Abstract
Background and purpose:
Recently, the use of inhaled insulin formulations for the treatment of type I and type II diabetes has been approved in Europe and in the United States. For regular use, it is critical that airway function remains unimpaired in response to insulin exposure.
Experimental approach:
We investigated the effects of insulin on airway smooth muscle (ASM) contraction and contractile prostaglandin (PG) production, using guinea-pig open-ring tracheal smooth muscle preparations.
Key results:
It was found that insulin (1 nM-1 μM) induced a concentration-dependent contraction that was insensitive to epithelium removal. These sustained contractions were susceptible to inhibitors of cyclooxygenase (indomethacin, 3 μM), Rho-kinase (Y-27632, 1 μM) and p42/44 MAP kinase (PD-98059, 30 μM and U-0126, 3 μM), but not of PI-3-kinase (LY-294002,10 μM). In addition, insulin significantly increased PGF2α-production which was inhibited by indomethacin, but not Y-27632. Moreover, the FP-receptor antagonist AL-8810 (10 μM) and the EP1-receptor antagonist AH-6809 (10 μM) strongly reduced insulin-induced contractions, supporting a pivotal role for contractile prostaglandins.
Conclusions and implications:
Collectively, the results show that insulin induces guinea-pig ASM contraction presumably through the production of contractile prostaglandins, which in turn are dependent on Rho-kinase for their contractile effects. The data suggest that administration of insulin as an aerosol could result in some acute adverse effects on ASM function.
Keywords: insulin, contraction, airway smooth muscle, Rho-kinase, guinea-pig, prostaglandins
Introduction
At present, the predominant mode of insulin administration is by subcutaneous (s.c.) injection, which is considered a major burden to diabetic patients (Hunt et al., 1997; Zambanini et al., 1999). Consequently, much effort is directed at developing new insulin delivery technologies, with inhaled formulations representing one strategy being recently approved in Europe and the United States for the treatment of type I and II diabetes (Lenzer, 2006). Several phase 2/3 trials on the use of inhaled insulin formulations in these patients have revealed comparable results to s.c. insulin regarding efficacy and toleration (Hollander et al., 2004; Quattrin et al., 2004; Garg et al., 2006). Although effects of insulin on forced expiratory volume in 1 s (FEV1) have been reported, both in type I (Quattrin et al., 2004) and type II diabetic (Hollander et al., 2004) patients, these effects were considered to be small and not clinically significant. However, diabetic patients suffering from respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), were excluded from these studies. Of importance, inhaled stimuli that cause no or a small fall in FEV1 in healthy subjects can induce significant effects in patients suffering from asthma and COPD (Fuller et al., 1987; Joos et al., 1987; Polosa and Holgate, 1990).
Although some epithelium-mediated relaxant effects of insulin on precontracted ASM have been described (Papayianni et al., 2001), acute contractile effects of insulin on airway myogenic tone have thus far not been determined. Other growth factors, including insulin-like growth factor-1 (IGF-1), angiotensin II, platelet-derived growth factor (PDGF) and epidermal growth factor (EGF), have been shown to induce airway smooth muscle (ASM) contraction of human and guinea-pig airway preparations in vitro (Gosens et al., 2004a; Schaafsma et al., 2005). Such contractions were found to be dependent on the production of contractile prostaglandins, not derived from the epithelium, and moreover these contractions were largely mediated by Rho-kinase (Schaafsma et al., 2005). As insulin also binds to and activates receptors with intrinsic tyrosine kinase activity, we hypothesized that insulin may have acute effects on ASM tone.
Methods
Animals
Outbred specified pathogen-free male Dunkin Hartley guinea-pigs (Harlan, Heathfield, UK), weighing 500–700 g, were used in this study. All protocols described in this study were approved by the University of Groningen Committee for Animal Experimentation.
Isometric tension measurements
After the animals had been killed by a blow to the head and rapid exsanguination the trachea was removed and transferred to Krebs–Henseleit (KH) buffer solution (composition in mM: NaCl 117.5, KCl 5.6, MgSO4 1.18, CaCl2 2.5, NaH2PO4 1.28, NaHCO3 25.00 and D-glucose 5.55; pregassed with 95% O2 and 5% CO2; pH 7.4) at 37°C. The trachea was carefully prepared free of serosa and connective tissue. In some cases, the airway epithelium was carefully removed by gently rubbing the mucosal side of the trachea using a 15-cm woollen thread. Epithelium removal was confirmed by histological examination after fixating cryostat sections (5 μm) in acetone and staining with haematoxylin eosin as shown previously (Schaafsma et al., 2005). Single open-ring tracheal preparations, exclusively obtained from the mid-section of the trachea, were prepared and mounted for isometric tension recording, using Grass FT-03 transducers, in 20 ml water-jacketed organ baths (37°C) containing KH solution. During a 90 min equilibration period, with washouts every 30 min, resting tension was gradually adjusted to 0.5 g. Subsequently, the preparations were precontracted with 20 and 40 mM KCl. Following two washouts, maximal relaxation was established by the addition of 0.1 μM isoprenaline to determine inherent tone. Tension was then re-adjusted to 0.5 g, immediately followed by two changes of fresh KH-buffer. After another equilibration period of 30 min, insulin (1, 10 nM, 0.1 or 1 μM) was applied or a cumulative concentration–response curve was constructed to stepwise increasing concentrations of histamine (1 nM–100 μM). When maximal histamine-induced contraction or insulin-induced steady state contraction was obtained, the tracheal rings were washed several times and maximal relaxation was established again using isoprenaline. When used, selective and effective concentrations of the inhibitors of Rho-kinase (Y-27632, 1 μM) (Uehata et al., 1997; Schaafsma et al., 2004, 2005; Gosens et al., 2006), p42/p44 MAPK-kinase (PD-98059, 30 μM; U-0126, 3 μM) (Davies et al., 2000; Hallsworth et al., 2001; Schaafsma et al., 2005), PI-3-kinase (LY-294002, 10 μM) (Gosens et al., 2002, 2004b; Tsang et al., 2002) or COX (indomethacin, 3 μM) (Range et al., 2000; Schaafsma et al., 2005) and antagonists for the FP-receptor (AL-8810, 10 μM) (Griffin et al., 1999; Kelly et al., 2003) and EP1-receptor (AH-6809, 10 μM) (Catalli and Janssen, 2004) were applied to the organ bath 30 min before agonist addition.
Measurement of prostaglandin F2α production
Contractile prostaglandin production was assessed as described previously (Schaafsma et al., 2005). In brief, guinea-pig tracheal rings were incubated using a 24-well plate at 37°C. Each well contained 1 ml KH-buffer and four tracheal rings. Following a 30 min preincubation period in the presence or absence of indomethacin (3 μM) or Y-27632 (1 μM), 100 μl of the medium was taken as the first sample. Subsequently, insulin (10 μM) was applied. Samples were collected at 30 min after insulin-addition. Sampling was performed under a 95% O2/5% CO2 atmosphere. PGF2α-production was determined using an ELISA-assay according to the manufacturer's protocol (R&D Systems, UK).
Data analysis
Unless indicated otherwise, insulin-induced contractions were related to maximal histamine-induced contraction, as determined each time in at least two parallel preparations from the same animal. Maximal histamine-induced guinea-pig tracheal smooth muscle contraction has been shown to be independent of basal (inherent) tone (Schaafsma et al., 2004). Therefore, basal tone, as assessed in each preparation, was expressed as a percentage of the maximum response to histamine (100 μM). All data represent means±s.e.m. from n separate animals. Responses for each animal were determined in duplicate. Statistical significance of difference was determined by the Student's t-test for paired observations. Differences were considered significant when P<0.05.
Materials
Insulin (from bovine pancreas), indomethacin, histamine dihydrochloride and (−)-isoprenaline hydrochloride were obtained from Sigma Chemical Co. (St Louis, MO, USA). (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide (Y-27632), 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD-98059), 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U-0126), 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY-294002) and 6-isopropoxy-9-xanthone-2-carboxylic acid (AH-6809) were obtained from Tocris Cookson Ltd. (Bristol, UK). 9α, 15R-dihydroxy-1 1β-fluoro-15-(2,3-dihydro-1H-inden-2-yl)-16, 17, 18, 19, 20-pentanor-prosta-5Z, 13E-dien-1-oic acid (AL-8810) was obtained from Cayman Chemical (Ann Arbor, MI, USA). All other chemicals were of analytical grade.
Results
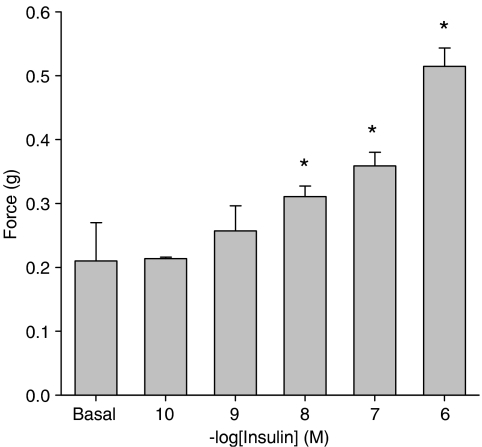
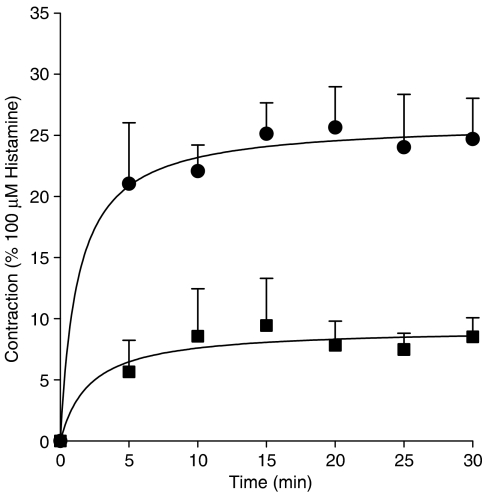
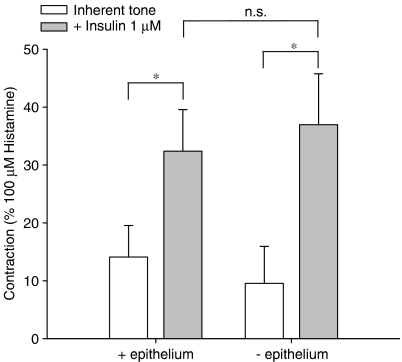
As shown in Figure 1, administration of insulin induced a concentration-dependent tracheal smooth muscle contraction that was statistically significant from basal (inherent) tone, which amounted to 0.21±0.06 g on average, from 0.01 μM onwards. Contraction induced by (1 μM) insulin, reached up to 0.51±0.04 g, corresponding to 33.2±1.9% of maximal histamine (100 μM)-induced contraction. Insulin-induced contractions, as shown for 0.1 and 1 μM, tended to develop slowly, but sustained over time (Figure 2). Previous studies indicated that growth factor-induced contractions are not dependent on the epithelium (Schaafsma et al., 2005). As the epithelium is a well-known source of contractile mediators, including eicosanoids (Watson et al., 1997), endothelins (Goldie and Fernandes, 2000) and acetylcholine (Wessler and Kirkpatrick, 2001), the effect of epithelium removal on insulin-induced ASM contractions was assessed. Inherent myogenic tone and the effects of insulin remained completely intact in epithelium-denuded preparations, suggesting a direct role for the ASM in these effects (Figure 3).
Figure 1.
Insulin induces airway smooth muscle contraction. Guinea-pig tracheal rings were mounted for isometric recording and exposed to increasing concentrations of insulin. Data shown represent means and s.e.m. (vertical lines) of three experiments, each performed in duplicate, obtained using three different animals. *P<0.05 compared to basal (inherent) tone.
Figure 2.
Insulin-induced contractions are sustained in nature. Responses shown (0.1 (squares) and 1 (circles) μM insulin) are corrected for inherent myogenic tone, which amounted to 0.23±0.02 g on average. Data shown represent means and s.e.m. (vertical lines) of three experiments, each performed in duplicate, obtained using three different animals.
Figure 3.
Insulin-induced contractions are independent of the epithelium. Responses shown (1 μM insulin) represent means and s.e.m. (vertical lines) of three experiments, each performed in duplicate, obtained using three different animals. *P<0.05. Basal tone amounted to 0.18±0.06 g (−epithelium) and 0.24±0.07 g (+epithelium).
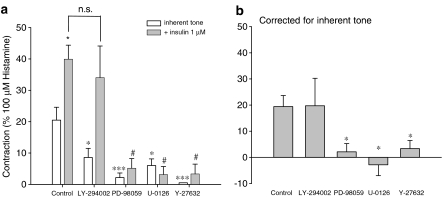
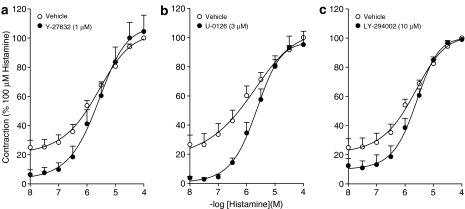
ASM contraction involves phosphorylation of the 20 kDa regulatory myosin light chain (MLC20), initiated by Ca2+-calmodulin-mediated activation of myosin light chain kinase (MLCK). Increased MLC20 phosphorylation can also occur as a consequence of Ca2+-sensitization (Fukata et al., 2001; Somlyo and Somlyo, 2003), which involves inhibition of myosin light chain phosphatase (MLCP) by the Rho/Rho-kinase pathway (Somlyo and Somlyo, 2003; Amrani et al., 2004; Gosens et al., 2006). Studies in human ASM have shown that growth factor-induced contractions are largely dependent on Rho-kinase (Gosens et al., 2004a). In addition, these contractions were found to depend on p42/44 MAPK in guinea-pig ASM (Schaafsma et al., 2005). To assess whether the intracellular signalling associated with insulin-induced contraction is similar to that induced by other growth factors, we studied the effects of p42/p44 MAPK inhibition (PD-98059, 30 μM; U-0126, 3 μM) and Rho-kinase inhibition (Y-27632, 1 μM). In addition, we determined the effects of PI-3-kinase inhibition (LY-294002, 10 μM), as this kinase also plays an important role in insulin signalling (Begum, 2003; Chang et al., 2004). Basal myogenic tone was almost abolished by Y-27632, and considerably reduced by PD-98059, U-0126 and LY-294002 (Figure 4a). Insulin-induced contraction was almost fully prevented in the presence of PD-98059, U-0126 and Y-27632, but was left intact in the presence of the PI-3-kinase inhibitor LY-294002 (Figure 4a and b), indicating an important role for p42/44 MAPK and Rho-kinase. Despite their effects on basal tone and on the lower part of the histamine cumulative concentration–response curve, no effects of Y-27632, U-0126 or LY-294002 were found on maximal contraction or potency of histamine (Figure 5).
Figure 4.
Intracellular signalling associated with insulin-induced airway smooth muscle contraction. Insulin (1 μM)-induced contractions were recorded in the absence and presence of LY-294002 (10 μM), PD 98059 (30 μM), U-0126 (3 μM) or Y-27632 (1 μM). Responses shown in (b) are corrected for inherent myogenic tone and are derived from the data shown in (a). All data shown represent means and s.e.m. (vertical lines) of five to six experiments, each performed in duplicate, obtained using five to six different animals. *P<0.05; ***P<0.001 compared to control. #P<0.05 compared to the control insulin response.
Figure 5.
Maximal histamine-induced contractions are independent of Rho-kinase (a), p42/44-MAPK (b) and PI-3-kinase (c). Data represent means and s.e.m. (vertical lines) of five to seven experiments, each performed in duplicate, obtained using five to seven different animals. Maximal histamine-induced contraction amounted to 1.53±0.1 g on average.
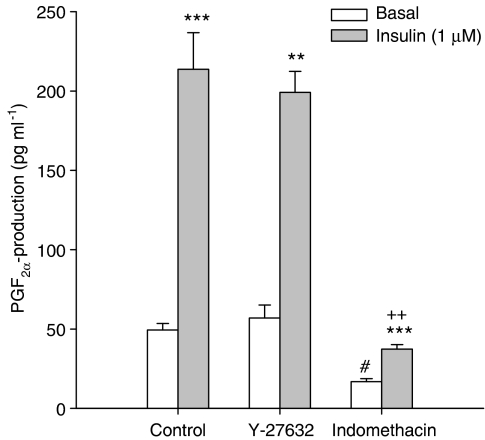
As we have previously demonstrated that growth factor-induced ASM contraction is mediated by the production of contractile prostaglandins (Schaafsma et al., 2005), we assessed the effects of insulin on prostaglandin F2α (PGF2α) production. Stimulation of tracheal smooth muscle preparations with insulin for 30 min greatly enhanced the release of PGF2α from 49.4±4.1 to 213.6±23.1 pg ml−1 per tracheal ring (P<0.01 at t=30 min; Figure 6). Importantly, both basal and insulin-induced release of PGF2α were significantly reduced in the presence of the cyclooxygenase inhibitor indomethacin (3 μM). In contrast to insulin-induced contraction, treatment with Y-27632 (1 μM) did not affect PGF2α release at all. These findings strongly suggest that as for other growth factors, insulin induces the synthesis of contractile prostaglandins, which in turn are dependent on Rho-kinase for their contractile effects.
Figure 6.
Insulin induces a cyclooxygenase-dependent release of PGF2α, which is insensitive to Rho-kinase inhibition. Responses shown represent PGF2α-production by single tracheal rings. Data represent means and s.e.m. (vertical lines) of four experiments, obtained using two different animals. **P<0.01, ***P<0.001 compared to basal. ++P<0.01 compared to control insulin response. #P<0.05 compared to control basal.
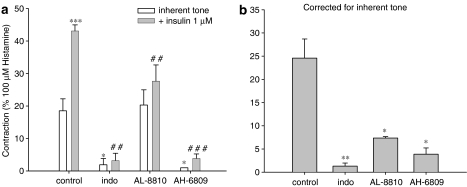
To establish the functional contribution of contractile prostaglandins, we assessed the role of COX in insulin-induced contraction of epithelium-denuded preparations, to ensure that mediators derived from the epithelium did not interfere in any way, by using indomethacin (3 μM; Figure 7). Also, we determined the functional contribution of the contractile PGE2-sensitive EP1-receptor and the contractile PGF2α-sensitive FP-receptor in these preparations, using the selective receptor antagonists AH-6809 and AL-8810 (both applied at 10 μM), respectively (Figure 7). Fully in line with its effects on prostaglandin production (Figure 6), indomethacin abrogated both inherent myogenic tone and insulin-induced contractions (Figure 7a). Of note, indomethacin does not reduce histamine-induced contractions in guinea-pig tracheal preparations (Schaafsma et al., 2004). EP1-receptor blockade had identical effects as indomethacin, indicating a pivotal role for EP1-mediated signalling. FP-receptor blockade did not significantly affect basal myogenic tone, but did significantly reduce insulin-induced contraction, suggesting that insulin-induced contractions are dependent on FP-receptor stimulation as well. Moreover, these findings demonstrate that contractile FP- and EP1-receptor stimulation involved in insulin-induced contraction occurs independently of epithelium.
Figure 7.
Role of contractile prostaglandin receptor stimulation in insulin-induced airway smooth muscle contraction. Insulin (1 μM)-induced contractions were recorded in the absence and presence of indomethacin (3 μM), the FP-receptor antagonist AL-8810 (10 μM) or the EP1-receptor antagonist AH-6809 (10 μM). Responses shown in (b) are corrected for inherent myogenic tone and are derived from the data shown in (a). All data shown represent means and s.e.m. (vertical lines) of three experiments. *P<0.05; **P<0.01; ***P<0.001 compared to control. ##P<0.01; ###P<0.001 compared to the control insulin response.
Discussion
In the present study, we demonstrate for the first time that insulin induces ASM contraction, which is not sensitive to epithelium removal. These contractions are sustained in nature, dependent on p42/p44 MAP kinase and Rho-kinase, and are mediated by the production of contractile prostaglandins, as COX inhibition and EP1- and FP-receptor blockade strongly reduced the insulin-induced effects on myogenic tone. Accordingly, insulin stimulation resulted in a significant release of PGF2α, which was fully prevented in the presence of indomethacin, but left completely unaffected in the presence of Y-27632. These mechanisms fully correspond to PDGF- and EGF-induced contractions that are also mediated by the production of non-epithelium derived contractile prostaglandins (Schaafsma et al., 2005). Prostaglandin production by growth factors is presumably the result of the consecutive actions of MEK, p42/p44 MAP kinase, cytosolic PLA2 and COX; these contractile prostaglandins produced are in turn largely dependent on Rho-kinase for their effects (Schaafsma et al., 2005). Growth factor-induced contractions of human bronchial smooth muscle preparations are also almost fully dependent on Rho-kinase (Gosens et al., 2004a), suggesting that the signalling underlying this ASM contraction is species-independent and shared by most growth factors, including insulin (Schaafsma et al., 2005). Furthermore, this mechanism might be characteristic for contractions induced by growth factors, as no effect of the applied inhibitors was found on maximal histamine-induced contraction.
In addition to our present finding that insulin directly affects myogenic ASM tone, we previously showed that insulin, applied to bovine tracheal smooth muscle cells, was weakly mitogenic by itself and synergistically potentiated mitogenesis induced by PDGF and EGF. Both effects could have implications for the use of inhaled insulin formulations, especially in inflamed airways. Several growth factors, including PDGF and EGF, have been implicated in airway inflammation as they are being generated by inflammatory cells, epithelial cells, extravasated plasma as well as the ASM itself (Hirst, 2000; McKay and Sharma, 2002). In addition to the acute pro-mitogenic effects of insulin, our previous study also showed that prolonged exposure of bovine tracheal smooth muscle to insulin results in the induction of a hypercontractile phenotypic state, with augmented maximal contractile responses to methacholine and KCl, but with reduced mitogenic activity to other growth factors (Gosens et al., 2003). However, the conclusions that can be made from our previous (Gosens et al., 2003) and current studies are limited, insofar as it is not known whether the insulin effects observed on bovine and guinea-pig ASM translate to human ASM. Further investigations are clearly warranted to identify acute and chronic effects of insulin on human ASM and to establish the effects of insulin inhalation on ASM function and phenotype under specific pathophysiological conditions (e.g. in asthma and COPD models).
Under physiological conditions blood insulin concentrations may rise to ∼1 nM, which may significantly increase under insulin resistant conditions (Wang et al., 2003). However, little is known about local insulin concentrations in the airways after insulin inhalation. Owing to the low biological availability of inhaled insulin and poor absorption by asthmatics in comparison to healthy subjects, effective dosing for inhaled delivery is more than 10-fold higher than that used for s.c. injections to achieve satisfactory glycaemic control (Heinemann et al., 2001; Steiner et al., 2002; Henry et al., 2003; Sakagami, 2004), suggesting that local insulin concentrations may rise substantially. In a very recent study, it was found that inhalation of insulin was accompanied by an increased incidence of cough, dyspnoea, sinusitis and pharyngitis (Lenzer, 2006). Moreover, it was shown that insulin caused a decrease in FEV1, which was indicated to be of small and uncertain clinical significance (Lenzer, 2006). As diabetic patients with asthma may need to inhale a higher dose of insulin as compared to nonasthmatic diabetics (Henry et al., 2003), and since patients suffering from asthma and COPD are also hyper-reactive to inhaled contractile stimuli (Fuller et al., 1987; Joos et al., 1987; Polosa and Holgate, 1990), it could be envisaged that these effects on FEV1 might be more significant in these patients. Not surprisingly, the use of aerosol formulations of insulin is not recommended for patients suffering from obstructive airway diseases (Lenzer, 2006).
Effects of insulin on airway hyper-responsiveness and airway inflammation have been studied in a rat model of diabetes (Belmonte et al., 1997, 1998). It was found that airway constriction in response to electrical field stimulation was decreased in diabetic rats due to an enhanced function of the neuronal inhibitory M2-muscarinic receptor in the lungs (Belmonte et al., 1997). Upon allergen challenge neuronal M2-receptor function was preserved and eosinophilia did not occur around airway nerves in these animals (Belmonte et al., 1998). Conversely, treatment of these diabetic animals with insulin induced M2-receptor dysfunction, airway hyper-responsiveness and eosinophilia after allergen challenge. Collectively, these data indicate that inhaled insulin could promote detrimental airway inflammation, airway hyper-responsiveness, and airway remodelling during prolonged use.
In conclusion, we demonstrate that insulin is able to induce a concentration-dependent ASM contraction, which is mediated by the generation of contractile prostaglandins. These prostaglandins, which are presumably produced by the consecutive actions of MEK and COX, are not primarily derived from the airway epithelium (strongly suggesting they are derived from the airway smooth muscle itself) and are dependent on Rho-kinase for their contractile effects. This mechanism of ASM contraction is very similar to that observed previously for other growth factors (Schaafsma et al., 2005).
Altogether, it can be envisaged that in addition to the adverse effects of insulin on ASM phenotype (Gosens et al., 2003), the present findings could have implications for the use of aerosol formulations of insulin, especially under conditions of airways inflammation.
Acknowledgments
This work was financially supported by the Netherlands Asthma Foundation, NAF-Grant 01.83.
Abbreviations
- ASM
airway smooth muscle
- KH
Krebs–Henseleit
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
Conflict of interest
The authors state no conflict of interest.
References
- Amrani Y, Tliba O, Deshpande DA, Walseth TF, Kannan MS, Panettieri RA., Jr Bronchial hyperresponsiveness: insights into new signaling molecules. Curr Opin Pharmacol. 2004;4:230–234. doi: 10.1016/j.coph.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Begum N. Insulin signaling in the vasculature. Front Biosci. 2003;8:s796–s804. doi: 10.2741/1146. [DOI] [PubMed] [Google Scholar]
- Belmonte KE, Fryer AD, Costello RW. Role of insulin in antigen-induced airway eosinophilia and neuronal M2 muscarinic receptor dysfunction. J Appl Physiol. 1998;85:1708–1718. doi: 10.1152/jappl.1998.85.5.1708. [DOI] [PubMed] [Google Scholar]
- Belmonte KE, Jacoby DB, Fryer AD. Increased function of inhibitory neuronal M2 muscarinic receptors in diabetic rat lungs. Br J Pharmacol. 1997;121:1287–1294. doi: 10.1038/sj.bjp.0701274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalli A, Janssen LJ. Augmentation of bovine airway smooth muscle responsiveness to carbachol, KCl, and histamine by the isoprostane 8-iso-PGE2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1035–L1041. doi: 10.1152/ajplung.00138.2004. [DOI] [PubMed] [Google Scholar]
- Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Dixon CM, Cuss FM, Barnes PJ. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am Rev Respir Dis. 1987;135:176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]
- Garg S, Rosenstock J, Silverman BL, Sun B, Konkoy CS, de la PA, et al. Efficacy and safety of preprandial human insulin inhalation powder versus injectable insulin in patients with type 1 diabetes. Diabetologia. 2006;49:891–899. doi: 10.1007/s00125-006-0161-3. [DOI] [PubMed] [Google Scholar]
- Goldie RG, Fernandes LB. A possible mediator role for endothelin-1 in respiratory disease. Monaldi Arch Chest Dis. 2000;55:162–167. [PubMed] [Google Scholar]
- Gosens R, Meurs H, Bromhaar MM, McKay S, Nelemans SA, Zaagsma J. Functional characterization of serum- and growth factor-induced phenotypic changes in intact bovine tracheal smooth muscle. Br J Pharmacol. 2002;137:459–466. doi: 10.1038/sj.bjp.0704889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol. 2003;481:125–131. doi: 10.1016/j.ejphar.2003.08.081. [DOI] [PubMed] [Google Scholar]
- Gosens R, Schaafsma D, Grootte Bromhaar MM, Vrugt B, Zaagsma J, Meurs H, et al. Growth factor-induced contraction of human bronchial smooth muscle is Rho-kinase-dependent. Eur J Pharmacol. 2004a;494:73–76. doi: 10.1016/j.ejphar.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Gosens R, Schaafsma D, Meurs H, Zaagsma J, Nelemans SA. Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype. Eur J Pharmacol. 2004b;483:71–78. doi: 10.1016/j.ejphar.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Gosens R, Schaafsma D, Nelemans SA, Halayko AJ. Rho-kinase as a drug target for the treatment of airway hyperresponsiveness in asthma. Mini Rev Med Chem. 2006;6:339–348. doi: 10.2174/138955706776073402. [DOI] [PubMed] [Google Scholar]
- Griffin BW, Klimko P, Crider JY, Sharif NA. AL-8810: a novel prostaglandin F2 alpha analog with selective antagonist effects at the prostaglandin F2 alpha (FP) receptor. J Pharmacol Exp Ther. 1999;290:1278–1284. [PubMed] [Google Scholar]
- Hallsworth MP, Moir LM, Lai D, Hirst SJ. Inhibitors of mitogen-activated protein kinases differentially regulate eosinophil-activating cytokine release from human airway smooth muscle. Am J Respir Crit Care Med. 2001;164:688–697. doi: 10.1164/ajrccm.164.4.2011004. [DOI] [PubMed] [Google Scholar]
- Heinemann L, Pfutzner A, Heise T. Alternative routes of administration as an approach to improve insulin therapy: update on dermal, oral, nasal and pulmonary insulin delivery. Curr Pharm Des. 2001;7:1327–1351. doi: 10.2174/1381612013397384. [DOI] [PubMed] [Google Scholar]
- Henry RR, Mudaliar SR, Howland WC, III, Chu N, Kim D, An B, et al. Inhaled insulin using the AERx Insulin Diabetes Management System in healthy and asthmatic subjects. Diabet Care. 2003;26:764–769. doi: 10.2337/diacare.26.3.764. [DOI] [PubMed] [Google Scholar]
- Hirst SJ. Airway smooth muscle as a target in asthma. Clin Exp Allergy. 2000;30 Suppl 1:54–59. [PubMed] [Google Scholar]
- Hollander PA, Blonde L, Rowe R, Mehta AE, Milburn JL, Hershon KS, et al. Efficacy and safety of inhaled insulin (exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabet Care. 2004;27:2356–2362. doi: 10.2337/diacare.27.10.2356. [DOI] [PubMed] [Google Scholar]
- Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients' fears and hopes about insulin therapy. The basis of patient reluctance. Diabet Care. 1997;20:292–298. doi: 10.2337/diacare.20.3.292. [DOI] [PubMed] [Google Scholar]
- Joos G, Pauwels R, van der Straeten M. Effect of inhaled substance P and neurokinin A on the airways of normal and asthmatic subjects. Thorax. 1987;42:779–783. doi: 10.1136/thx.42.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CR, Williams GW, Sharif NA. Real-time intracellular Ca2+ mobilization by travoprost acid, bimatoprost, unoprostone, and other analogs via endogenous mouse, rat, and cloned human FP prostaglandin receptors. J Pharmacol Exp Ther. 2003;304:238–245. doi: 10.1124/jpet.102.042556. [DOI] [PubMed] [Google Scholar]
- Lenzer J. Inhaled insulin is approved in Europe and United States. BMJ. 2006;332:321. doi: 10.1136/bmj.332.7537.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S, Sharma HS. Autocrine regulation of asthmatic airway inflammation: role of airway smooth muscle. Respir Res. 2002;3:11. doi: 10.1186/rr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayianni M, Gourgoulianis KI, Molyvdas PA. Insulin NO-dependent action on airways smooth muscles. Nitric Oxide. 2001;5:72–76. doi: 10.1006/niox.2000.0317. [DOI] [PubMed] [Google Scholar]
- Polosa R, Holgate ST. Comparative airway response to inhaled bradykinin, kallidin, and [des-Arg9]bradykinin in normal and asthmatic subjects. Am Rev Respir Dis. 1990;142:1367–1371. doi: 10.1164/ajrccm/142.6_Pt_1.1367. [DOI] [PubMed] [Google Scholar]
- Quattrin T, Belanger A, Bohannon NJ, Schwartz SL. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabet Care. 2004;27:2622–2627. doi: 10.2337/diacare.27.11.2622. [DOI] [PubMed] [Google Scholar]
- Range SP, Pang L, Holland E, Knox AJ. Selectivity of cyclo-oxygenase inhibitors in human pulmonary epithelial and smooth muscle cells. Eur Respir J. 2000;15:751–756. doi: 10.1034/j.1399-3003.2000.15d20.x. [DOI] [PubMed] [Google Scholar]
- Sakagami M. Insulin disposition in the lung following oral inhalation in humans : a meta-analysis of its pharmacokinetics. Clin Pharmacokinet. 2004;43:539–552. doi: 10.2165/00003088-200443080-00004. [DOI] [PubMed] [Google Scholar]
- Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Allergic sensitization enhances the contribution of Rho-kinase to airway smooth muscle contraction. Br J Pharmacol. 2004;143:477–484. doi: 10.1038/sj.bjp.0705903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma D, Gosens R, Bos S, Meurs H, Zaagsma J, Nelemans A. Role of contractile prostaglandins and Rho-kinase in growth factor-induced airway smooth muscle contraction. Respir Res. 2005;6:85. doi: 10.1186/1465-9921-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinases, and Myosin Phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Steiner S, Pfutzner A, Wilson BR, Harzer O, Heinemann L, Rave K. Technosphere/Insulin – proof of concept study with a new insulin formulation for pulmonary delivery. Exp Clin Endocrinol Diabet. 2002;110:17–21. doi: 10.1055/s-2002-19989. [DOI] [PubMed] [Google Scholar]
- Tsang F, Choo HH, Dawe GS, Wong WS. Inhibitors of the tyrosine kinase signaling cascade attenuated thrombin-induced guinea pig airway smooth muscle cell proliferation. Biochem Biophys Res Commun. 2002;293:72–78. doi: 10.1016/S0006-291X(02)00170-5. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–2569. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- Watson N, Magnussen H, Rabe KF. Inherent tone of human bronchus: role of eicosanoids and the epithelium. Br J Pharmacol. 1997;121:1099–1104. doi: 10.1038/sj.bjp.0701244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. The Non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]