Abstract
Background and purpose:
Mitogen-activated protein kinases (MAPK) are centrally involved in several mechanisms important for heart failure such as apoptosis, activation of inflammatory responses and cell proliferation. We therefore evaluated the effect of the selective p38 MAPK inhibitor SB 239063 on progression of left ventricular remodelling after myocardial infarction (MI) in rats.
Experimental approach:
Rats were treated for 9 weeks with placebo or SB 239063 by gavage (15mg kg-1) twice daily starting 7 days after ligation of the left anterior descending artery. Serial transthoracic echocardiography was performed at days 7, 36 and 70.
Key results:
Over the 9 weeks, mortality was not different between the groups. On echocardiography, animals after myocardial infarction exhibited significant left ventricular dilatation as expected (week 10, end-systolic diameter, placebo sham 5.21± 0.34 vs. placebo MI 8.44± 0.57 mm). However, there was no difference between placebo and SB 239063-treated rats (week 10, end-systolic diameter, SB MI 7.76± 0.74mm, not significantly different from placebo MI). Haemodynamics changed accordingly. Moreover, SB 239063 had no effect on left ventricular hypertrophy. Treatment with SB 239063 significantly reduced cytokine expression of tumour necrosis factor and interleukin-1β after myocardial infarction. However, collagen content was not influenced by the treatment.
Conclusion:
Despite a reduction of inflammation, treatment with the p38 inhibitor SB 239063 does not affect cardiac remodelling and cardiac function when treatment is started 7 days after myocardial infarction.
Keywords: myocardial infarction, p38, remodeling, hypertrophy
Introduction
Large myocardial infarctions lead to post-infarction ventricular remodelling, a process that is characterized by replacement of necrotic myocardium with fibrotic tissue, increased size of myocytes and fibrosis in the remote area, as well as alterations in the left ventricular structure. Several mechanisms have been proposed for left ventricular remodelling including inflammation with mitogen-activated protein kinases (MAPK) as central mediators of the inflammatory response.
MAPK are evolutionary conserved enzymes connecting cell-surface receptors to critical intracellular targets. Their activity is tightly controlled by a consecutive activation of MAPK kinases and MAPKK kinases (Chang and Karin, 2001). The MAPK signalling pathways ultimately phosphorylate and activate the terminal kinases p38, c-Jun N-terminal kinases , and extracellular signal-regulated kinases (ERKs). MAPK serve as pivotal transducers of a number of biological functions including cell growth, differentiation, proliferation and apoptosis. In the myocardium all MAPKs are expressed. They have been implicated in a variety of important physiological and pathophysiological processes: MAPK activity is significantly induced in experimental heart failure (Petrich and Wang, 2004) and inhibition of p38 MAPK reduces hypertensive cardiac hypertrophy and end-organ damage (Behr et al., 2001). Their precise role in cardiac hypertrophy has not been exactly defined, despite the use of several transgenic animals to delineate further the role of p38 in cardiac hypertrophy. Although expression of a dominant-negative mutant of p38 does not affect cardiac hypertrophy induced by pressure overload (Zhang et al., 2003), the dominant-negative mutants used in the laboratory of Braz et al. (2003) displayed enhanced myocyte growth, a process involving calcineurin-NFAT signalling, which suggests that p38 is an antagonist of the hypertrophic response. Thus, important questions regarding p38 signalling and cardiac remodelling still need to be answered.
MAPK signalling cascades represent an attractive intermediate signalling transduction target for pharmacological intervention as they have an important role in apoptosis, induction of inflammatory responses and cell proliferation, all mechanisms involved in the development of heart failure. We tested the hypothesis that inhibition of p38 MAPK with SB 239063 improves left ventricular remodelling after myocardial infarction (MI) in rats by preventing the inflammatory responses.
Methods
Experimental MIs
All procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Male Wistar rats weighing 180–200 g underwent MI or sham operations. Left coronary artery ligation was induced by a previously described technique (Gaudron et al., 1997). Sham operation was performed using an identical procedure, except that the suture was passed under the coronary artery without ligation. Animals were randomly allocated to receive twice daily doses, by gavage, of placebo or the MAPK inhibitor SB 239063 (15 mg kg−1 bodyweight) starting 7 days after MI as previously described (Frantz et al., 2004).
In vivo haemodynamic measurements
Ten weeks after left coronary artery ligation or sham operation, haemodynamic measurements were performed as described previously (Hu et al., 1998). Left ventricular systolic and end-diastolic pressures (LVSP, LVEDP), mean arterial pressure (MAP) and heart rate (HR) were measured under light isoflurane anaesthesia and spontaneous respiration.
Echocardiography
Ultrasound analyses were performed by a single researcher experienced in rodent echocardiography, unaware of the treatment, at days 7, 36 and 70 immediately prior to killing the animals, as recently described (Frantz et al., 2004). From two-dimensional short-axis imaging, endocardial borders were traced at end-systole and end-diastole, utilizing a prototype off-line analysis system (NICE, Tomtech, The Netherlands). Measurements were performed at the mid-papillary and the apical muscle level. The end-systolic (smallest) and end-diastolic (largest) cavity areas were determined. Using the end-systolic and end-diastolic areas, fractional area changes were calculated [(end-diastolic area – end-systolic area)*end-diastolic area−1]. From two-dimensionally targeted M-mode tracings, end-diastolic diameter and end-systolic diameter were measured. Fractional shortening was calculated. Only animals with an infarct size between 30 and 60% of the left ventricle were included.
Sample collection and determination of infarct size
After echocardiographic measurements, hearts were excised and dissected into atria, right and left ventricle, including septum. The left ventricle was cut into three transverse sections: apex, middle ring and base as previously described (Bauersachs et al., 2001). From the middle ring, 5 μm-sections were cut and stained with picrosirius red. The boundary lengths of the infarcted and non-infarcted endocardial and epicardial surfaces were traced with a planimeter digital image analyser. Infarct size (fraction of the infarcted left ventricle) was calculated as the average of percentage of length of epicardial and endocardial circumference.
Real-time PCR
Myocardial RNA isolation and real-time PCR measurements were performed as previously described (Frantz et al., 2005) with TaqMan probes for 18S, interleukin-1β (IL-1β), tumour necrosis factor (TNF), collagen 1, atrial natriuretic peptide (ANP) and myosin heavy chain (MHC). RNA samples were normalized to 18S rRNA.
p38 acitivity
The activation of p38 was measured by the activation of its downstream target, the MAPKAP-2, in aortic tissue. The tissue was taken from a study after 8 weeks of treatment with the p38 inhibitor SB 239063 as recently presented by us (Widder et al., 2004).
Materials
SB 239063 (trans-1-(4-hydroxycyclohexyl)-4-(4-fluorophenyl methoxypyridimidin-4-yl) imidazole) was provided by GlaxoSmithKline (King of Prussia, PA, USA). TaqMan probes of IL-1β, ANP, collagen 1 and TNF were obtained from Applied Biosystems (Foster City, CA, USA).
Statistical analysis
All data are expressed as mean and s.e.m. Absolute differences between groups were compared using a two-way analysis of variance adjusted by the Fisher rule. Statistical significance was achieved when two-tailed P<0.05. Statistical analyses were carried out using StatView statistics programme (Abacus Concepts, Inc., Berkley, CA, USA).
Results
Mortality and organ weights
Rats were randomly allocated into SB 239063 or a placebo group, 7 days after MI. Mortality after coronary artery ligation was not different between the two groups: all animals in the SB 239063 group and all animals in the placebo group survived until day 70.
Body weights were slightly, but not significantly reduced in animals treated with SB 239063 (Table 1). Infarct size determined 10 weeks after MI was comparable in both groups (placebo vs SB 239063, 43.8±3.2 vs 42.6±2.0%).
Table 1.
Global parameters and haemodynamic measurements
| Sham | Sham | MI | MI | |
|---|---|---|---|---|
| placebo | SB 239063 | placebo | SB 239063 | |
| n | 15 | 16 | 5 | 8 |
| MI size (%) | 43.8±3.2 | 42.6±2.0 | ||
| bw | 377±4 | 358±4 | 380±9 | 358±9 |
| Heart weight bw−1 | 2.2±0.1 | 2.0±0.5 | 2.3±0.5 | 2.9±0.4 |
| LVSP (mm Hg) | 133±3 | 137±4 | 123±7 | 135±8 |
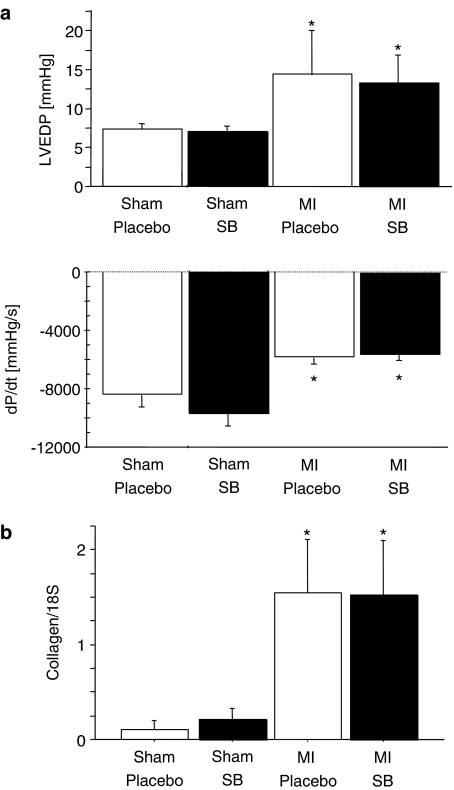
| LVEDP (mm Hg) | 7±1 | 7±1 | 14±6* | 13±3* |
| dP/dtmax (mm Hg* s−1) | 9104±728 | 11 020±732 | 7920±563 | 7589±625* |
| dP/dtmin (mm Hg* s−1) | −8391±832 | −9775±867 | −5279±545* | −5600±462* |
Abbreviations: bw, body weight; dP/dtmax, the maximum rate of pressure rise; dP/dtmin, the maximum rate of pressure decline; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure.
Data are mean±s.e.m., n indicates number of animals studied.
P<0.05 Sham vs MI.
Echocardiographic and haemodynamic measurements
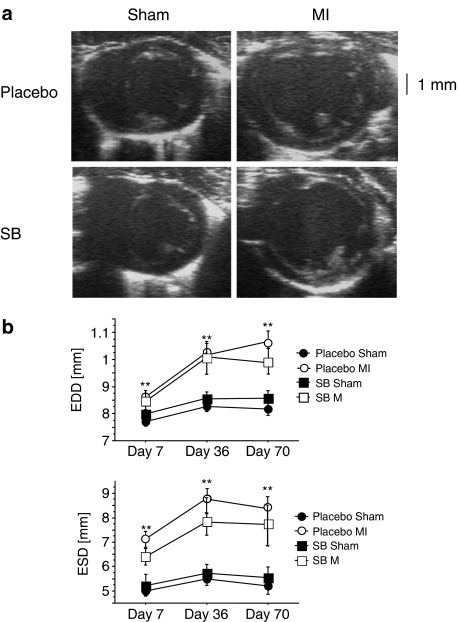
Animals underwent echocardiography at days 7, 36 and 70 post-MI. All measurements were recorded at two levels: at the apical level, which shows changes within the infarcted region, and at the mid-papillary level, which shows changes in the dimensions of the surviving un-infarcted myocardium. Left ventricles of infarcted animals were dilated significantly when compared to those from sham-operated animals (see Figure 1, Table 2). However, treatment with SB 239063 did not improve left ventricular dilatation.
Figure 1.
Echocardiographic analyses. The development of left ventricular dilatation (b) and representative examples (a) of two-dimensional echocardiography of sham-operated and infarcted animals 10 weeks after MI. These images were obtained from short-axis imaging at the mid-papillary level. In the infarcted animal, dilatation of the left ventricular cavity can be detected. SB 239063 treatment had no effect on left ventricular dilatation (**P⩽0.01, sham vs MI).
Table 2.
Echocardiographic measurements at week 10
| Sham | Sham | MI | MI | |
|---|---|---|---|---|
| placebo | SB 239063 | placebo | SB 239063 | |
| n | 13 | 14 | 5 | 9 |
| Heart rate (b.p.m.) | 232±8 | 229±5 | 235±4 | 233±17 |
| Papillary | ||||
| ESD (mm) | 5.21±0.34 | 5.66±0.46 | 8.44±0.57** | 7.76±0.74** |
| EDD (mm) | 8.12±0.25 | 8.65±0.29 | 10.60±0.54** | 9.91±0.49** |
| FS (%) | 36.6±2.3 | 35.6±3.3 | 20.6±1.6* | 23.3±4.5* |
| Apical | ||||
| ESD (mm) | 4.83±0.39 | 5.55±0.51 | 8.34±0.57** | 7.57±0.81** |
| EDD (mm) | 7.85±0.26 | 8.05±0.26 | 10.14±0.44** | 9.52±0.43** |
| FS (%) | 37.7±2.3 | 36.5±2.9 | 17.4±2.8** | 21.3±3.5** |
Abbreviations: EDD, end-diastolic diameter; ESD, end-systolic diameter; FS, fractional shortening.
Data are mean±s.e.m., n indicates number of animals studied.
P<0.05
P<0.01 sham vs MI.
Ten weeks after ligation of the left coronary artery, haemodynamic measurements were obtained from the animals. Consistent with the echocardiographic data, LVSP, dP/dtmax and dP/dtmin were significantly lower and LVEDP significantly higher in rats after MI. Treatment with SB 239063 did not improve these haemodynamic variables (see Table 1 and Figure 2).
Figure 2.
Haemodynamic measurements. (a) dP/dtmin (the maximum rate of pressure decline) and LVEDP in sham-operated rats and in rats treated with SB 239063 or placebo (*P⩽0.05, sham vs MI). (b) Collagen was not influenced by the treatment (*P⩽0.05, placebo vs MI).
Molecular measurements
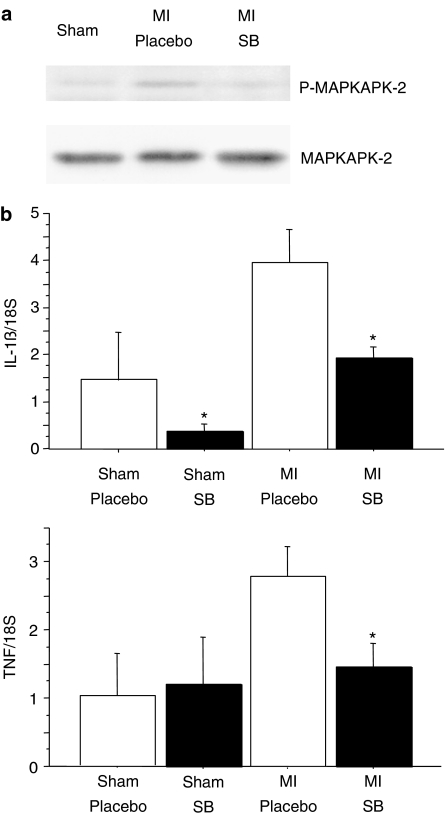
Activation of MAPKAP-2, the downstream target of p38 (see Figure 3) was reduced in rats, treated for 8 weeks with the p38 inhibitor SB239063 after MI, as previously shown (Widder et al., 2004).
Figure 3.
MAPKAP-2 and cytokine activation. (a) Phosphorylation of MAPKAP-2, the downstream target of activated p38, is significantly reduced after treatment with the p38 inhibitor SB 239063. (b) Interleukin-1β as well as TNF are significantly downregulated in animals treated with the p38 inhibitor SB 239063 after myocardial infarction (*P⩽0.05, placebo vs SB 239063).
Molecular indices of left ventricular hypertrophy, MHC and ANP, were not affected by SB 239063 treatment (βMHC αMHC−1, placebo MI vs SB 239063 MI, 2.1±0.6 vs 2.1±0.7, NS; ANP, placebo MI vs SB 239063 MI, 2.4±0.9 vs 2.2±0.5, NS). Collagen I RNA expression was not influenced by the treatment (Figure 2, normalized to 18S, placebo MI vs SB 239063 MI, 1.5±0.6 vs 1.5±0.6, NS), but was significantly higher than in sham-operated animals (placebo normalized to 18S, placebo vs SB 239063, 0.1±0.09 vs 0.2±0.1).
However, as expected, the proinflammatory cytokine interleukin 1β (Figure 3, normalized to 18S, placebo MI vs SB 239063 MI, 3.9±0.7 vs 1.9±0.2, P=0.006) as well as TNF (Figure 3, normalized to 18S, placebo MI vs SB 239063 MI, 2.8±0.4 vs 1.5±0.4, P=0.04) were significantly reduced in animals treated with SB 239063 after myocardial infarction.
Discussion
p38 MAPK signalling has been implicated in the progression of chronic heart failure and hypertrophy (See et al., 2004). This study failed to prove the hypothesis that treatment with the p38 MAPK inhibitor SB 239063 prevents left ventricular remodelling and mortality after myocardial infarction, although treatment with SB 239063 did reduce the inflammatory response.
p38 MAPK is rapidly activated in rats after myocardial infarction (Shimizu et al., 1998). Sustained p38 activation has been described in animal models (Behr et al., 2001) and in humans (Cook et al., 1999) with heart failure. Moreover, p38 plays an important role in cardiac remodelling. Mice with targeted activation of p38 MAP kinase in ventricular myocytes develop signs of heart failure and cardiac fibrosis, and ultimately die prematurely at 7–9 weeks (Liao et al., 2001). In spontaneously hypertensive stroke-prone rats, treatment with the p38 MAPK inhibitor SB 239063 has been shown to reduce pro-inflammatory gene expression and enhance survival (Behr et al., 2001). Furthermore, vascular p38 MAPK is markedly activated in rats after MI, and treatment with SB 239063 prevents post-infarction endothelial vasomotor dysfunction (Widder et al., 2004). Thus, there was a strong rationale to hypothesize that p38 MAPK inhibition would improve left ventricular remodelling and prevent the progression of heart failure.
Why did we not detect protective effects against MI with the MAPK inhibitor SB239063 despite it significantly reducing the production of proinflammatory cytokines? Firstly, several mechanisms have been suggested to contribute to left ventricular remodelling. These include, among others, alterations in left ventricular function (Carluccio et al., 2006), myocyte apoptosis, myocyte regeneration, neurohumoral activation and inflammatory responses. However, even though we found that the inflammatory response in MI was reduced by SB 239063, the remodelling process was not altered by this treatment. Thus, either the inflammatory cascade was only partially blocked by SB239063 or, what seems to be more likely inflammation cannot entirely explain the complex pathophysiology of ischaemic remodelling.
Secondly, SB 239063, the drug used in our experiments, is a second-generation and highly selective p38 MAPK inhibitor (IC50 for p38 inhibition: 0.044 μM, ERK1/2>10 μM) (Barone et al., 2001). However, p38 has an α- and a β-isoform with distinct functions: for example, myocyte apoptosis is enhanced by p38-α, whereas in contrast, survival is improved by p38-β (Wang et al., 1998). With regard to ischaemia, only mice with expression of the dominant-negative mutant form of p38α have so far been tested in a cardiac ischaemic model and found to be protected from ischaemic injury (Ren et al., 2005). Thus, p38 isoform-specificity of the drug might influence the results and explain the different results obtained in our study from that of See et al. (2004): they found protective effects when rats were treated for 3 weeks after myocardial infarction with the p38 inhibitor, RWJ-67657, which inhibits both p38 isoforms. Yet, in this latter study, infarct sizes were only small and only minor protective echocardiographic effects were found; this might also account for the different results obtained between the two studies.
Thirdly, the effects seem to be species-specific. Indeed, the p38 MAPK inhibitor, SB 239063, used in the present study reduced ischaemia/reperfusion injury in a rat model, when infused minutes before ischaemia (Kaiser et al., 2005). In contrast, SB 239063 did not reduce ischaemia/reperfusion injury in a pig model (Kaiser et al., 2005). Thus, our results in rats could be different to results in mice.
Fourthly, studies exploring effects on left ventricular remodelling after MI need to be divided into those where treatment is given before or at the time of MI and those where the treatment is delayed until after myocardial infarction. Early treatment may reduce infarct size with consequent improvement of remodelling. In contrast, studies with late treatment look at remodelling independently of infarct size reduction. Most of the studies that started p38 inhibition before or early after cardiac injury reported protective effects: ischaemia/reperfusion injury was significantly reduced in rats pretreated with the same p38 inhibitor as that used in our study, SB 239063 (Gao et al., 2002). Mice treated immediately after permanent coronary artery ligation with the p38 inhibitor SC-409 were protected from the effects of ischaemic injury (Liu et al., 2005). The p38 MAPK inhibitor SB 203580 only reduced infarct size when administered early after myocardial infarction but not after a delay (Gorog et al., 2004). We started treatment 1 week after myocardial infarction to avoid effects on infarct size and early activation of inflammatory responses. Thus, p38 seems to affect the acute healing phase after ischaemic injury, but may be less important for left ventricular remodelling when viewed independently of its effects on infarct size.
In conclusion, we did not detect significant effects of p38 MAPK inhibition on left ventricular remodelling after MI. Although other animal studies suggest that p38 might be an attractive target for improving heart failure progression, the data available indicate that inhibition of this kinase does not always induce a clear, protective effect. However, the timing and dosing regime seem to be important determinants of the effectiveness of a p38 inhibitor in protecting the heart from the injurious effects of MI, as well as the type of drug and its specificity, especially with regard to p38 α and β isoforms. The function of these inhibitors and their role in cardiac remodelling need to be evaluated further.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (TB and SF, Sonderforschungsbereich 688, TPA10, JB SFB355, TPB10). We thank H Wagner, B Bayer, and N Kehl for their technical support.
Abbreviations
- ANP
atrial natriuretic peptide
- bw
body weight
- dP/dtmax
maximum rate of pressure rise
- dP/dtmin
maximum rate of pressure decline
- EDD
end-diastolic diameter
- ESD
end-systolic diameter
- ERK
extracellular signal-regulated kinase
- FS
fractional shortening
- HR
heart rate
- IL
interleukin
- JNK
c-Jun N-terminal kinases
- LV
left ventricle
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic
- MAP
mean arterial pressure
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinases
- MHC
myosin heavy chain
- MI
myocardial infarction
- TNF
tumour necrosis factor
Conflict of interest
The authors state no conflict of interest.
References
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, et al. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–985. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- Behr TM, Nerurkar SS, Nelson AH, Coatney RW, Woods TN, Sulpizio A, et al. Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation. 2001;104:1292–1298. doi: 10.1161/hc3601.094275. [DOI] [PubMed] [Google Scholar]
- Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carluccio E, Biagioli P, Alunni G, Murrone A, Giombolini C, Ragni T, et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization: evidence that dyssynergy might directly induce cardiac remodeling. J Am Coll Cardiol. 2006;47:969–977. doi: 10.1016/j.jacc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cook SA, Sugden PH, Clerk A. Activation of c-Jun N-terminal kinases and p38-mitogen-activated protein kinases in human heart failure secondary to ischaemic heart disease. J Mol Cell Cardiol. 1999;31:1429–1434. doi: 10.1006/jmcc.1999.0979. [DOI] [PubMed] [Google Scholar]
- Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, et al. Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J. 2005;19:591–593. doi: 10.1096/fj.04-2459fje. [DOI] [PubMed] [Google Scholar]
- Frantz S, Hu K, Widder J, Bayer B, Witzel CC, Schmidt I, et al. Peroxisome proliferator activated-receptor agonism and left ventricular remodeling in mice with chronic myocardial infarction. Br J Pharmacol. 2004;141:9–14. doi: 10.1038/sj.bjp.0705585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yue TL, Shi DW, Christopher TA, Lopez BL, Ohlstein EH, et al. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc Res. 2002;53:414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Gaudron P, Hu K, Schamberger R, Budin M, Walter B, Ertl G. Effect of endurance exercise training early or late after coronary artery occlusion on left ventricular remodeling, hemodynamics, and survival in rats with chronic transmural myocardial infarction. Circulation. 1997;89:402–412. doi: 10.1161/01.cir.89.1.402. [DOI] [PubMed] [Google Scholar]
- Gorog DA, Tanno M, Cao X, Bellahcene M, Bassi R, Kabir AM, et al. Inhibition of p38 MAPK activity fails to attenuate contractile dysfunction in a mouse model of low-flow ischemia. Cardiovasc Res. 2004;61:123–131. doi: 10.1016/j.cardiores.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Hu K, Gaudron P, Anders HJ, Weidemann F, Turschner O, Nahrendorf M, et al. Chronic effects of early started angiotensin converting enzyme inhibition and angiotensin AT1-receptor subtype blockade in rats with myocardial infarction: role of bradykinin. Cardiovasc Res. 1998;39:401–412. doi: 10.1016/s0008-6363(98)00090-x. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Lyons JM, Duffy JY, Wagner CJ, McLean KM, O'Neill TP, et al. Inhibition of p38 reduces myocardial infarction injury in the mouse but not pig following ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H2747–H2751. doi: 10.1152/ajpheart.01280.2004. [DOI] [PubMed] [Google Scholar]
- Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, et al. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Card Fail. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Wang Y. Stress-activated MAP kinases in cardiac remodeling and heart failure; new insights from transgenic studies. Trends Cardiovasc Med. 2004;14:50–55. doi: 10.1016/j.tcm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Yoshiyama M, Omura T, Hanatani A, Kim S, Takeuchi K, et al. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc Res. 1998;38:116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Widder J, Behr T, Fraccarollo D, Hu K, Galuppo P, Tas P, et al. Vascular endothelial dysfunction and superoxide anion production in heart failure are p38 MAP kinase-dependent. Cardiovasc Res. 2004;63:161–167. doi: 10.1016/j.cardiores.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]