Abstract
Background and purpose:
Nitric oxide (NO) and vasoactive intestinal peptide (VIP) are considered transmitters of non-adrenergic, non-cholinergic (NANC) relaxations in guinea-pig trachea, whereas the role of carbon monoxide (CO) is unknown. This study was designed to assess the participation of CO, and to investigate the localization of haem oxygenase-2 (HO-2), the CO-producing enzyme, in tracheal neurons.
Experimental approach:
NANC responses to electrical field stimulation (EFS) at 3 and 10 Hz were evaluated in epithelium-free whole tracheal segments as intraluminal pressure changes. Drugs used were: L-nitroarginine methyl ester (L-NAME, 100 μ M) to inhibit NO synthase (NOS), α-chymotrypsin (2 U ml−1) to inactivate VIP, zinc protoporphyrin-IX (ZnPP-IX, 10 μM) to inhibit HO-2, and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 μM), a soluble guanylyl cyclase inhibitor. For immunohistochemistry, tissues were exposed to antibodies to PGP 9.5, a general neuronal marker, HO-2 and NOS, and processed with an indirect immunofluorescence method.
Key results:
α-Chymotrypsin did not affect NANC relaxations. ODQ inhibited NANC responses by about 60%, a value similar to that obtained by combining L-NAME and ZnPP-IX. The combination of ODQ, L-NAME and ZnPP-IX reduced the responses by 90%. Subpopulations of HO-2 positive neurons containing NOS were detected in tracheal sections.
Conclusions and Implications:
In the guinea-pig trachea, NANC inhibitory responses at 3 and 10 Hz use NO and CO as main transmitters. Their participation is revealed following inhibition of NOS, HO-2 and soluble guanylyl cyclase. The involvement of CO as a relaxing transmitter paves the way for novel therapeutic approaches in the treatment of airway obstruction.
Keywords: trachea, guinea-pig, NANC inhibitory innervation, electrical field stimulation, immunohistochemistry, nitric oxide, vasoactive intestinal peptide, carbon monoxide
Introduction
The intrinsic non-adrenergic, non-cholinergic (NANC) inhibitory innervation has been characterized in the airways of different species, including the guinea-pig (Coburn and Tomita, 1973; Chesrown et al., 1980; Yip et al., 1981; Moffatt et al., 1999). Experimental findings indicate a leading role for nitric oxide (NO) (Tucker et al., 1990; Li and Rand, 1991; Venugopalan et al., 1998) with a variable participation of vasoactive intestinal peptide (VIP) (Ellis and Farmer, 1989a, 1989b) as transmitters of electrically induced NANC relaxation in the guinea-pig trachea. Conversely, ATP does not participate as an inhibitory transmitter in this preparation, as indicated by the ineffectiveness of apamin (Zacour et al., 1987) and reactive blue 2 (Ellis and Farmer, 1989b). The evidence that in most studies nitric oxide synthase (NOS) inhibitors and α-chymotrypsin (α-CT)(or VIP antisera) reduced, but did not abolish NANC relaxations in guinea-pig tracheal strips, suggests that transmitters other than NO and VIP are involved in this response.
In both the central (Glaum and Miller, 1993; Shinomura et al., 1994) and the peripheral (Rattan and Chakder, 1993; Alcon et al., 2001) nervous systems, carbon monoxide (CO) produced by constitutive haem oxygenase-2 (HO-2) has been found to act as a transmitter by enhancing cGMP production via guanylyl cyclase stimulation (Barañano et al., 2001; Morse et al., 2002; Gibbons and Farrugia, 2004). In the periphery, a role for CO as a NANC inhibitory transmitter has been proposed in the opossum internal anal sphincter (Rattan and Chakder, 1993) and the canine gallbladder (Alcon et al., 2001). In the airways, HO-2 was found to colocalize with choline-acetyltransferase (ChAT) in the parasympathetic ganglia of human and guinea-pig trachea and bronchi, but was not observed in nerve fibres innervating the smooth muscle, lamina propria and epithelium (Canning and Fischer, 1998). From a functional point of view, zinc protoporphyrin-IX (ZnPP-IX), an inhibitor of HO-2, when administered alone in the guinea-pig trachea did not affect NANC relaxations to electrical field stimulation (EFS) (Undem et al., 1996).
The aims of the present study were: (i) to characterize the role of NO, VIP and CO by quantifying their participation in the NANC relaxations to EFS in guinea-pig isolated whole tracheal segments, according to the method described by Tanihata and Uchiyama (1996) and (ii) to investigate the localization of HO-2 immunoreactivity in the tracheal neuronal pathways and its relationship with the immunoreactivity to NOS, a marker of intrinsic inhibitory innervation. Our findings suggest that, under our experimental conditions, CO participates together with NO in tracheal NANC relaxations evoked by low frequency of stimulation, whereas VIP does not. The involvement of CO as a relaxing transmitter paves the way for novel therapeutic approaches in the treatment of airway obstruction, provided that CO participates as a NANC inhibitory transmitter in human airways.
Methods
Animals
Male albino guinea-pigs weighing 500–600 g (Bettinardi, Momo, Italy) were used for functional studies, whereas animals weighing 200–300 g (Simonsen Labs, San Diego, CA, USA) were used for immunohistochemistry. Guinea-pigs were housed in standard animal facilities, providing constant temperature (21±1°C), relative humidity (50–55%), and alternating 12 h light and dark cycles. Animals were provided with food and water ad libitum. Care and handling of the animals were in accordance with the European Union Directive 86/609 and National Institutes of Health recommendations for the humane use of animals. All experimental procedures were reviewed and approved by the appropriate Animal Use Committee of the Institutions (University of Pavia, Italy, and UCLA, Los Angeles, CA, USA) where the experiments were performed. The number of animals used was kept to the minimum necessary for a meaningful interpretation of the data and animal discomfort was kept to a minimum. For functional studies, animals were killed by cervical dislocation and rapid exsanguination. For immunohistochemistry, animals were killed by an intraperitoneal (i.p.) injection of sodium pentobarbitone (Nembutal: 100 mg kg−1).
Functional studies
The trachea was excised and transferred to a Petri dish containing oxygenated (95% O2 and 5% CO2) standard Tyrode solution. A 3-cm-long tracheal tube was prepared by gently removing the mucosa (D'Agostino et al., 1990), to avoid the electrical stimulation of epithelial cells, which may release substantial amounts of prostanoids, ATP and NO that may distort the nerve-mediated response to EFS. As this technique allows an histologically proved total mucosa ablation, the use of prostanoid synthesis inhibitors (e.g. indomethacin) is no longer needed. Then, the tracheal tube was cannulated at each extremity using two polyvinyl chloride (PVC) tubes (outer diameter, 2.0 mm; inner diameter, 1.35 mm), and was set up horizontally in a 10 ml organ bath containing standard Tyrode solution, maintained at 37°C and bubbled with a mixture of 95% O2 and 5% CO2. The preparation was flushed intraluminally with a peristaltic pump delivering Tyrode solution at 0.4 ml min−1 for 30 min. Then, one end of the preparation was occluded and the other one was connected to a pressure transducer for intraluminal pressure recording. Signals were recorded using a PowerLab data acquisition system (ADInstruments Ltd, Crowborough, UK), and analysed using PowerLab Chart v4.1.1 software.
After 1 h of equilibration, the tracheal tube was stimulated via two platinum electrodes placed in parallel, 1 cm apart, and connected to an electrical stimulator (MARB ST 87). Trains of rectangular pulses (0.5 ms duration, 3–10 Hz frequency at 60 V) were delivered for 5 s at 10 min intervals. EFS-induced NANC relaxations were measured as a reduction in the intratracheal pressure. They consisted of an initial fast response (peak response) followed by a late slow recovery of the tone up to the basal value. The amplitude of the peak response was calculated as pressure variation from baseline (Pascal: Pa), whereas to evaluate the overall inhibitory response (i.e. both the peak and duration of the relaxant response), the area under the curve (AUC) was calculated as the integral from baseline for each response (Pa s). All the experiments were carried out in the presence of hyoscine (1 μM) to block muscarinic acetylcholine receptors, and piperoxan (1 μM) and propranolol (1 μM) to block α- and β-adrenoceptors. NANC relaxations to EFS were elicited before (control) and 45–60 min after the administration of the following drugs, administered as single agents: the NOS inhibitor, L-nitroarginine methyl ester (L-NAME) (100 μM); the endogenous peptide digesting enzyme, α-CT (2 U ml−1); the HO-2 inhibitor, ZnPP-IX (10 μM); and the soluble guanylyl cyclase inhibitor, 1H[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (10 μM). Other experiments were conducted by giving L-NAME and ZnPP-IX or L-NAME, ZnPP-IX and ODQ to inhibit NO- and CO-producing enzymes and to prevent the formation of cGMP, the second messenger common to both NO and CO systems, involved in smooth muscle relaxation. As ZnPP-IX is photosensitive, all experiments using this substance were performed in complete darkness (Zygmunt et al., 1994). Tetrodotoxin (TTX) (1 μM) was given in a subset of experiments to ensure that EFS-evoked inhibitory responses were neurogenic.
Immunohistochemistry
The trachea was quickly dissected and fixed in 4% paraformaldehyde in 0.1 M, phosphate buffer (Fisher Scientific, Fair Lawn, NJ, USA), pH 7.4 (PB) for 2 h at room temperature and then stored in 25% sucrose/PB for cryoprotection at 4°C for at least 12 h. Specimens were imbedded in Tissue-Tek OCT Compound (Sakura, Torrance, CA, USA), frozen with dry ice and cut into 12 μM thick sections using a cryostat. Sections were then processed for indirect immunofluorescence as described previously. Briefly, sections were washed in PB, incubated in 10% normal donkey serum for 1 h at room temperature and then incubated overnight at 4°C in a mixture of rabbit HO-2 antiserum (1:1500; Stressgen Biotech. Corp., Victoria, Canada) and one of the following: mouse monoclonal antibody to protein gene product 9.5 (PGP 9.5) (1:500 Neuromics Antibodies, Northfield, MN, USA), as a general neuronal marker, mouse monoclonal antibody to NOS (N31020; 1:50; Transduction Laboratories, Lexington, KY, USA), the enzyme that synthesizes NO, a main inhibitory transmitter. Following washing, sections were incubated for 2 h at room temperature with a mixture of affinity-purified donkey anti-rabbit IgG coupled with Alexa 488 (1:1000; Molecular Probes, Eugene, OR, USA) and donkey anti-mouse IgG conjugated with Rhodamine Red-X (1:300; Jackson Immunolabs, West Grove, PA, USA), washed again and attached to coverslips with Acqua Poly/Mount (Polysciences Inc., Warrington, PA, USA). Fluorescent images were acquired with a Zeiss Laser Scanning Microscope 510 META (Zeiss, Thornwood, NY, USA) equipped with a Plan Apochromat × 63 (1.4 NA) oil objective. A 488 nm argon laser line and a 543 nm He-Ne laser line were used for excitation. The images were acquired as 12-bit signals. All post-image processing was performed using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA, USA).
Statistical analysis
Data are given as raw data or as the mean±s.e.m. of the percent residual response after pharmacological treatment compared to control response (100%). Statistical analysis was performed by means of Student's t-test for paired or unpaired data or by means of analysis of variance followed by Bonferroni's test for multiple comparisons. A P-value <0.05 was considered statistically significant.
Drugs
α-CT, hyoscine, isoprenaline, L-NAME, propranolol, TTX (all purchased from Sigma–Aldrich, St Louis, MO, USA) and piperoxan (Rhône-Poulenc, France) were dissolved in distilled water. ZnPP-IX (Sigma–Aldrich) was prepared (and maintained) in the dark by first dissolving it in 0.2 N sodium hydroxide solution and then diluting in distilled water. The pH of the solution was adjusted to 7.4 with 0.2 N HCl. ODQ (Sigma–Aldrich) was prepared by first dissolving it in dimethylsulphoxide (DMSO) and then diluting in distilled water. Sodium pentobarbitone (Nembutal) was from Abbott Laboratories (Chicago, IL, USA).
Results
Functional studies
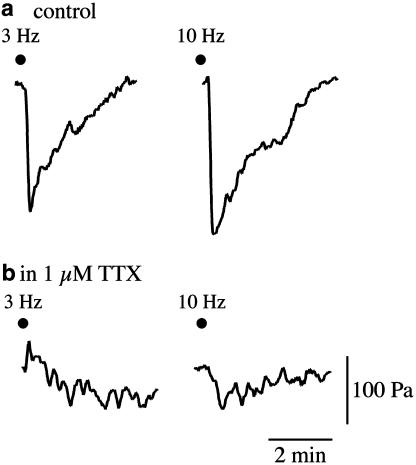
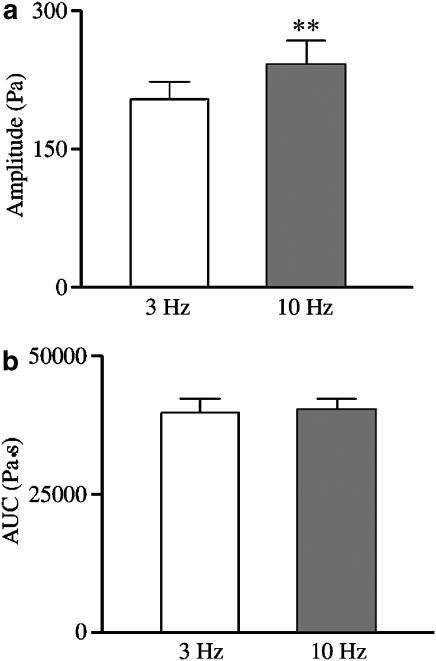
Under NANC conditions, EFS at 3 and 10 Hz induced biphasic relaxant responses, consisting of an initial fast response followed by a late slow response up to the recovery of the basal tone, which were reproducible over a 7 h time interval and were abolished by 1 μM TTX (n=4) (Figure 1). EFS-induced relaxations were frequency-dependent and submaximal compared with the response evoked by 10 μM isoprenaline (45 and 67%, respectively, n=4). Conversely, when assessed as AUC the two types of response were indistinguishable (Figure 2).
Figure 1.
Representative tracing showing EFS-induced NANC relaxations evoked by frequencies of 3 and 10 Hz in the guinea-pig isolated whole trachea in the absence (a) and in the presence (b) of TTX.
Figure 2.
EFS-induced NANC relaxations evoked by frequencies of 3 and 10 Hz in the guinea-pig isolated whole trachea were evaluated either as amplitude of the fast initial response (a) or as AUC (b) of the overall inhibitory response, including both the fast and the slow component. Note that the amplitude of the responses evoked by 10 Hz was significantly higher than that evoked by 3 Hz, whereas in terms of AUC the two responses were superimposable. Data represent mean and vertical lines show s.e.m. of 43 experiments. **P<0.01.
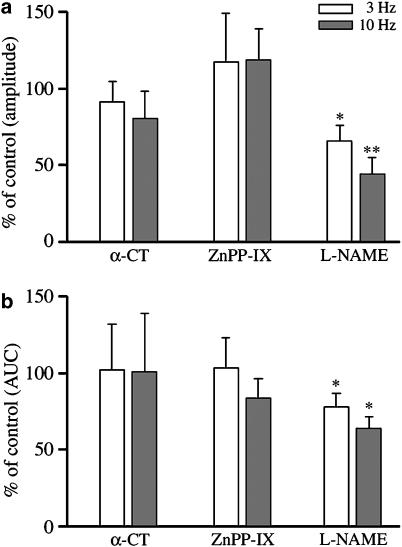
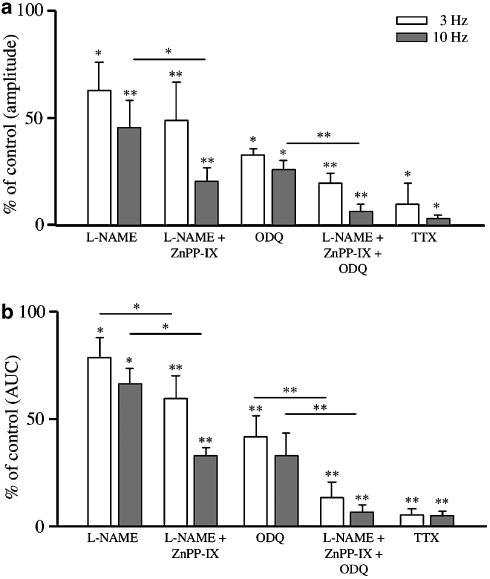
Treatment with α-CT (2 U ml−1) did not affect NANC relaxations at 3 and 10 Hz, whereas L-NAME (100 μM) reduced NANC relaxations at both frequencies (Figure 3) regarding either the amplitude of responses or the AUC. As an individual treatment, ZnPP-IX (10 μM) failed to affect NANC relaxations (Figure 3). However, when ZnPP-IX was added after L-NAME to the bath, a reduction of NANC relaxations greater than that caused by L-NAME alone was observed, with the exception of the amplitude of relaxations elicited at 3 Hz (Figure 4). The extent of the reduction caused by ZnPP-IX and L-NAME was similar to that produced by 10 μM ODQ (Figure 4). Another series of experiments was carried out to assess the effect caused by the inhibition of NO- and CO-producing enzymes, and soluble guanylyl cyclase to prevent the formation of cGMP, the second messenger common to both NO and CO systems. In the presence of L-NAME, ZnPP-IX and ODQ, NANC relaxations to 3 and 10 Hz were inhibited by approximately 90%, a value similar to that evoked by 1 μM TTX (Figure 4).
Figure 3.
Effect of α-CT, ZnPP-IX and L-NAME on EFS-induced NANC relaxations evoked by frequencies of 3 and 10 Hz in the guinea-pig isolated whole trachea. α-CT (2 U ml−1) and ZnPP-IX (10 μM) were ineffective, whereas L-NAME (100 μM) significantly reduced both the amplitude (a) and the AUC (b) of the inhibitory responses. Data represent mean and vertical lines show s.e.m. of 5–8 experiments. *P<0.05, **P<0.01.
Figure 4.
Effects of various pharmacological treatments with single drugs (ODQ and TTX), or with a combination of drugs (L-NAME+ZnPP-IX or L-NAME+ZnPP-IX+ODQ) on EFS-induced NANC relaxations evoked by frequencies of 3 and 10 Hz in the guinea-pig isolated whole trachea. For comparison purposes, the effect of L-NAME (as already presented in Figure 2) on both the amplitude (a) and the AUC (b) of the inhibitory responses has also been included. L-NAME+ZnPP-IX significantly reduced both the amplitude and the AUC of NANC responses either vs control relaxations or vs L-NAME alone (with the exception of the amplitude of relaxations elicited at 3 Hz). The effect of ODQ alone was similar to that of L-NAME+ZnPP-IX. The combination of L-NAME+ZnPP-IX+ODQ inhibited the responses in terms of amplitude and AUC to an extent similar to that of TTX (1 μM). Data represent mean and vertical lines show s.e.m. of 4–10 experiments. *P<0.05, **P<0.01.
Immunohistochemistry
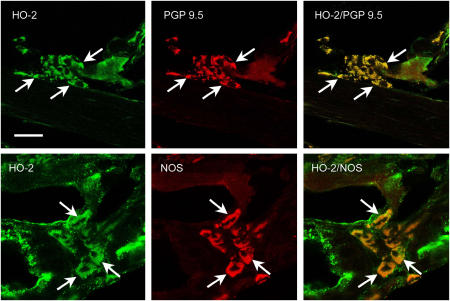
Clusters of cells containing HO-2 immunoreactivity were observed within the tracheal wall. Double labelling immunohistochemistry showed that all HO-2 immunoreactive cells contained immunoreactivity for PGP 9.5, a general neuronal marker, indicating that HO-2 was expressed by neurons. Furthermore, a subpopulation of HO-2-positive neurons contained NOS, indicating that CO- and NO-producing enzymes can be present in the same cell (Figure 5).
Figure 5.
Confocal images of neurons of the guinea-pig trachea showing HO-2 (green fluorescence), PGP 9.5 and NOS (red fluorescence) immunoreactivity in the cytoplasm of neuronal cell bodies in tracheal ganglion cells. HO-2/PGP 9.5 and HO-2/NOS show colocalization of HO-2 with PGP 9.5 or NOS immunoreactivity, respectively. Arrows point to examples of neurons that contain HO-2 and PGP 9.5 or NOS immunoreactivity. Calibration bar: 50 μm.
Discussion and conclusions
The present study provides evidence that, besides the well-documented participation of NO, another gaseous transmitter, CO, is involved in the NANC relaxations to EFS at low frequencies of stimulation (3 or 10 Hz) in an isolated whole preparation of guinea-pig trachea. Under our experimental conditions, a peptidergic component of the NANC response mediated by VIP, or related peptides, was not apparent. Based on our findings, the role of NO seems to be less prominent than that previously described in isolated tracheal preparations from the same animal species. In particular, in our experiments L-NAME inhibited NANC relaxations in the range of 21–53%, whereas other studies conducted with precontracted tracheal strips revealed that the participation of NO ranged from 50 to 100% at frequencies up to 10 Hz (Tucker et al., 1990; Li and Rand, 1991; Moffatt et al., 1999). Our findings are also at variance with those of Tanihata and Uchiyama (1996), who found that the responses up to 10 Hz were abolished by L-NAME in an experimental model similar to ours. In another study carried out in vivo by measuring intratracheal pressure, Venugopalan et al. (1998) observed a reduction by 35% of the NANC relaxations due to vagal nerve stimulation following treatment with L-NAME.
Early studies with precontracted guinea-pig tracheal strips (Ellis and Farmer, 1989a, 1989b; Tucker et al., 1990; Li and Rand, 1991) suggested that a proportion of the NANC inhibitory response, ranging from 28 to 40%, was attributable to VIP or related peptides, although findings inconsistent with VIP being a transmitter have also been reported (Undem et al., 1996). More recently, α-CT was found to inhibit by 60% the non-NO-related component of the NANC relaxation evoked by long trains at low frequency or short trains at high frequency of stimulation (Moffatt et al., 1999). In contrast, using α-CT, we were unable to demonstrate a peptidergic component of NANC relaxations evoked by short (5 s) trains at low frequency (3 and 10 Hz), an observation compatible with that of Tanihata and Uchiyama (1996), who reported the involvement of VIP only at frequencies higher than 10 Hz. Differences in experimental findings may depend on the preparation used, the presence or the absence of the mucosa, the characteristics of electrical stimulation and the presence of pharmacological manipulation of muscular tone.
As previously found in the guinea-pig airways (Undem et al., 1996), the HO-2 inhibitor ZnPP-IX failed to affect EFS-induced relaxations when given alone, suggesting that CO does not participate in the NANC inhibitory response. However, when ZnPP-IX was used in combination with L-NAME NANC responses were reduced by 43–80%, as compared with 21–53% inhibition caused by L-NAME alone. This finding leads us to propose that the participation of CO in the NANC relaxation is not apparent in a preparation with an intact (active) nitrergic innervation, but the contribution of CO can be unmasked after inhibition of the nitrergic pathways. How can this finding be explained? Our immunohistochemical findings revealed that HO-2 is colocalized with NOS in the cytoplasm of neuronal cell bodies in tracheal ganglion cells. This supports the involvement of the HO-2 pathway in neuronal signalling (Verma et al., 1993), and the concept that, as in the central nervous system (CNS) (Snyder et al., 1998) and in the gastrointestinal tract (Donat et al., 1999; Xue et al., 2000), NO and CO may act as co-transmitters in the tracheal innervation. Both compounds are known to mediate most of their effects, including smooth muscle relaxation, by stimulating soluble guanylyl cyclase in the effector cells. However, the stimulation of soluble guanylyl cyclase activity by CO is relatively weaker (by a factor of about 30–100) than that of NO, the classical regulator of soluble guanylyl cyclase (Furchgott and Jothianandan, 1991; Stone and Marletta, 1994). This means that when both NO and CO are co-released upon electrical stimulation, CO in the presence of NO has little or no chance of stimulating guanylyl cyclase activity and contributing to NANC relaxation. Our findings are in agreement with those of Zakhary et al. (1996), who found that tin protoporphyrin-IX, a metalloporphyrin similar to ZnPP-IX inhibited endothelium-dependent and acetylcholine-dependent vasodilatation in porcine isolated aortic rings only after chemical inhibition of NOS activity. This has led to the hypothesis that CO may act as a regulator of soluble guanylyl cyclase under conditions where NO is low or absent (Ryter et al., 2004). The cross-relationship between NO and CO signalling has been exhaustively discussed elsewhere (Ryter et al., 2004). Interestingly, in the guinea-pig isolated tracheal rings CO has been shown to participate in the relaxing response due to chemical stimulation by pituitary adenylate cyclase-activating peptide 38 (PACAP 38) via a mechanism sensitive to ZnPP-IX and Rp-8Br-cyclicGMPS, an inhibitor of cGMP (Kinhult et al., 2001).
We are aware that the use of metalloporphyrins in CO research is not devoid of criticism owing to the effects of metalloporphyrins distinct from the inhibition of haem oxygenase. Two of these effects, namely inhibition of NOS and soluble guanylyl cyclase, usually obtained at concentrations higher than 10 μM (Grundemar and Ny, 1997), are not compatible with our results. The fact that ZnPP-IX was also found to inhibit relaxations to applied VIP in rat aorta (Ny et al., 1995), cat lower oesophageal sphincter (Ny et al., 1996) and guinea-pig trachea (Undem et al., 1996) deserves some comment. In the first two sets of experiments, ZnPP-IX was used at 100 μM, a concentration 10-fold higher than that used in our study. Conversely, in the guinea-pig trachea ZnPP-IX was able to reduce relaxations produced by exogenous VIP even at 10 μM, but failed to modify electrically evoked NANC relaxations in the range of 1–16 Hz (Undem et al., 1996), allowing the authors to question the participation of VIP in the NANC response (see above).
A series of experiments was performed to assess the inhibition of NO- and CO-producing enzymes together with the inhibition of soluble guanylyl cyclase. In the presence of L-NAME, ZnPP-IX and ODQ, the relaxations evoked by both 3 and 10 Hz were reduced by approximately 90%, a value similar to that obtained with TTX. This implies that a near-complete suppression of NANC responses is achieved when the production of NO and CO is blocked together with that of cGMP, the second messenger common to both transmitters.
In human airways, HO-2 immunoreactivity was detected in ganglion nerve cell bodies supplying the trachea and bronchi (Canning and Fischer, 1998). With regard to the NANC inhibitory innervation of the human airways, NO is considered to be the main transmitter (Belvisi et al., 1992), whereas a role for CO has not yet been established. Nevertheless, CO might play a role in pathophysiological conditions, as suggested by the involvement of CO in hypoxic bronchodilatation in vivo (Cardell et al., 1998a) and by the relaxing effect of exogenous CO observed in animal studies (Cardell et al., 1998b).
In conclusion, the NANC inhibitory system activated by low frequencies of stimulation in the guinea-pig trachea consists of two main components, identified by their L-NAME, ZnPP-IX and ODQ sensitivity, having NO and CO as transmitters and cGMP as a second messenger. The colocalization of NO and CO in the intrinsic tracheal inhibitory innervation is a novel finding about the neurochemical content of these neurons. The participation of CO in the NANC relaxation is unmasked after inhibition of nitrergic transmission, and this should be taken into consideration when assessing the role of CO as a potential component of NANC inhibitory transmission.
Acknowledgments
This study was supported by a grant from the Italian Ministry of University and Research (PRIN 2003 to AD, MT, SMC) and by NIH Grant DK 41301, Morphology and Imaging Core to CS.
Abbreviations
- α-CT
α-chymotrypsin
- AUC
area under the curve
- ChAT
choline-acetyltransferase
- CNS
central nervous system
- CO
carbon monoxide
- DMSO
dimethylsulphoxide
- EFS
electrical field stimulation
- HO-2
haem oxygenase-2
- L-NAME
L-nitroarginine methyl ester
- NANC
non-adrenergic, non-cholinergic
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PACAP 38
pituitary adenylate cyclase-activating peptide 38
- PGP 9.5
protein gene product 9.5
- PVC
polyvinyl chloride
- TTX
tetrodotoxin
- VIP
vasoactive intestinal peptide
- ZnPP-IX
zinc protoporphyrin-IX
Conflict of interest
The authors state no conflict of interest.
References
- Alcon S, Morales S, Camello PJ, Salido GM, Miller SM, Pozo MJ. Relaxation of canine gallbladder to nerve stimulation involves adrenergic and non-adrenergic non-cholinergic mechanisms. Neurogastroenterol Motil. 2001;13:555–566. doi: 10.1046/j.1365-2982.2001.00286.x. [DOI] [PubMed] [Google Scholar]
- Barañano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Stretton CD, Miura M, Verleden GM, Tadjkarimi S, Yacoub MH, et al. Inhibitory NANC nerves in human tracheal smooth muscle: a quest for the neurotransmitter. J Appl Physiol. 1992;73:2505–2510. doi: 10.1152/jappl.1992.73.6.2505. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Fischer A. Localization of heme oxygenase-2 immunoreactivity to parasympathetic ganglia of human and guinea-pig airways. Am J Respir Cell Mol Biol. 1998;18:279–285. doi: 10.1165/ajrcmb.18.2.3029. [DOI] [PubMed] [Google Scholar]
- Cardell LO, Lou YP, Takeyama K, Ueki IF, Lausier J, Nadel JA. Carbon monoxide, a cyclic GMP-related messenger, involved in hypoxic bronchodilation in vivo. Pulm Pharmacol Ther. 1998a;11:309–315. doi: 10.1006/pupt.1998.0152. [DOI] [PubMed] [Google Scholar]
- Cardell LO, Ueki IF, Stjarne P, Agusti C, Takeyama K, Linden A, et al. Bronchodilatation in vivo by carbon monoxide, a cyclic GMP related messenger. Br J Pharmacol. 1998b;24:1065–1068. doi: 10.1038/sj.bjp.0701878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesrown SE, Venugopalan CS, Gold WM, Drazen JM. In vivo demonstration of nonadrenergic inhibitory innervation of the guinea pig trachea. J Clin Invest. 1980;65:314–320. doi: 10.1172/JCI109674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn RF, Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973;224:1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, Chiari MC, Grana E, Subissi A, Kilbinger H. Muscarinic inhibition of acetylcholine release from a novel in vitro preparation of the guinea-pig trachea. Naunyn Schmiedeberg's Arch Pharmacol. 1990;342:141–145. doi: 10.1007/BF00166956. [DOI] [PubMed] [Google Scholar]
- Donat ME, Wong K, Staines WA, Krantis A. Heme oxygenase immunoreactive neurons in the rat intestine and their relationship to nitrergic neurons. J Auton Nerv Syst. 1999;77:4–12. doi: 10.1016/s0165-1838(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Farmer SG. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br J Pharmacol. 1989a;96:521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JL, Farmer SG. The effects of vasoactive intestinal peptide (VIP) antagonists, and VIP and peptide histidine isoleucine antisera on non-adrenergic, non-cholinergic relaxations of tracheal smooth muscle. Br J Pharmacol. 1989b;96:513–520. doi: 10.1111/j.1476-5381.1989.tb11847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol. 2004;556:325–336. doi: 10.1113/jphysiol.2003.056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Zinc protoporphyrin-IX blocks the effects of metabotropic glutamate receptor activation in the rat nucleus tractus solitarii. Mol Pharmacol. 1993;43:965–969. [PubMed] [Google Scholar]
- Grundemar L, Ny L. Pitfalls using metalloporphyrins in carbon monoxide research. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- Kinhult J, Uddman R, Cardell LO. The induction of carbon monoxide-mediated airway relaxation by PACAP 38 in isolated guinea pig airways. Lung. 2001;179:1–8. doi: 10.1007/s004080000043. [DOI] [PubMed] [Google Scholar]
- Li CG, Rand MJ. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991;102:91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JD, Dumsday B, McLean JR. Characterization of non-adrenergic, non-cholinergic inhibitory responses of the isolated guinea-pig trachea: differences between pre- and post-ganglionic nerve stimulation. Br J Pharmacol. 1999;128:458–464. doi: 10.1038/sj.bjp.0702786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D, Sethi J, Choi AMK. Carbon monoxide-dependent signalling. Crit Care Med. 2002;30:S12–S17. [PubMed] [Google Scholar]
- Ny L, Alm P, Ekstrom P, Larsson B, Grundemar L, Andersson KE. Localization and activity of haem oxygenase and functional effects of carbon monoxide in the feline lower oesophageal sphincter. Br J Pharmacol. 1996;118:392–399. doi: 10.1111/j.1476-5381.1996.tb15415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Andersson KE, Grundemar L. Inhibition by zinc protoporphyrin-IX of receptor-mediated relaxation of the rat aorta in a manner distinct from inhibition of haem oxygenase. Br J Pharmacol. 1995;115:186–190. doi: 10.1111/j.1476-5381.1995.tb16337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Morse D, Choi AMK. Carbon monoxide: to boldly go where NO has gone before. Sci STKE. 2004;230:RE6. doi: 10.1126/stke.2302004re6. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nakao S, Mori K. Reduction of depolarization-induced glutamate release by heme oxygenase inhibitor: possible role of carbon monoxide in synaptic transmission. Neurosci Lett. 1994;166:131–134. doi: 10.1016/0304-3940(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Jaffrey SR, Zakhary R. Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res Rev. 1998;26:167–175. doi: 10.1016/s0165-0173(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- Tanihata S, Uchiyama T. Role of nitric oxide in nonadrenergic, noncholinergic relaxation of whole tracheal tube preparations isolated from guinea pigs. Gen Pharmacol. 1996;27:827–832. doi: 10.1016/0306-3623(95)02083-7. [DOI] [PubMed] [Google Scholar]
- Tucker JF, Brave SR, Charalambous L, Hobbs AJ, Gibson A. L-NG-nitroarginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990;100:663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Ellis JL, Meeker S, Fischer A, Canning BJ. Inhibition by zinc protoporphyrin-IX of vasoactive intestinal peptide-induced relaxations of guinea pig isolated trachea. J Pharmacol Exp Ther. 1996;278:964–970. [PubMed] [Google Scholar]
- Venugopalan CS, Krautmann MJ, Holmes EP, Maher TJ. Involvement of nitric oxide in the mediation of NANC inhibitory neurotransmission of guinea-pig trachea. J Auton Pharmacol. 1998;18:281–286. doi: 10.1046/j.1365-2680.1998.18595.x. [DOI] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip P, Palombini B, Coburn RF. Inhibitory innervation to the guinea pig trachealis muscle. J Appl Physiol. 1981;50:374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]
- Zacour ME, Collier B, Martin G. Apamin and nonadrenergic inhibition of guinea pig trachealis. Agents Actions. 1987;22:75–81. doi: 10.1007/BF01968820. [DOI] [PubMed] [Google Scholar]
- Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Hogestatt ED, Grundemar L. Light-dependent effects of zinc protoporphyrin IX on endothelium-dependent relaxation resistant to N omega-nitro-L-arginine. Acta Physiol Scand. 1994;152:137–143. doi: 10.1111/j.1748-1716.1994.tb09793.x. [DOI] [PubMed] [Google Scholar]