocker of the effects of both 5α- and 5β-reduced steroids (Shu et al., 2004).
Functional reversal of steroid effects by γ-cyclodextrin
If cyclodextrins are to be useful for acutely reducing steroid effects in situ, then their effects on neuronal function are of interest. To determine whether γ-cyclodextrin sequesters steroid effects at neuronal synapses, we examined recurrent, autaptic GABA ipscs generated in microcultures of hippocampal neurons. γ-Cyclodextrin applied to naïve neurons had no effect on the peak amplitude of the ipsc (2±2% depression from a mean baseline amplitude of 7.1±2.0 nA, n=11 GABAergic neurons) and a modest effect on the decay time course of ipscs (15±5% decrease in the 10–90% decay time: 336±63 to 271±46 ms, P=0.04, paired t-test, n=11 cells). This effect was clear and reversible in some cells (Figure 5a) but not apparent in others, and might result either from an endogenous steroid-like tone or another nonspecific effect on pre-synaptic or post-synaptic function. We found no effect of 0.5 mM γ-cyclodextrin on amplitude or decay of 2 glutamatergic neurons tested (data not shown).
Figure 5.
Functional use of γ-cyclodextrin to reverse exogenous steroid effects in neurons. (a) The ipscs generated from autaptic hippocampal neurons cultured in synaptic isolation. γ-Cyclodextrin (0.5 mM) has little effect on ipscs under basal conditions. (b) In the same cell as in (a), the ipsc is prolonged by 100 nM 3α5αP in the absence, but not in the presence of 0.5 mM γ-cyclodextrin. The dotted trace is a re-plot of the baseline ipsc from (a).
We reasoned that if an endogenous steroid is responsible for the very small effect of γ-cyclodextrin on ipsc decays, we might be able to augment the effect of cyclodextrin by blocking 3α-hydroxysteroidoxidoreductase enzymes responsible for metabolizing neurosteroids (Belelli and Herd, 2003). However, in three neurons incubated for >15 min in 1 μM of medroxyprogesterone, a potent 3α-hydroxysteroidoxidoreductase inhibitor (Belelli and Herd, 2003), we failed to find any effect of γ-cyclodextrin on ipsc decays. These results, although somewhat inconclusive, are consistent with our previous findings that levels of endogenous neurosteroids are too low in dissociated cell culture to measure reliably by bioassay (Xu et al., 2000).
In contrast to modest and variable basal effects of γ-cyclodextrin on synaptic function, effects of exogenous 3α5αP were dramatically reduced when it was co-applied with γ-cyclodextrin (Figure 5b). In four isolated GABAergic neurons from which we elicited recurrent ipscs, 100 nM 3α5αP potentiated 10–90% decay time by 98±33% (504±143 ms 10–90% decay time at baseline to 905±182 ms in the presence of 100 nM 3α5αP). Co-application of 0.5 mM γ-cyclodextrin reversed the 3α5αP-mediated slowing of the ipsc decays (450±109 ms; Figure 5b). These results demonstrate that at least acutely, γ-cyclodextrin can be used to manipulate steroid effects relatively selectively without grossly affecting basal pre-synaptic and post-synaptic function.
γ-Cyclodextrin effects on PS
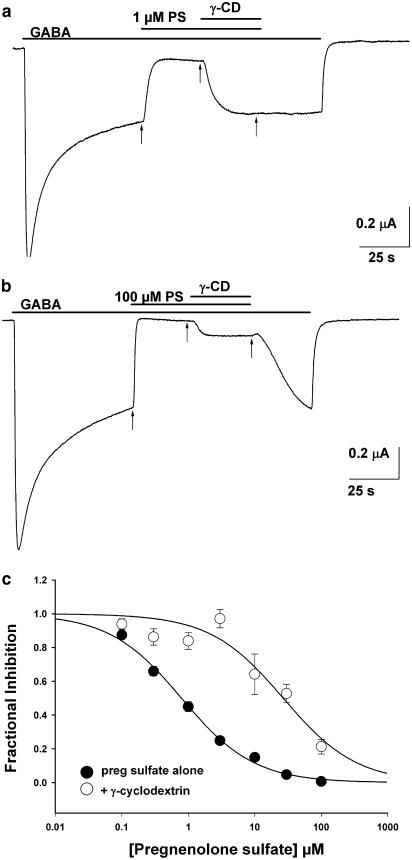
We tested the ability of sulphated neuroactive steroids to form complexes with γ-cyclodextrin to determine whether a sulphate group at C3 affects this. Our goal in these experiments was to compare sulphated steroids directly with the non-sulphated potentiating steroids, using inhibition and potentiation, respectively, as bioassays for the efficiency of complex formation. Sulphated steroids typically exhibit significantly less potency for their inhibiting actions than 3α-hydroxysteroids exhibit for potentiation, thus making direct comparisons difficult. However, because the IC50 for sulphated steroid inhibition is dependent on the GABA concentration (Eisenman et al., 2003), we were able to titrate GABA concentration to yield an IC50 for sulphated steroid inhibition similar to the EC50's found for potentiating steroids (∼0.5–1 μM; note that the EC50 for steroid potentiation in oocytes is higher than in native cells (Shu et al., 2004) for reasons that are unclear). Pilot experiments showed a GABA concentration of 30 μM to be appropriate, yielding an IC50 concentration for inhibition of PS of 0.8 μM (Figure 6c and Eisenman et al., 2003). Figure 6a shows a typical example in which 1 μM PS depressed the GABA response in the absence of cyclodextrin. In this example, the degree of depression of sustained GABA response was larger (83%) than the mean depression (Figure 6c), yet co-application of PS and γ-cyclodextrin reversed the inhibition to 90% of the original sustained GABA response. By contrast, 100 μM PS abolished the sustained GABA response (99% reduction), and the reversal of PS effects by 0.5 mM γ-cyclodextrin was weak (83% depressed relative to the sustained GABA response; Figure 6b). As with potentiating steroids, the concentration–response curve of PS was shifted to the right by γ-cyclodextrin (Figure 6c). When data from individual cells were fitted with the Hill equation, the IC50 for PS shifted from 0.9±0.2 μM in the absence of γ-cyclodextrin to 27.4±6.9 μM when co-applied with γ-cyclodextrin (P<0.01, Figure 6c). This shift in IC50 predicts a dissociation constant for the PS/cyclodextrin interaction of 16.1 μM, similar to the range of affinities estimated for potentiating steroids (see above).
Figure 6.
PS and cyclodextrins. (a) Effect of γ-cyclodextrin on a low (1 μM) concentration of PS during the steady-state response of an oocyte to 30 μM GABA. Co-application with 0.5 mM γ-cyclodextrin (γ-CD) completely reversed PS inhibition. (b) The effect of γ-cyclodextrin at a saturating concentration of 100 μM PS. Arrows in (a) and (b) indicate the time points from which measurements were taken. (c) The concentration–response curve of PS was shifted to the right in the presence of γ-cyclodextrin compared with the curve in the absence of 0.5 mM γ-cyclodextrin. The solid line indicates a fit of the Hill equation to the average data points shown in the Figure. IC50 of PS in the presence of γ-cyclodextrin shifted from 0.8 μM (baseline) to 27 μM (in the presence of 0.5 mM γ-cyclodextrin) (n=15).
Cyclodextrin effects on other commonly used neuroactive steroids
Figure 7a summarizes the effect of 0.5 μM of various commonly used neuroactive steroids applied alone or with 0.5 mM γ-cyclodextrin. It is clear that several chemical substitutions are unimportant for the ability of inclusion complexes to form between γ-cyclodextrin and steroid. Notably, substitutions at C11 in alphaxalone or C21 in 3α5βTHDOC (see Figure 1) were unimportant for the ability of cyclodextrin to sequester steroid (Figure 7a). For steroid-mediated inhibition of GABA currents, substitution of a pregnane backbone for the pregnene backbone of PS (Figure 1b) did not appreciably affect the ability of γ-cyclodextrin to interact with sulphated steroids (Figure 7b). As with 3α-hydroxysteroids, 5β reduction of sulphated steroids (3α5βPS), a 5β A/B ring fusion, did not affect complex formation (Figure 7b). We conclude that commonly used neuroactive steroids active at GABAA receptors are effectively sequestered by γ-cyclodextrin.
Interaction of synthetic 5α-reduced and 5β-reduced neuroactive steroids with γ-cyclodextrin
Based on previous structural information about steroid–cyclodextrin inclusion complexes (Adam et al., 2002) and our own data suggesting that an NBD substitution at C11 reduced inclusion complex formation (Figure 3a and b), we hypothesized that the ability of steroids to form inclusion complexes with cyclodextrin would be particularly sensitive to substituents located on the steroid C-ring. We tested several synthetic steroid analogues with substitutions at C11 (Figure 1e–g; ZCM compound series). All of these compounds retained at least some potentiation at GABAA receptors, allowing us to assay presumed inclusion complex formation. In the 5α-reduced series, we found that all but the largest substitutions at C11 still permitted γ-cyclodextrin modulation (Figure 7c). For 5β-reduced steroids, compounds containing the same structural modification at C11 (Figure 7d; ZCM 27, ZCM 33, ZCM 40) were also studied. We found that γ-cyclodextrin effects for both the 5α- and 5β-reduced series of C11-modified steroids were similar (Figure 7c vs d), confirming that 5β reduction does not affect the ability of inclusion complexes to form, nor does 5β reduction appear to interact with C11 modifications to influence inclusion complex formation.
Differences in rate-limiting factors for potentiating and inhibitory steroids
We have recently suggested that cellular retention of steroid unbound to GABAA receptors is responsible for long-lived currents directly gated by GABA-active steroids in the absence of GABA (Shu et al., 2004). The results presented here (Figure 2b) suggest that removal of potentiation is also cyclodextrin-sensitive, implying that non-receptor-based cellular retention rate limits the potentiating effects of steroids. Does cellular retention also rate limit the effects of steroidal GABAA receptor antagonists? To determine directly and quantitatively whether cellular retention is responsible for rate-limiting steroid potentiation and inhibition of GABA responses by 3α-hydroxysteroids and sulphated steroids, respectively, we examined whether γ-cyclodextrin could speed recovery from the effects of the two classes of steroids at GABAA receptors. We used hippocampal neurons for these studies to optimize solution exchange times.
Consistent with our previous results from studying interactions between 3α-hydroxysteroids and GABAA receptors in the absence of GABA, we found that the recovery from the potentiating actions of 3α-hydroxysteroids was sensitive to wash with γ-cyclodextrin (Figure 8a). Responses to GABA (0.5–2 μM) were potentiated by 300 nM 3α5αP by 9.2±2.3-fold. Upon removal of steroid, the relaxation from the potentiated response back towards the original GABA response was described by a time constant of 30.6±12.6 s (n=11). This was decreased to 0.9±0.1 s in the same cells washed with GABA plus γ-cyclodextrin (Figure 8a, P<0.05).
A similar protocol employing 2 μM GABA and 5 μM PS was used to study the removal of steroid inhibition. PS inhibited GABA responses by 75±3%. Recovery from inhibition was much faster than recovery from potentiation (τ=3.7±0.6 s, n=5), and this recovery was not affected by the presence of γ-cyclodextrin (τ=4.6±0.6, P>0.05, Figure 8b). Taken together, these results suggest that similar factors (i.e. cellular retention of steroid) are responsible for rate limiting both major actions of 3α-hydroxysteroids: potentiation of GABA-gated currents and direct activation of receptors in the absence of GABA. A different factor, probably the dissociation rate of steroid from receptors, is responsible for rate limiting the effect of the sulphated inhibitory steroids.
Discussion and conclusions
We have tested the ability of cyclodextrins functionally to sequester neuroactive steroids. We found that all commonly used neuroactive steroids that we tested exhibited approximately equivalent binding to γ-cyclodextrin, assayed using GABA currents in oocytes expressing recombinant GABAA receptor subunits. Neither the configuration of the A/B ring fusion nor addition of sulphate groups to pregnene or pregnane steroid backbones affected cyclodextrin binding. Large, hydrophobic substituents at C11, however, affected the ability of γ-cyclodextrin to bind steroid. We conclude that γ-cyclodextrin is a useful, albeit nonspecific, tool for terminating the actions of multiple classes of neuroactive steroids.
In the course of these studies, we determined several novel characteristics of the interaction of steroids with GABAA receptors, and several features of γ-cyclodextrin-mediated sequestration of neuroactive steroids. Firstly, we directly showed that γ-cyclodextrin, acutely applied, functionally reversed effects of exogenous steroid at synapses, with little influence on presynaptic or postsynaptic function at baseline. Secondly, we showed that different factors govern the time course of the two major classes of neuroactive steroids at GABAA receptors. GABAA receptor potentiation (present work), like direct gating (Shu et al., 2004; Akk et al., 2005), is rate limited by accumulation in cyclodextrin-accessible reservoirs rather than by inherent drug dissociation rates from receptors, which are not influenced by γ-cyclodextrin. In contrast, the offset rate of GABA inhibition by sulphated steroids is faster than potentiation offset, and is governed by a cyclodextrin-inaccessible mechanism, probably the drug–receptor dissociation rate. This conclusion is consistent with the negative charge on PS, which probably prevents this steroid from accumulating in the same reservoirs, including intracellular compartments, that 3α5αP accesses. This finding is also consistent with our previous suggestion that rates of block by sulphated steroids are influenced by receptor conformation (Eisenman et al., 2003) rather than by cellular retention.
Nonspecific effects need to be carefully considered when working with cyclodextrins. Cyclodextrins have been used for other purposes, such as extracting cholesterol from membranes (Yancey et al., 1996; Haynes et al., 2000; Westover et al., 2003), for sponging lipophilic fluorescent markers from plasma membranes (Kay et al., 1999), for terminating the actions of peripherally active steroids (Adam et al., 2002) and for complexing solvents like DMSO (Aree and Chaichit, 2002). We focused our analysis on γ-cyclodextrin, because it appears to be less effective than β-cyclodextrins at removing cholesterol from membranes (Ohtani et al., 1989), thus reducing the possibility that effects on membrane cholesterol might contribute to our results.
β-Cyclodextrins have recently been suggested to have direct potentiating effects on GABAA receptors (Pytel et al., 2006), which would confound our results. However, we have found that γ-cyclodextrin alone does not affect responses to exogenous GABA in either hippocampal neurons or oocytes (Shu et al., 2004) and does not potentiate non-equilibrium synaptic GABA responses. Also, our results have eliminated an important potentiating effect of γ-cyclodextrin on synaptic, non-synaptic and recombinant α1β2γ2L receptors. It is possible that subunit combinations not identified in our hippocampal cultures or oocytes are potentiated by the β-cyclodextrin used by Pytel et al. (2006). It is also possible that patch excision in the fast application studies of Pytel et al. (2006) renders receptors sensitive to β-cyclodextrin. However, preliminary results suggest that up to 5 mM methyl-β cyclodextrin has no effect on intracluster channel kinetics of α1β2γ2L receptors in cell attached patches (G Akk, personal communication). Although only structural methods such as nuclear magnetic resonance or crystallography can definitively determine inclusion complex formation, our results, including control experiments (data shown in Figures 2 and 3), strongly support our conclusion that the attenuation of the steroid effects results directly from cyclodextrin–steroid interactions rather than from direct effects of cyclodextrin on GABAA receptors or other targets.
Cyclodextrins are also used to dissolve and deliver steroids systemically (Wallimann et al., 1997). At first glance, this use of cyclodextrins seems paradoxical in the light of our demonstration that cyclodextrins inhibit the actions of steroids. How can cyclodextrins be effective delivery agents if they inhibit the ability of the drug to act, as shown in the present work? It is important to consider the differences in paradigms when considering the different uses of cyclodextrins. Cyclodextrins are often used to deliver steroids by dissolving a high ratio of steroid to cyclodextrin. The low affinity of the steroid for cyclodextrin (15–60 μM estimated in the Results) thus favours delivery of high steroid concentrations to tissues. In vivo cyclodextrin complexes may also be protected from enzymatic degradation, imparting some of their usefulness as drug delivery vehicles (Wallimann et al., 1997). In our in vitro assay, excess free cyclodextrin will out-compete receptors and lipophilic reservoirs for steroid retention. It should be noted that delivery of free cyclodextrins in vivo has been used to speed recovery from the steroid neuromuscular blocking agent rocuronim (de Boer et al., 2006; Sorgenfrei et al., 2006).
Assayed by phase solubility, dissociation constants of steroids for cyclodextrins range considerably (Wallimann et al., 1997). Our bioassay of neuroactive steroids showed that over a relatively wide range of structures, neuroactive steroids have a similar affinity for γ-cylcodextrin, but this affinity is reflected in estimated dissociation constants of tens of micromolar. Thus, sequestration of steroids by γ-cyclodextrin under the conditions of our experiments is a relatively low-affinity interaction, and this limitation needs to be considered in potential uses of cyclodextrins.
In summary, we have found that cyclodextrins are useful tools for reversing the effects of a wide range of neuroactive steroids, including both potentiators and inhibitors of GABAA receptor function. The utility of cyclodextrins is limited by their effects on other cellular processes, notably the cholesterol content of membranes. However, in the short term, we have directly demonstrated that cyclodextrins are useful for functionally sequestering steroids, as they have few effects during brief exposures on other cellular processes such as those governing acute signalling. Further, we have used cyclodextrin to elucidate differences in the mechanisms governing the time course of steroid potentiation vs inhibition of GABA actions.
Acknowledgments
We thank Alex Evers, Joe Henry Steinbach, Gustav Akk, Keith Isenberg, and lab members for discussion. We thank Ann Benz and Amanda Taylor for technical help. This work was supported by GM47969 (DFC, CFZ), AA12951 (CFZ), AA12952 (SM), and a grant from the Bantly Foundation (CFZ).
Abbreviations
- γ-CD
γ-cyclodextrin
- DMSO
dimethylsulphoxide
- NBD
7-nitro-2,1,3-benzoxadiazol-4-amino
- 3α5αP
(3α,5α)-3-hydroxypregnan-20-one
- 3α5βP
(3α,5β)-3-hydroxypregnan-20-one
- PS
pregnenolone sulphate
- 3α5βTHDOC
(3α,5β)-3,21-dihydroxypregnan-20-one
Conflict of interest
The authors state no conflict of interest.
References
- Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure–activity relationships. J Med Chem. 2002;45:1806–1816. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, et al. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aree T, Chaichit N. Crystal structure of β-cyclodextrin-dimethylsulfoxide inclusion complex. Carbohydrate Res. 2002;337:2487–2494. doi: 10.1016/s0008-6215(02)00335-x. [DOI] [PubMed] [Google Scholar]
- Barker JL, Harrison NL, Lange GD, Owen DG. Potentiation of γ-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids. J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc London B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer HD, van Egmond J, van de Pol F, Bom A, Booij LH. Reversal of profound rocuronium neuromuscular blockade by sugammadex in anesthetized rhesus monkeys. Anesthesiology. 2006;104:718–723. doi: 10.1097/00000542-200604000-00016. [DOI] [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S. Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J Physiol. 2003;550:679–691. doi: 10.1113/jphysiol.2003.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MP, Phillips MC, Rothblat GH. Efflux of cholesterol from different cellular pools. Biochemistry. 2000;39:4508–4517. doi: 10.1021/bi992125q. [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, et al. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion B, Wang M, Shute AA, et al. Selective antagonism of 5α-reduced neurosteroid potentiation of GABAA receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186:17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Phillipps GH. Structure–activity relationships in steroidal anaesthetics. J Steroid Biochem. 1975;6:607–613. doi: 10.1016/0022-4731(75)90041-2. [DOI] [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, et al. On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Pytel M, Mercik K, Mozrzymas JW. Interaction between cyclodextrin and neuronal membrane results in modulation of GABAA receptor conformational transitions. Br J Pharmacol. 2006;148:413–422. doi: 10.1038/sj.bjp.0706747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- Redenti E, Szente L, Szejtli J. Cyclodextrin complexes of salts of acidic drugs. Thermodynamic properties, structural features, and pharmaceutical applications. J Pharm Sci. 2001;90:979–986. doi: 10.1002/jps.1050. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Zeng CM, Wang C, Covey DF, Zorumski CF, Mennerick S. Cyclodextrins form inclusion complexes with multiple classes of steroids. Soc Neurosci Abstr. 2005;261:9. [Google Scholar]
- Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104:667–674. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Barros HM, Manitiu A, Miczek KA. Ultrasonic vocalizations in rat pups: modulation at the γ-aminobutyric acidA receptor complex and the neurosteroid recognition site. J Pharmacol Exp Ther. 1997;282:318–325. [PubMed] [Google Scholar]
- Wallimann P, Marti T, Furer A, Diederich F. Steroids in molecular recognition. Chem Rev. 1997;97:1567–1608. doi: 10.1021/cr960373b. [DOI] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, et al. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Wahlstrom G, Backstrom T. Pregnenolone sulphate and pregnenolone do not interact with 5β-pregnanolone- and hexobarbitone-induced anaesthesia in the rat. Br J Anaesth. 1997;78:328–331. doi: 10.1093/bja/78.3.328. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cormier R, Fu T, Covey DF, Isenberg KE, Zorumski CF, et al. Slow death of postnatal hippocampal neurons by GABAA receptor overactivation. J Neurosci. 2000;20:3147–3156. doi: 10.1523/JNEUROSCI.20-09-03147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Rodrigueza WV, Kilsdonk EP, Stoudt GW, Johnson WJ, Phillips MC, et al. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J Biol Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]