Abstract
Background and purpose:
5α,8α-Epidioxy-22E-ergosta-6, 22-dien-3β-ol (ergosterol peroxide) is a major antitumour sterol produced by edible or medicinal mushrooms. However, its molecular mechanism of action has yet to be determined. Here, we examine the anticancer and anti-inflammatory effects of ergosterol peroxide.
Experimental approach:
After treating RAW264.7 macrophages with LPS and purified ergosterol peroxide or ergosterol, we determined LPS-induced inflammatory cytokines, nuclear DNA binding activity of transcription factors and phosphorylation of MAP kinases (MAPKs). HT29 colorectal adenocarcinoma cells were treated with ergosterol peroxide for 5 days. To investigate the antitumour properties of ergosterol peroxide, we performed DNA microarray and RT-PCR analyses and determined the reactive oxygen species (ROS) in HT29 cells.
Key results:
Ergosterol peroxide suppressed LPS-induced TNF-α secretion and IL-1α/β expression in RAW264.7 cells. Ergosterol peroxide and ergosterol suppressed LPS-induced DNA binding activity of NF-κB and C/EBPβ, and inhibited the phosphorylation of p38, JNK and ERK MAPKs. Ergosterol peroxide down-regulated the expression of low-density lipoprotein receptor (LDLR) regulated by C/EBP, and HMG-CoA reductase (HMGCR) in RAW264.7 cells. In addition, ergosterol peroxide showed cytostatic effects on HT29 cells and increased intracellular ROS. Furthermore, ergosterol peroxide induced the expression of oxidative stress-inducible genes, and the cyclin-dependent kinase inhibitor CDKN1A, and suppressed STAT1 and interferon-inducible genes.
Conclusion and Implication:
Our results suggest that ergosterol peroxide and ergosterol suppress LPS-induced inflammatory responses through inhibition of NF-κB and C/EBPβ transcriptional activity, and phosphorylation of MAPKs. Moreover, ergosterol peroxide appears to suppress cell growth and STAT1 mediated inflammatory responses by altering the redox state in HT29 cells.
Keywords: ergosterol peroxide, inflammation, RAW264.7 macrophage-like cells, NF-κB, C/EBPβ, HT29 human colorectal adenocarcinoma cells, reactive oxigen species (ROS)
Introduction
Mushrooms have been used as traditional medicines since ancient times, and the results of recent studies have elucidated the anticancer actions of various mushroom components. The best known of these is the immunomodulatory effect of polysaccharides belonging to the β-glucans. The β-glucans have been shown to stimulate the mononuclear phagocyte system by binding to complement receptor CR3 and some lymphocyte to produce cytokines such as interferons (IFNs) and interleukins (ILs) (Zaidman et al., 2005). In clinical trials, the β-D-glucans, such as Lentinan from Lentinus edodes and PSK (or PSP) from Trametes versicolor, were shown to have some beneficial effects against cancer (Kidd, 2000; Zaidman et al., 2005). The β-D-glucans are not particularly effective, but, because they are non-toxic and well tolerated, they are expected to enhance the potency of chemotherapy or radiation therapy (Kidd, 2000). Many of the secondary metabolites have been shown to inhibit cancer cell growth in vitro (Zaidman et al., 2005). The fungal sterol, ergosterol, is abundant in mushrooms and is known to be pro-vitamin D2. Ergosterol was shown to inhibit phorbol-12-myristate 13-acetate (TPA)-induced inflammatory ear oedema in mice (Yasukawa et al., 1994), and vitamin D2 has been shown to contribute to prevention of prostate and colon cancer (Guyton et al., 2003). The peroxide of ergosterol, 5α,8α-epidioxy-22E-ergosta-6, 22-dien-3β-ol (ergosterol peroxide), is common in mushrooms (Figure 1a) (Bok et al., 1999; Yaoita et al., 2002; Takei et al., 2005), and has been shown to inhibit the growth of some cancer cells and to induce apoptosis of HL60 human leukaemia cells (Bok et al., 1999; Takei et al., 2005). Ergosterol peroxide inhibits TPA-induced inflammation and tumour promotion in mice (Yasukawa et al., 1994), and also decreases lipid peroxidation of rat liver microsomes and suppresses proliferation of mouse and human lymphocytes stimulated with mitogens (Fujimoto et al., 1994; Kim et al., 1999; Kuo et al., 2003). Thus, ergosterol peroxide shows antitumour, antioxidative and immunosuppressive properties, but the molecular mechanism of its antitumour action has not been clarified.
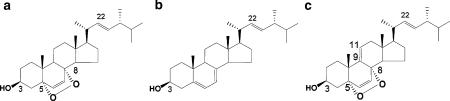
Figure 1.
Structures of ergosterol peroxide (a), ergosterol (b) and 9,11-dehydroergosterol peroxide (c).
In our previous study, we screened edible mushroom extracts for apoptosis-inducing effects, and our results indicated that the extract of Sarcodon aspratus (Berk.) S. Ito strongly induces apoptosis in HL60 leukaemia cells (Takei et al., 2005). We purified and identified ergosterol peroxide as a major antitumour sterol present in S. aspratus extract (Takei et al., 2005). To determine the possible utility of ergosterol peroxide as a chemopreventive agent or a dietary factor that contributes to cancer prevention, we examined the anticancer and anti-inflammatory effects of purified ergosterol peroxide.
Methods
Purification of ergosterol peroxide, ergosterol and 9,11-ergosterol peroxide
Ergosterol peroxide, ergosterol and 9,11-dehydroergosterol peroxide were purified from S. aspratus (Berk.) S. Ito as described previously with some modifications (Takei et al., 2005, Kobori et al. 2006). Briefly, S. aspratus was extracted with acetone, and ergosterol peroxide and 9,11-dehydroergosterol peroxide were isolated from the S. aspratus acetone extract by OASIS HLB column chromatography (Waters, Milford, MA, USA), silica gel column chromatography (Merck, Darmstadt, Germany) and reverse-phase HPLC using a C18 column (Inertsil ODS-3 column, GL Science Inc., Tokyo, Japan). Ergosterol was precipitated from the eluate of the silica gel column. Ergosterol peroxide, ergosterol and 9,11-dehydroergosterol peroxide were identified by ultraviolet spectroscopy, mass spectrometry and 13C- and 1H-NMR.
Cells and cell culture
RAW264.7 mouse macrophage-like cells (ATCC TIB-71) and HT29 human colorectal adenocarcinoma cells (ATCC HTB38) were purchased from Dainippon Pharmaceutical Co. (Osaka, Japan). CACO-2 human colon carcinoma cells (RCB0988) and WI38 human lung embryonal fibroblasts (RCB702) were provided by RIKEN Cell Bank (Tsukuba, Japan). RAW264.7 cells were maintained in RPM1640 medium (Invitrogen Japan KK, Tokyo, Japan). HT29 cells were grown in McCoy 5A medium (Invitrogen). WI38 cells were maintained in minimal essential medium (MEM; Invitrogen) and CACO-2 cells were maintained in MEM with 0.1 mM non-essential amino acids. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air in medium supplemented with 10% heat-inactivated fetal calf serum (ICN Biomedicals, Inc., Aurora, OH, USA or JRH Bioscience, Lenexa, KS, USA).
Treatment of cells with ergosterol peroxide
RAW264.7 cells were seeded at 5 × 104 cells ml−1 on a 96-well plate, incubated for 24 h and then treated with ergosterol peroxide (0, 15, 30 or 60 μM) and 1 ng ml−1 lipopolysaccharide (LPS; from Escherichia coli Serotype 0111:B4; Sigma-Aldrich Co., St Louis, MO, USA) for 6 h to examine the suppressive effects on inflammatory responses. Effects of ergosterol and 9,11-dehydroergosterol peroxide on tumour necrosis factor-α (TNF-α) production induced by LPS in RAW264.7cells were determined under the same conditions as used for ergosterol peroxide. Otherwise RAW264.7 cells were seeded at 1 × 105 cells ml−1 on a 96-well plate and incubated with LPS for 2 h before addition of ergosterol peroxide to the medium. The cells were then further incubated for 6 h before determining TNF-α production and cell viability. The TNF-α levels in the culture medium were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience, Inc., San Diego, CA, USA). HT29, CACO-2 and WI38 cells were seeded at 1 × 104 cells ml−1 on a 96-well plate, incubated for 24 h to allow the cells to adhere to the bottom of the culture dishes and then treated with ergosterol peroxide for 5 days. Cell viability was determined spectrophotometrically using WST-1 reagent (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer's instructions.
Cell cycle analysis and determination of apoptosis
Ht29 cells (1 × 105 cells ml−1) were incubated for 5 days. Cells were stained with propidium iodide by a Cycle Test Plus DNA Reagent Kit according to the manufacturer's instructions (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), and then analysed by FACSort (Becton Dickinson Immunocytometry Systems) with ModFit LT (Ver.2.0, Verity Software House Inc. Topsham, ME, USA) software. The distribution of DNA content was expressed as the percentage of total diploid cells. The proportion of hypodiploid cells was expressed as a percentage of the total cell population.
Determination of nuclear factor-κB and C/EBPβ DNA-binding activity
RAW264.7 cells (1 × 105 cells ml−1) were treated with ergosterol peroxide (0, 30 or 60 μM) or ergosterol (0, 30 or 60 μM) for 6 h and 2 ng ml−1 LPS for 30 min. Nuclear extract of the cells was then prepared by a Nuclear Extraction Kit according to the manufacturer's instructions (Active Motif, Carlsbad, CA, USA). The protein concentration of the extract was determined by a Bradford-based assay (Bio-Rad Protein assay, Bio-Rad Laboratories, Richmond, CA, USA). DNA-binding activity of nuclear factor-κB (NF-κB) in 0.5 μg of protein of each nuclear extract was determined using a TransAM NF-κB Chemi kit (Active Motif). The NF-κB p65 bound to the immobilized oligonucleotide that contained a p65-binding site was detected by ELISA, with chemiluminescent reagent, according to the manufacturer's instructions. DNA-binding activity of C/EBPβ using 2 μg of protein from each nuclear extract was determined using a TransAM C/EBPβ kit (Active Motif). The C/EBPβ bound to the immobilized oligonucleotide, that contained a C/EBP-binding site, was detected spectrophotometrically by ELISA, according to the manufacturer's instructions. All assays were performed in triplicate and data are expressed as means±s.d.
Western blotting
RAW264.7 cells (1 × 105 cells ml−1) were treated with ergosterol peroxide or ergosterol for 6 h and LPS for 15 min. The cells were lysed in a loading buffer (10 mM Tris-HCl, pH 6.8, 1% sodium dodecyl sulphate (SDS), 1% 2-mercaptoethanol and 10% glycerol). Whole-cell lysates (2 × 104 cells lane−1) were separated by 12% SDS–polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane (Amersham, Chalfont St Giles, UK). Mitogen-activated protein kinases (MAPKs) and phospho-MAPKs were detected using antibodies (rabbit anti-MAPKs (p38, Jun N-terminal kinase (JNK) and Jun N-terminal kinase (ERK) (p44/42)); Cell Signalling Technology, Danvers, MA, USA) and anti-phopho-MAPKs (phospho-p38, phospho-JNK, phospho-ERK(p44/42); Cell Signalling Technology) and ECL Plus detection system (Amersham). α-Tubulin was determined as loading control using mouse anti- α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
RNA isolation and cDNA microarray analysis
HT29 cells (1 × 104 cells ml−1) were treated with 2 or 7 μM ergosterol peroxide for 5 days. Total RNA was extracted from the cells (3 × 105 cells) using TRIzol reagent (Invitrogen) and then isolated using an RNeasy Mini Kit (Qiagen KK, Tokyo, Japan) according to the manufacturer's instructions. Double-stranded cDNA was synthesized using the SuperScript Choice System (Invitrogen) with a T7-(dT)24 primer (Affymetrix Japan KK, Tokyo, Japan), and then biotin-labelled cRNA was synthesized using an Enzo BioArray RNA Transcript Labelling kit (Affymetrix). The biotin-labelled cRNA was further purified and fragmented using the GeneChip Sample Cleanup Module (Affymetrix). Aliquots of 15 μg of fragmented cRNA were hybridized to an array (HG-Focus array, Affymetrix) at 45°C for 16 h. After hybridization, the gene chips were washed and stained using a GeneChip Fluidics Station 400 (Affymetrix), and then scanned with an Aglient GeneArray Scanner (Affymetrix) with Affymetrix Microarray Suite Ver. 5.0. Data analysis was performed with a Microarray Suite and GeneSpring Ver.7.0 (Silicon Genetics, Redwood City, CA, USA). Statistical analysis of differences in the dosages was performed by one-way ANOVA. The up- or down-regulated genes that showed differences in expression of more than twofold and P<0.05 are shown in Table 1. Data are expressed as means±s.d. of triplicate cultures. The extent of changes (-fold) was calculated as the normalized intensity in ‘ergosterol peroxide-treated' vs. ‘control' cells.
Table 1.
Alterations in gene expression by ergosterol peroxide in HT29 cells
|
Fold of change in EPO-treated cells |
||||||
|---|---|---|---|---|---|---|
| GenBank Accession No. | Gene symbol | Gene name | Signal of control cells | 2 μM EPO | 7 μM EPO | P-value |
| Up-regulated genes | ||||||
| U05598 | AKR1C2 | Aldo-keto reductase family 1, member C2 | 186.1±26.4 | 2.80±0.37 | 18.68±1.68 | 0.0188 |
| N30649 | SQSTM1 | Sequestosome 1 | 62.4±8.7 | 1.45±0.20 | 6.54±0.70 | 0.0257 |
| NM_001353 | AKR1C1 | Aldo-keto reductase family 1, member C1 | 273.1±41.0 | 4.06±0.48 | 25.74±5.44 | 0.0343 |
| M24779 | PIM1 | — | 55.9±1.6 | 1.11±0.21 | 2.54±0.06 | 0.0343 |
| M57731 | CXCL2 | Chemokine (C-X-C motif) ligand 2 | 29.9±2.7 | 0.67±0.34 | 6.01±0.42 | 0.0348 |
| NM_000691 | ALDH3A1 | Aldehyde dehydrogenase 3 family, memberA1 | 66.3±6.9 | 2.06±0.14 | 5.16±0.58 | 0.0366 |
| Down-regulated genes | ||||||
| M97935 | STAT1 | Signal transducer and activator of transcription 1 | 605.3±236.9 | 0.36±0.00 | 0.19±0.00 | 0.00135 |
| NM_005532 | IFI27 | Interferon, alpha-inducible protein 27 | 4768.5±372.8 | 0.56±0.07 | 0.08±0.01 | 0.0186 |
| NM_002462 | MX1 | Myxovirus (influenza virus) resistance 1 | 1606.6±175.4 | 0.52±0.05 | 0.04±0.01 | 0.0222 |
| NM_005101 | G1P2 | Interferon, alpha-inducible protein (clone IFI-15 K) | 7039.4±427.6 | 0.74±0.12 | 0.18±0.01 | 0.0222 |
| AA749101 | IFITM1 | Interferon induced transmembrane protein 1 (9-27) | 2482.5±362.9 | 0.34±0.04 | 0.02±0.00 | 0.0222 |
| NM_006820 | C1orf29 | Interferon-induced protein 44-like | 793.9±42.3 | 0.35±0.06 | 0.01±0.00 | 0.0226 |
| NM_016817 | OAS2 | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | 602.3±16.1 | 0.28±0.05 | 0.06±0.01 | 0.0226 |
| NM_002463 | MX2 | Myxovirus (influenza virus) resistance 2 | 713.1±123.9 | 0.40±0.02 | 0.07±0.01 | 0.0226 |
| NM_004509 | SP110 | SP110 nuclear body protein | 657.4±50.4 | 0.66±0.05 | 0.17±0.02 | 0.0226 |
| BF338947 | IFITM3 | Interferon induced transmembrane protein 3 (1-8U) | 3787.0±635.5 | 0.64±0.05 | 0.12±0.01 | 0.0226 |
| NM_006187 | OAS3 | 2′-5′-oligoadenylate synthetase 3, 100kDa | 1506.1±165.0 | 0.62±0.05 | 0.17±0.02 | 0.0226 |
| NM_001549 | IFIT4 | Interferon-induced protein with tetratricopeptide repeats 3 | 1220.6±79.7 | 0.43±0.02 | 0.08±0.02 | 0.023 |
| NM_006435 | IFITM2 | Interferon induced transmembrane protein 2 (1-8D) | 2102.4±321.6 | 0.58±0.06 | 0.13±0.01 | 0.0239 |
| AI825926 | PLSCR1 | Phospholipid scramblase 1 | 1963.2±39.2 | 0.74±0.11 | 0.31±0.02 | 0.0257 |
| NM_001548 | IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 1139.6±147.4 | 0.41±0.12 | 0.05±0.01 | 0.0272 |
| NM_022873 | G1P3 | Interferon, alpha-inducible protein (clone IFI-6-16) | 843.6±199.1 | 0.51±0.05 | 0.08±0.01 | 0.0348 |
| NM_014314 | RIG-I | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 292.3±58.4 | 0.46±0.07 | 0.09±0.01 | 0.0348 |
| NM_006084 | ISGF3G | Interferon-stimulated transcription factor 3, gamma 48 kDa | 648.7±35.1 | 0.82±0.06 | 0.31±0.03 | 0.0487 |
| NM_006417 | IFI44 | Interferon-induced protein 44 | 922.5±74.9 | 0.52±0.08 | 0.07±0.02 | 0.0496 |
HT29 cells were treated with 2 or 7 μM ergosterol peroxide (EPO) for 5 days, and subjected to DNA microarray analysis using HG-Focus array (Affymetrix). The degree of change (-fold) was calculated as the normalized intensity in ‘ergosterol peroxide-treated' vs ‘control' cells. Values of gene expression are the means±s.d.of triplicate cultures.
Quantitative real-time reverse transcription–polymerase chain reaction analysis
RAW264.7 cells (1 × 106 cells) were treated with 1 ng ml−1 LPS alone or together with 30 μM ergosterol peroxide for 6 h. HT29 cells (1 × 104 cells ml−1) were incubated with 7 or 12 μM ergosterol peroxide for 3 or 5 days. Total RNA was extracted from the RAW264.7 cells or the HT29 cells using TRIzol reagent and reverse transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) using TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's specifications. The TaqMan primers and probes for IL-1α (assay identification number Mm0039620_m1), IL-1β (Mm00434228_m1), low-density lipoprotein receptor (LDLR) (Mm00440169_m1), 3-hydroxy-3-methylglutamyl-coenzyme A reductase (HMGCR) (Mm01282491_m1), AKR1C1,C2 (Hs00413886_m1), signal transducers and activators of transcription 1 (STAT1) (Hs00234829_m1) and cyclin-dependent kinase inhibitor p21(WAF1/Cip1) (CDKN1A) (Hs00355782_m1) were TaqMan Gene Expression Assay products (Applied Biosystems). The mouse and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control (Mouse- and Human-GAPDH MGB, Applied Biosystems). The thermal cycler conditions were as follows: 2 min at 50°C and then 10 min at 95°C, followed by two-step PCR for 40 cycles consisting of 95°C for 15 s followed by 60°C for 1 min. All assays were performed in triplicate. The results are expressed relative to the GAPDH internal control.
Determination of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) were detected using the ROS-sensitive fluorescent dye, 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) (Molecular Probes, Eugene, OR, USA). HT29 cells (1 × 104 cells ml−1) were treated with or without (control) ergosterol peroxide for 5 days, collected and washed with phosphate-buffered saline (PBS(−); Nissui Pharmaceutical, Tokyo, Japan), and then incubated with 10 μM CM-H2DCFDA in PBS at 37°C for 30 min. After incubation, the cells were washed with PBS and the fluorescence intensities of the stained cells were determined by flow cytometry (FACSort).
Results
Suppression of LPS-induced inflammatory responses in RAW264. 7 mouse macrophage-like cells by ergosterol peroxide
Ergosterol peroxide was isolated from the edible mushroom, S. aspratus (Berk.) S. Ito, by acetone extraction, OASIS HLB column chromatography, silica gel column chromatography and reverse-phase HPLC, to a final purity of 98–100% (Figure 1a). Purified ergosterol peroxide strongly inhibited the growth of human leukaemia (HL60) cells but not that of neonatal normal human dermal fibroblasts and Chinese hamster lung cells after 24 h incubation (data not shown). The growth suppressive effect of ergosterol peroxide on HT29 human colon adenocarcinoma cells and B16 mouse melanoma cells was less than that on HL60 cells after 24 h incubation (data not shown).
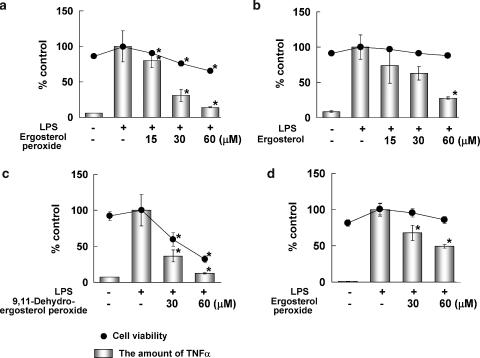
Suppression of the expression of genes involved in inflammatory responses is expected to reduce inflammatory disease and the development of cancer (Itzkowitz and Yio, 2004). Therefore, we examined the effects of ergosterol peroxide on the inflammatory responses using a macrophage-like cell line, RAW264.7. RAW264.7 cells were treated with ergosterol peroxide and bacterial LPS for 6 h, and the level of TNF-α secreted from the cells was determined by ELISA. Ergosterol peroxide inhibited the LPS-induced production of TNF-α by RAW264.7 cells with little effect on cell viability (Figure 2a). To examine the structure–activity relationship, we purified ergosterol and 9,11-dehydroergosterol peroxide from S. aspratus (Berk.) S. Ito (Figure 1b and c) and compared their effects on the LPS-induced production of TNF-α by RAW264.7 cells. Ergosterol inhibited LPS-induced TNF-α production by RAW264.7 cells without suppression of cell viability (Figure 2b). The other antitumour ergosterol derivative, 9,11-dehydroergosterol peroxide, strongly suppressed the cell viability of RAW264.7 cells after the 6 h incubation (Figure 2c). The suppression of TNF-α production by 9,11-dehydroergoterol peroxide correlated directly to the level of cell death (Figure 2c). This observation suggested that ergosterol peroxide and ergosterol, but not 9,11-dehydroergosterol peroxide, inhibit the LPS-induced inflammatory responses in RAW264.7 cells. We then examined whether ergosterol peroxide had an effect on TNF-α production after stimulation by LPS. RAW264.7 cells were treated with LPS for 2 h and then ergosterol peroxide was added into the medium. After 6 h incubation, suppression of LPS-induced TNF-α production was observed with little affect on cell viability (Figure 2d).
Figure 2.
Effects of ergosterol peroxide (a), ergosterol (b) and 9,11-dehydroergosterol peroxide (c) on LPS-induced TNF-α production in RAW264.7 macrophage-like cells. (a–c) Cells (5 × 104 cells ml−1) were treated with LPS (1 ng ml−1) and ergosterol peroxide (ergosterol or 9,11-dehydroergosterol peroxide) for 6 h. (d) Cells (5 × 104 cells ml−1) were treated with LPS (1 ng ml−1) for 2 h, and then treated with ergosterol peroxide for 6 h. The amount of TNF-α in the medium was then determined by ELISA (Mouse TNFα Ready-SET-Go, eBioscience). Cell viability was determined using WST-1 reagent (Cell Counting Kit, Dojin, Japan). TNF-α production and cell viability are expressed as percentages of those of the group treated with LPS alone (control). Values shown are the means±s.d. of triplicate cultures. *P<0.01 (two-sided), significantly different from the group treated with LPS alone by Bonferroni-type multiple t-test.
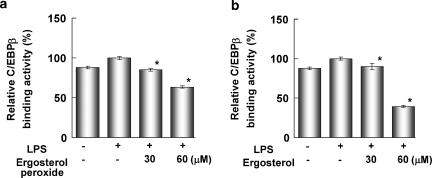
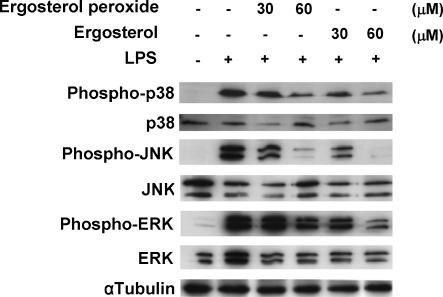
LPS induces production of TNF-α and expression of various inflammatory genes through induction of NF-κB and C/EBP transcriptional activity. We then examined the effects of ergosterol peroxide on LPS-induced NF-κB p65 DNA binding activity in RAW264.7 cells. The cells were treated with ergosterol peroxide for 6 h and stimulated with LPS for 30 min. The NF-κB promoter binding activity in the nuclear extract was determined by monitoring its affinity to an immobilized oligonucleotide containing an NF-κB consensus binding site and detected by ELISA. Ergosterol peroxide and ergosterol suppressed the LPS-induced NF-κB p65 DNA-binding activity in the nuclear extract (Figure 3a and b). Ergosterol peroxide and ergosterol also suppressed the LPS-induced C/EBPβ DNA-binding activity in the nuclear extract (Figure 4a and b). TNF-α expression was also induced by LPS-stimulated activation of MAP kinases (MAPKs). Ergosterol peroxide and ergosterol suppressed the phosphorylation of p38, JNK and ERK MAPKs in a dose-dependent manner (Figure 5).
Figure 3.
Ergosterol peroxide (a) and ergosterol (b) suppressed the NF-κB DNA-binding activity in RAW264.7cells. Cells (1 × 105 cells ml−1) were treated with ergosterol peroxide or ergosterol for 6 h and LPS for 30 min. Then, the NF-κB p65 DNA-binding activity in the nuclear extract (0.5 μg protein) was determined using a TransAM NF-κB p65 Chemi Kit (Active Motif). Relative NF-κB binding activity is expressed as a percentage of that of the group treated with LPS alone (control). All assays were performed in triplicate and data are expressed as means±s.d. *P<0.01 (two-sided), significantly different from the group treated with LPS alone by Bonferroni-type multiple t-test.
Figure 4.
Ergosterol peroxide (a) and ergosterol (b) suppressed the C/EBPβ DNA-binding activity in RAW264.7cells. Cells (1 × 105 cells ml−1) were treated with ergosterol peroxide or ergosterol for 6 h and LPS for 30 min. Then, the C/EBPβ DNA-binding activity in the nuclear extract (2 μg protein) was determined using a TransAM C/EBPβ Kit (Active Motif). Relative C/EBPβ binding activity is expressed as a percentage of that of the group treated with LPS alone (control). All assays were performed in triplicate and data are expressed as means±s.d. *P<0.01 (two-sided), significantly different from the group treated with LPS alone by Bonferroni-type multiple t-test.
Figure 5.
Ergosterol peroxide and ergosterol inhibited phosphorylation of MAPKs in RAW264.7 cells. Cells (1 × 105 cells ml−1) were treated with ergosterol peroxide or ergosterol for 6 h and LPS for 15 min. The protein levels of MAPKs, phosphorylated-MAPKs and α-tubulin in the cells were examined by Western blot analysis.
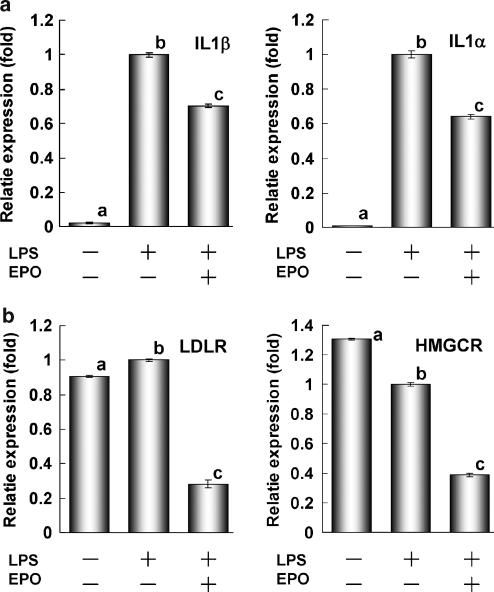
Next, we performed real-time RT–PCR analysis to confirm the suppressive effect of ergosterol peroxide on LPS-induced inflammatory gene expression in RAW264.7 cells. Ergosterol peroxide inhibited the LPS-induced expression of IL-1β and IL-1α after 6 h of incubation (Figure 6a). The results of preliminary DNA microarray analysis (Affymetrix, Mouse U74Av2 array) suggested that ergosterol peroxide inhibited the expression of LDLR, 3-hydroxy-3-methylglutamyl-coenzyme A synthase (HMGCS) and the molecules involved in cholesterol synthesis (data not shown). LDLR expression was transcriptionally regulated by C/EBPβ. HMGCR expression was regulated by sterol regulatory element binding protein (SREBP) as well as LDLR and HMGCS and the molecules involved in cholesterol synthesis (Brown and Goldstein, 1997). Therefore, we examined LDLR and HMGCR mRNA expression in RAW264.7 cells treated with LPS and ergosterol peroxide by RT–PCR. Although LPS had little effect on the expression of LDLR or HMGCR, ergosterol peroxide significantly suppressed the expression of both molecules (Figure 6b).
Figure 6.
Suppression of gene expression by ergosterol peroxide in RAW264.7 cells. (a) Ergosterol peroxide suppressed LPS-induced IL-1β and IL-1α expression. (b) Ergosterol peroxide suppressed the expression of LDLR and HMGCR. Cells (1 × 106 cells) were treated with 1 ng ml−1 LPS alone or together with 30 μM ergosterol peroxide for 6 h. Expression levels determined by RT–PCR were normalized to GAPDH and plotted relative to those of cells treated with LPS (control). All assays were performed in triplicate and data are expressed as means±s.d. Alphabets show significant difference (P<0.01, (two-sided) by Bonferroni-type multiple t-test).
Ergosterol peroxide shows the cytostatic effect on HT29 human colorectal adenocarcinoma cells
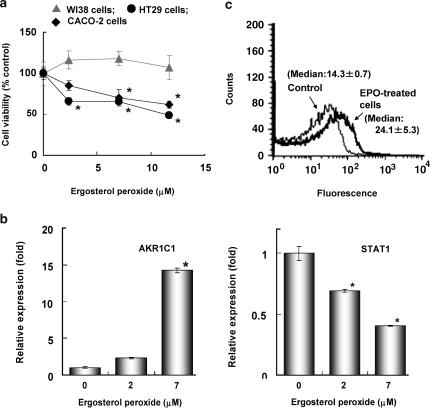
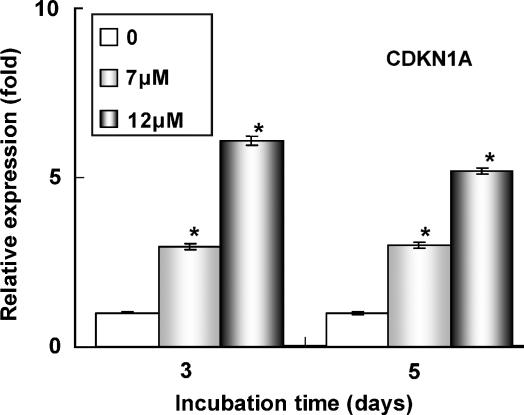
Ergosterol peroxide, which is mostly excreted in the faeces, probably affects colon cancer. We treated HT29 cells, CACO-2 human colon carcinoma cells or WI38 normal human embryonic fibroblasts for 5 days with ergosterol peroxide. Although the cytotoxicity of ergosterol peroxide on HT29 cells was less than that on HL60 cells after 24 h of incubation, it showed a cytostatic effect on HT29 and CACO-2 colon cancer cells at doses of 2–12 μM without suppressing the growth of normal diploid WI38 cells after 5 days (Figure 7a). Cell cycle analysis showed that 7 μM ergosterol peroxide decreased the number of S phase cells from 21.9±1.0 to 16.6±0.7% of the total diploid cell population and increased the proportion of cells in G1/G0 phase from 71.1±1.3 to 76.8±1.0% after 5 days of incubation. The proportion of hypodiploid cells increased from 0.7±0.8 to 16.8±2.1% of the total cells after ergosterol peroxide treatment. To determine the molecular mechanism of the cytostatic effect of ergosterol peroxide on HT29 cells, we examined the effects of ergosterol peroxide on gene expression in HT29 cells after 5 days of incubation. HT29 cells were treated with 0, 2 and 7 μM ergosterol peroxide for 5 days and gene expression patterns were determined by DNA microarray analysis using Human Genome Focus arrays (Affymetrix). Table 1 shows genes that were significantly up- or down-regulated after treatment with ergosterol peroxide, with differences in expression of more than twofold (P<0.05 by one-way one-way analysis of variance). Ergosterol peroxide strongly induced expression of the aldo-keto reductases AKR1C1 and AKR1C2, enzymes involved in detoxification (Table 1). Ergosterol peroxide also upregulated the oxidative stress-inducible genes SQSTM1, CXCL2 and ALDH3A1 (Table 1). The induction of AKR1C1,C2 expression was also detected by RT–PCR analysis (Figure 7b). Treatment of HT29 cells with 7 μM ergosterol peroxide for 5 days increased the expression of AKR1C1,C2 mRNA by ca. 15-fold as compared with control cells (Figure 7b). The levels of expression of interferon-inducible genes and STAT1 were reduced after treatment with ergosterol peroxide (Table 1). RT–PCR analyses indicated that 7 μM ergosterol peroxide reduced the level of STAT1 expression to 0.4-fold that in control cells (Figure 7b). Intracellular ROS have been shown previously to upregulate the expressions of AKR1C1,C2 and oxidative stress-inducible genes, and downregulate the expression of STAT1 (Simon et al., 1998; Burczynski et al., 1999, 2001; Chen et al., 2003). Intracellular ROS were determined in HT29 cells treated with ergosterol peroxide using the ROS-sensitive fluorescent dye, CM-H2DCFDA. Figure 7c shows the fluorescence intensities of control cells and those treated with 7 μM ergosterol peroxide for 5 days. Results are representative of the cultures examined in triplicate. Median channel fluorescence was significantly (P<0.05, Student's t-test) increased from 14.3±0.7 to 24.1±5.3 after treatment with ergosterol peroxide. These observations indicated that ergosterol peroxide upregulated the levels of intracellular ROS in HT29 cells at a concentration of 7 μM after 5 days of incubation. The CDKN1A induced by ROS was shown to arrest the cell cycle and induce apoptosis in HT29 cells. Therefore, we examined the effects of ergosterol peroxide on CDKN1A expression by RT–PCR. After 3 days of incubation, ergosterol peroxide induced CDKN1A mRNA expression in HT29 cells (Figure 8).
Figure 7.
Effects of ergosterol peroxide on cell growth (a), AKR1C1,C2(AKR1C1) and STAT1 gene expression (b), and ROS generation (c) in HT29 human colon adenocarcinoma cells. (a) HT29 human colon adenocarcinoma cells (1 × 104 cells ml−1), CACO-2 human colon carcinoma cells (1 × 104 cells ml−1) and WI38 human fibroblast cells (1 × 104 cells ml−1) were incubated with ergosterol peroxide for 5 days. Values shown are the means±s.d. of triplicate cultures. *P<0.01 (two-sided), significantly different from the untreated control group by Bonferroni-type multiple t-test. (b) HT29 cells (1 × 104 cells ml−1) were incubated with 7 μM ergosterol peroxide for 5 days. Then, RT–PCR was performed and the AKR1C1,C2 (AKR1C1) and STAT1 expression levels were normalized to GAPDH and plotted relative to those of control cells. All assays were performed in triplicate and data are expressed as means±s.d. *P<0.01 (two-sided), significantly different from the untreated control group by Bonferroni-type multiple t-test. (c) Intracellular ROS level. HT29 cells (1 × 104 cells ml−1) were treated with 7 μM ergosterol peroxide (EPO) for 5 days. The cells were then harvested and stained with CM-H2DCFDA at 30°C for 30 min. The thin and thick lines represent the fluorescence traces from control cells and from cells treated with ergosterol peroxide, respectively. Medial channel fluorescence was indicated in the histogram.
Figure 8.
Ergosterol peroxide induces CDKN1A gene expression in HT29 human colon adenocarcinoma cells. HT29 cells (1 × 104 cells ml−1) were incubated with 7 or 12 μM ergosterol peroxide for 3 or 5 days. Then, RT–PCR was performed and the CDKN1A expression levels were normalized to GAPDH and plotted relative to those of control cells. All assays were performed in triplicate and data are expressed as means±s.d. *P<0.01 (two-sided), significantly different from the untreated control group by Bonferroni-type multiple t-test.
Discussion
Ergosterol peroxide is a major antitumour sterol present in edible and medicinal mushrooms. The results of the present study indicate that ergosterol peroxide, isolated from an edible mushroom, suppresses LPS-induced pro-inflammatory gene expression in macrophages and the growth of human colon adenocarcinoma cells. In our study, ergosterol peroxide suppressed the LPS-induced production of TNF-α by RAW264.7 macrophage-like cells with little inhibition of cell viability after a 6-h incubation period. To differentiate between the suppressive effect on LPS-induced TNF-α production and cytotoxicity, we determined the effect of ergosterol and 9,11-dehydroergosterol peroxide on the LPS-induced TNF-α production by RAW264.7 cells. Ergosterol does not suppress the growth of HL60 leukemia cells and other cancer cells (Takei et al., 2005; Kobori et al., 2006 and unpublished data). In the present study, ergosterol was shown to suppress the LPS-induced TNF-α production by RAW264.7 cells with little effect on cell viability. Although the suppression of TNF-α production by ergosterol peroxide was accompanied by a slight reduction in cell viability, this was probably due to the peroxide group and was independent of the suppressive effect on TNF-α production. Because the cytotoxic effect of 9,11-dehydroergosterol peroxide was stronger than that of ergosterol peroxide, suppression of LPS-induced TNF-α production by 9,11-dehydroergosterol peroxide almost mirrored the level of cell death. These observations suggest that the structure of ergosterol, but not the peroxide group, is important for the suppressive effect on LPS-induced TNF production. To examine the effect of treatment with ergosterol peroxide subsequent to LPS stimulation, we first incubated the cells with LPS for 2 h and then treated them with ergosterol peroxide for 6 h. Ergosterol peroxide was also found to be effective at inhibiting the LPS-induced TNF production when incubated with cells previously treated with LPS. By contrast, ergosterol peroxide did not have a significant effect on cell viability.
LPS induces TNF-α and other inflammatory gene expression by activating the MAPKs and transcription factor NF-κB and C/EBP in macrophages (Pope et al., 1994; Swantek et al., 1997). We showed that ergosterol peroxide and ergosterol suppressed the LPS-induced NF-κB p65 and C/EBPβ DNA-binding activity in the nuclear extract. Furthermore, ergosterol peroxide and ergosterol dose-dependently suppressed LPS-induced phosphorylation of MAPKs p38, JNK and ERK. Our results suggest that ergosterol peroxide and ergosterol suppressed LPS-induced TNF-α production by inhibiting the activation of the MAPKs and transcription factor NF-κB and C/EBP in RAW264.7 cells.
To determine the detailed molecular mechanisms responsible for the suppressive effects, we performed gene expression analysis using RT–PCR. In our preliminary experiments, we compared the gene expressions of the RAW264.7 cells, the cells treated with 2 ng ml−1 LPS for 6 h and the cells treated with ergosterol peroxide and LPS for 6 h using DNA microarrays (Affymetrix, Mouse U74Av2 array). Our results suggested that ergosterol peroxide suppresses the LPS-induced expression of inflammatory cytokines, such as IL-1α, IL-1β and TNF-α. Therefore, we determined the effect of ergosterol peroxide on the expression of IL-1α and IL-1β induced by LPS using RT–PCR. At a concentration of 30 μM, ergosterol peroxide suppressed the expression of IL-1α and IL-1β in RAW264.7 cells. The results show that ergosterol peroxide suppresses not only the production of TNF-α but also the production of other inflammatory cytokines, such as IL-1α and IL-1β. The results of preliminary DNA microarray analysis also suggested that ergosterol peroxide inhibited the expression of LDLR, HMGCS and the molecules involved in cholesterol synthesis. We showed that ergosterol peroxide strongly suppressed the expression of LDLR and HMGCR at the same dose, whereas LPS was less effective at altering their expression (assessed by RT–PCR analysis). The expression of LDLR is transcriptionally induced by C/EBP (Liu et al., 2000). Therefore, ergosterol peroxide probably suppresses the expression of LDLR by inhibiting C/EBPβ transcriptional activity. HMGCR is a key enzyme in cholesterol synthesis and HMGCR inhibitors not only suppress cholesterol synthesis but also inhibit the transcriptional activity of NF-κB, which induces the expression of pro-inflammatory cytokines, such as TNF-α, IL-1α and IL-1β (Dichtl et al., 2003; Madonna et al., 2005). Although HMGCR inhibitors are thought to inhibit NF-κB activity by affecting isoprenoid-mediated intracellular signal transduction, the molecular mechanism has not yet been clarified (Madonna et al., 2005). Inhibition of MAPKs also causes suppression of NF-κB transcriptional activity (Campbell et al., 2004). Therefore, ergosterol peroxide could suppress NF-κB transcriptional activity by inhibiting the activation of MAPKs and expression of HMGCR. The transcription factor SREBP induces the expression of LDLR, HMGCR, HMGCS and other molecules involved in cholesterol synthesis. The plant sterol stigmasterol, but not sitosterol, was shown to inhibit the transcriptional activity of SREBP-2 and the expression of HMGCR, HMGCS and LDLR (Yang et al., 2004). The fungal sterol, ergosterol peroxide, probably suppresses the expression of LDLR and HMGCR by inhibiting the transcriptional activity of SREBP.
Most of the ergosterol administered orally has been shown to be excreted in the faeces in rats without absorption through the intestine (Tsugawa et al., 1992). Ergosterol peroxide consumed orally in edible mushrooms may affect colon cancer. In the present study, we showed that 5 days of treatment with ergosterol peroxide suppresses the growth of HT29 and CACO-2 human colon adenocarcinoma cells but not that of WI38 normal diploid cells. To examine the molecular mechanism of the cytostatic effect of ergosterol peroxide on HT29 cells, we performed a DNA microarray analysis. The DNA microarray analysis showed that 5 days of incubation with ergosterol peroxide strongly upregulated the expression of AKR1C1 and AKR1C2. The results of RT–PCR analysis confirmed the induction of AKR1C1,C2 mRNA expression. The aldo-keto reductases, AKR1Cs, are xenobiotic metabolizing enzymes. AKR1C1 has been found to be induced by electrophilic Michael acceptors, phenolic antioxidants and ROS in HepG2 human hepatoma cells, and HT29 colon adenocarcinoma cells through an apparent antioxidant response element (ARE) (Burczynski et al., 1999, 2001). Isothiocyanates, which are dietary chemopreventive agents that increase drug-metabolizing enzyme activity, were suggested to induce the expression of AKR1C1 in LS-174 and CACO-2 human colon adenocarcinoma cells via the transcription factor Nrf2 through the ARE (Bonnesen et al., 2001). Other upregulated genes, with the exception of PIM1, have been shown to be regulated by oxidative stress or Nrf2 (Ishii et al., 2000; Thimmulappa et al., 2002; Jaramillo et al., 2005). However, with the exception of STAT1, all of the downregulated genes are inducible by interferon (IFN) (Aebi et al., 1989; Guldner et al., 1992; Hovnanian et al., 1998; Zhou et al., 2000; Cui et al., 2004). Both IFN-α and IFN-γ induce gene expression through STAT1 transcriptional activity (Bromberg et al., 1996). We confirmed, using RT–PCR analysis, that ergosterol peroxide downregulated the STAT1 mRNA expression. Our results indicate that ergosterol peroxide suppresses the expression of STAT1, and consequently of STAT1-mediated gene expression in HT29 cells. STAT1 signalling has been shown to be regulated by ROS (Simon et al., 1998; Chen et al., 2003). The peroxisome proliferator-activated receptor γ (PPARγ) agonist, 15-deoxy-Δ12,14-prostaglandin J2, inhibits IFN-γ-induced gene expression through inhibition of STAT1 signalling by ROS in RAW264.7 macrophages and HT29 cells transfected with PPARγ (Chen et al., 2003). As HT29 cells treated with ergosterol peroxide for 5 days showed accumulation of intracellular ROS, the downregulation of STAT1 and interferon-inducible genes were likely to be mediated by ROS. Ergosterol peroxide itself shows antioxidant activity (Kim et al., 1999). However, it probably generates intracellular ROS directly or through alteration of the redox state. Thus, ergosterol peroxide was suggested to upregulate the oxidative stress-sensitive genes and downregulate STAT1 and interferon-inducible genes by increasing the intracellular levels of ROS.
Ergosterol peroxide increased the ratio of hypodiploid cells and reduced the number of S phase cells. Aspirin, butyrate and other chemopreventive agents have been shown to increase ROS production and inhibit the growth of HT29 cells (Archer et al., 1998; Giardina and Inan 1998). Dietary antitumour flavonoids were shown to induce apoptosis by elevating the intracellular ROS levels and the subsequent induction of CDKN1A expression (Shen et al., 2004). CDKN1A arrests the cell cycle in G0/G1 or G2/M, and induces apoptosis in HT29 cells (Agarwal et al., 2003; Geller et al., 2004). Therefore, we examined the effect of ergosterol peroxide on the expression of CDKN1A using RT–PCR. Although upregulation of CDKN1A expression by ergosterol peroxide was not significantly detected by DNA microarray analysis, it was clearly detected by RT–PCR analysis. IFN-γ also induces the expression of CDKN1A and apoptosis in HT29 cells through STAT signalling (Xu et al., 1998; Karpf et al., 1999). Our results suggest that ergosterol peroxide induces apoptosis and cell cycle arrest through the generation of ROS, although the effect was partly counteracted by inhibition of STAT1 signalling.
In conclusion, ergosterol peroxide suppresses LPS-induced pro-inflammatory gene expression in RAW264.7 macrophage-like cells and the growth of HT29 colon adenocarcinoma cells. Furthermore, these effects appear to be correlated with the structures of ergosterol and the corresponding peroxide. In fact, ergosterol has been found to suppress the TPA-induced inflammatory response in vivo (Yasukawa et al., 1994). Our results suggest that ergosterol peroxide inhibits pro-inflammatory gene expression by inhibiting NF-κB p65 and C/EBPβ transcriptional activity and MAPKs activation in RAW264.7 cells, whereas ergosterol peroxide generates intracellular ROS in HT29 cells. The accumulation of ROS probably results in the suppression of HT29 cell growth and STAT1-regulated inflammatory gene expression. Ergosterol peroxide markedly induces expression of the xenobiotic metabolizing enzymes, the AKR1Cs. The genes regulated by ergosterol peroxide were found to be different from those reported previously to be regulated by sodium butyrate and aspirin (Iacomino et al., 2001; Hardwick et al., 2004).
Acknowledgments
We thank Dr T Takei (Fukushima Prefectural Forestry Research Centre) for help with procurement of S. aspratus and purification of ergosterol peroxide and ergosterol. This work is financially supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries (MAFF) Food Research Project ‘Integrated Research on Safety and Physiological Function of Food.'
Abbreviations
- AKR1C1
aldo-keto reductase family 1 member C1
- CDKN1A
cyclin-dependent kinase inhibitor p21(WAF1/Cip1)
- ergosterol peroxide
5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HMGCR
3-hydroxy-3-methylglutamyl-coenzyme A reductase
- HMGCS
3-hydroxy-3-methylglutamyl-coenzyme A synthase
- IL-1
interleukin-1
- LDLR
low-density lipoprotein receptor
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- ROS
reactive oxygen species
- RT–PCR
real-time reverse transcription–polymerase chain reaction
- S. aspratus
Sarcodon aspratus (Berk.) S. Ito
- SREBP
sterol regulatory element binding protein
- TNF-α
tumour necrosis factor-α
- TPA
phorbol-12-myristate 13-acetate
- 9,11-dehydroerogosterol peroxide
5α,8α-epidioxy-22E-ergosta-6, 9(11), 22-trien-3β-ol
Conflict of interest
The authors state no conflict of interest.
References
- Aebi M, Fah J, Hurt N, Samuel CE, Thomis D, Bazzigher L, et al. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GH. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci USA. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Burczynski ME, Lin HK, Penning TM. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999;59:607–614. [PubMed] [Google Scholar]
- Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive oxygen species-and Michael acceptor-inducible human aldo-keto reductase AKR1C1 reduces the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. J Biol Chem. 2001;276:2890–2897. doi: 10.1074/jbc.M006655200. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ciesielski CJ, Hunt AE, Horwood NJ, Beech JT, Hayes LA, et al. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004;173:6928–6937. doi: 10.4049/jimmunol.173.11.6928. [DOI] [PubMed] [Google Scholar]
- Chen CW, Chang YH, Tsi CJ, Lin WW. Inhibition of IFN-gamma-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor gamma agonist, 15-deoxy-delta 12,14-prostaglandin J2, involves inhibition of the upstream Janus kinase/STAT1 signaling pathway. J Immunol. 2003;171:979–988. doi: 10.4049/jimmunol.171.2.979. [DOI] [PubMed] [Google Scholar]
- Cui XF, Imaizumi T, Yoshida H, Borden EC, Satoh K. Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells. Biochem Cell Biol. 2004;82:401–405. doi: 10.1139/o04-041. [DOI] [PubMed] [Google Scholar]
- Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MP, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Nakayama M, Nakayama Y, Yamazaki M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem Pharm Bull. 1994;42:694–697. doi: 10.1248/cpb.42.694. [DOI] [PubMed] [Google Scholar]
- Geller JI, Szekely-Szucs K, Petak I, Doyle B, Houghton JA. P21Cip1 is a critical mediator of the cytotoxic action of thymidylate synthase inhibitors in colorectal carcinoma cells. Cancer Res. 2004;64:6296–6303. doi: 10.1158/0008-5472.CAN-04-0863. [DOI] [PubMed] [Google Scholar]
- Giardina C, Inan MS. Nonsteroidal anti-inflammatory drugs, short-chain fatty acids, and reactive oxygen metabolism in human colorectal cancer cells. Biochim Biophys Acta. 1998;1401:277–288. doi: 10.1016/s0167-4889(97)00140-7. [DOI] [PubMed] [Google Scholar]
- Guldner HH, Szostecki C, Grotzinger T, Will H. IFN enhance expression of Sp100, an autoantigen in primary biliary cirrhosis. J Immunol. 1992;149:4067–4073. [PubMed] [Google Scholar]
- Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–238. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, van Santen M, van den Brink GR, van Deventer SJ, Peppelenbosch MP. DNA array analysis of the effects of aspirin on colon cancer cells: involvement of Rac1. Carcinogenesis. 2004;25:1293–1298. doi: 10.1093/carcin/bgh118. [DOI] [PubMed] [Google Scholar]
- Hovnanian A, Rebouillat D, Mattei MG, Levy ER, Marie I, Monaco AP, et al. The human 2′,5′-oligoadenylate synthetase locus is composed of three distinct genes clustered on chromosome 12q24.2 encoding the 100-, 69-, and 40-kDa forms. Genomics. 1998;52:267–277. doi: 10.1006/geno.1998.5443. [DOI] [PubMed] [Google Scholar]
- Iacomino G., Tecce MF, Grimaldi C, Tosto M, Russo GL. Transcriptional response of a human colon adenocarcinoma cell line to sodium butyrate. Biochem Biophys Res Commun. 2001;285:1280–1289. doi: 10.1006/bbrc.2001.5323. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- Jaramillo M, Godbout M, Olivier MJ. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. Immunol. 2005;174:475–484. doi: 10.4049/jimmunol.174.1.475. [DOI] [PubMed] [Google Scholar]
- Karpf AR, Peterson PW, Rawlins JT, Dalley BK, Yang Q, Albertsen H, Jones DA. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc Natl Acad Sci USA. 1999;96:14007–14012. doi: 10.1073/pnas.96.24.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- Kim S, Park S, Min T, Yu K. Antioxidant activity of ergosterol peroxide (5, 8-epidioxy-5α, 8α-ergosta-6, 22E-dien-3β-ol) in Armillariella mellea. Bull Korean Chem Soc. 1999;20:819–823. [Google Scholar]
- Kobori M, Yoshida M, Ohnishi-Kameyama M, Takei T, Shinmoto H. 5α,8α-Epidioxy-22E- ergosta-6, 9(11), 22-trien-3β-ol from an edible mushroom suppresses growth of HL60 leukemia and HT29 colon adenocarcinoma cells. Biol Pharm Bull. 2006;29:755–756. doi: 10.1248/bpb.29.755. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Weng SC, Chou CJ, Chang TT, Tsai WJ. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br J Pharmacol. 2003;140:895–906. doi: 10.1038/sj.bjp.0705500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ahlborn TE, Briggs MR, Kraemer FB. Identification of a novel sterol-independent regulatory element in the human low dencity lipoprotein receptor promoter. J Biol Chem. 2000;275:5214–5221. doi: 10.1074/jbc.275.7.5214. [DOI] [PubMed] [Google Scholar]
- Madonna R, Di Napoli P, Massaro M, Grilli A, Felaco M, De Caterina A, et al. Simvastatin attenuates expression of cytokine-inducible nitric-oxide synthase in embryonic cardiac myoblasts. J Biol Chem. 2005;280:13503–13511. doi: 10.1074/jbc.M411859200. [DOI] [PubMed] [Google Scholar]
- Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol Appl Pharmacol. 2004;197:84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei T, Yoshida M, Ohnishi-Kameyama M, Kobori M. Ergosterol peroxide, an apoptosis-inducing component isolated from Sarcodon aspratus (Berk.) S. Ito. Biosci Biotechnol Biochem. 2005;69:212–215. doi: 10.1271/bbb.69.212. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Tsugawa N, Okano T, Takeuchi A, Kayama M, Kobayashi T. Metabolism of orally administered ergosterol and 7-dehydrocholesterol in rats and lack of evidence for their vitamin D biological activity. J Nutr Sci Vitaminol. 1992;38:15–25. doi: 10.3177/jnsv.38.15. [DOI] [PubMed] [Google Scholar]
- Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998;58:2832–2837. [PubMed] [Google Scholar]
- Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813–822. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y, Yoshihara Y, Kakuda R, Machida K, Kikuchi M. New sterols from two edible mushrooms, Pleurotus eryngii and Panellus serotinus. Chem Pharm Bull. 2002;50:551–553. doi: 10.1248/cpb.50.551. [DOI] [PubMed] [Google Scholar]
- Yasukawa K, Aoki T, Takido M, Ikekawa T, Saito H, Matsuzawa T. Inhibitory effects of ergosterol isolated from the edible mushroom Hypsizigus marmoreus on TPA-induced inflammatory ear oedema and tumour promotion in mice. Phytother Res. 1994;8:10–13. [Google Scholar]
- Zaidman BZ, Yassin M, Mahajna J, Wasser SP. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol. 2005;67:453–468. doi: 10.1007/s00253-004-1787-z. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhao J, Al-Zoghaibi F, Zhou A, Wiedmer T, Silverman RH, et al. Transcriptional control of the human plasma membrane phospholipid scramblase 1 gene is mediated by interferon-alpha. Blood. 2000;95:2593–2599. [PubMed] [Google Scholar]