Abstract
Background and purpose:
Protease-activated receptor-4 (PAR4), the most recently discovered member of the PARs family, is activated by thrombin, trypsin and cathepsin G, but can also be selectively activated by small synthetic peptides (PAR4-activating peptide, PAR4-AP). PAR4 is considered a potent mediator of platelet activation and inflammation. As both PAR1 and PAR2 have been implicated in the modulation of nociceptive mechanisms, we investigated the expression of PAR4 in sensory neurons and the effects of its selective activation on nociception.
Experimental approach and key results:
We demonstrated the expression of PAR4 in sensory neurons isolated from rat dorsal root ganglia by reverse transcription-polymerase chain reaction and immunofluorescence. We found that PAR4 colocalized with calcitonin gene-related peptide and substance P. We also showed that a selective PAR4-AP was able to inhibit calcium mobilization evoked by KCl and capsaicin in rat sensory neurons. Moreover, the intraplantar injection of a PAR4-AP significantly increased nociceptive threshold in response to thermal and mechanical noxious stimuli, while a PAR4 inactive control peptide had no effect. The anti-nociceptive effects of the PAR4-AP were dose-dependent and occurred at doses below the threshold needed to cause inflammation. Finally, co-injection of the PAR4-AP with carrageenan significantly reduced the carrageenan-induced inflammatory hyperalgesia and allodynia, but had no effect on inflammatory parameters such as oedema and granulocyte infiltration.
Conclusions and implications:
Taken together, these results identified PAR4 as a novel potential endogenous analgesic factor, which can modulate nociceptive responses in normal and inflammatory conditions.
Keywords: thrombin, protease-activated receptor-4, analgesia, inflammation, cathepsin G
Introduction
Protease-activated receptors (PARs) are G protein-coupled receptors characterized by a unique mechanism of activation involving serine proteases. A specific proteolytic cleavage of the amino-terminal sequence unmasks a new amino-terminal sequence consisting of a tethered ligand that binds to and activates the receptor (Hollenberg and Compton, 2002). Four PARs (PAR1–4), each of which can be activated by different or common proteases, have been cloned (Vu et al., 1991; Nystedt et al., 1995; Ishihara et al., 1997; Kahn et al., 1998a; Xu et al., 1998). PAR2 can be activated by trypsin and human mast cell tryptase, whereas PARs1,3,4 are all considered thrombin receptors (Ossovskaya and Bunnett, 2004). However, PAR4 can also be activated by trypsin, cathepsin G, the activated factor X of the coagulation cascade and trypsin IV (Sambrano et al., 2000; Camerer et al., 2000; Cottrell et al., 2004). With the exception of PAR3, PARs can be activated by short synthetic peptides of five or six amino acids, known as activating peptides (APs), that mimic the tethered ligand (Hollenberg and Compton, 2002). For example, the PAR4-AP, AYPGKF-NH2, which was designed based on the mouse PAR4 tethered ligand sequence, has been shown to be a selective and potent PAR4 agonist, being more potent than the mouse tethered ligand sequence itself (GYPGKF-NH2), that does not affect either PAR1 or PAR2 (Faruqi et al., 2000; Hollenberg et al., 2004; Mule et al., 2004). In addition, we have recently shown that the effects of the PAR4 agonist AYPGKF-NH2, in vivo, are abolished by a PAR4 antagonist, further suggesting the specificity of this peptide (Houle et al., 2005). For these reasons, AYPGKF-NH2 was used in the present study to characterize the effects of PAR4 activation on nociception.
The expression and function of each receptor vary between tissues. PAR2 has a role in regulation of vascular tone, a variety of pro- or anti-inflammatory effects and is pro-nociceptive in models of somatic or visceral pain (Macfarlane et al., 2001; Vergnolle et al., 2001b; Coelho et al., 2002). PAR1 has been implicated in haemostasis, platelet signalling, proinflammatory effects and can induce analgesia either from peripheral or spinal activation (Vergnolle et al., 2001b; Asfaha et al., 2002; Fang et al., 2003). Less is known about the physiological functions of PAR3 and PAR4. PAR3 may not be a fully functional receptor, but rather acts as a co-factor or tethering protein for its ligand thrombin (Nakanishi-Matsui et al., 2000). Like PAR1, PAR4 has also been shown to be a receptor for thrombin-mediated platelet activation. Given the high concentrations of thrombin that are needed to activate PAR4 as compared with PAR1, it has been postulated that PAR4 may be a low-affinity receptor through which thrombin is able to elicit its actions once PAR1 desensitization has occurred (Shapiro et al., 2000). PAR4 agonists have also been shown to induce leukocyte rolling and adherence, suggesting a pro-inflammatory role for this receptor (Vergnolle et al., 2002; Hollenberg et al., 2004). PAR4 may also mediate the pro-adhesive properties of thrombin on leukocyte–endothelial cell interactions (Vergnolle et al., 2002).
There is increasing evidence to suggest that PARs play a major role in peripheral nerve functions (Cenac and Vergnolle, 2005). Indeed, PAR1 and PAR2 are expressed in primary spinal afferent neurons and can modulate nociception (Steinhoff et al., 2000; de Garavilla et al., 2001; Vergnolle et al., 2001a; Asfaha et al., 2002). Activation of PAR2 by sub-inflammatory doses of agonists has been shown to elicit hyperalgesia to both thermal and mechanical stimuli via a neurokinin-1 receptor and prostaglandin-dependent mechanism (Vergnolle et al., 2001a; Coelho et al., 2002). In contrast, PAR1 activation by sub-inflammatory doses of a selective AP has an analgesic effect, in both normal and inflammatory conditions, after application of a thermal or a mechanical stimulus (Asfaha et al., 2002). Surprisingly, even though thrombin could reproduce the analgesic effect of the PAR1-AP against a mechanical stimulus, it induced hyperalgesia in response to a thermal stimulus in normal and inflammatory conditions (Asfaha et al., 2002). This discrepancy could be explained by the fact that thrombin can activate other receptors that may modulate its nociceptive activity. PAR4 is an obvious candidate, as its activation mediates excitatory responses in myenteric neurons (Gao et al., 2002). Therefore, the aims of this study were to (1) characterize PAR4 expression and function in dorsal root ganglia (DRG) sensory neurons and (2) investigate the effects of sub-inflammatory doses of the selective PAR4-AP, AYPGKF-NH2, on nociceptive responses to both thermal and mechanical stimuli in normal and inflammatory conditions.
Methods
Animals
Male Wistar rats (175–200 g) were obtained from Charles River Laboratories (Montréal, Québec, Canada). The rats had free access to food and water and were housed under constant temperature (22°C) and photoperiod (12 h light–dark cycle). All experimental procedures were approved by the Animal Care Committee of the University of Calgary and were performed in accordance with the guidelines established by the Canadian Council on Animal Care and the Committee for Research and Ethical Issues of IASP published in PAIN (1983;16:109).
Isolation of rat DRG sensory neurons
The isolation of rat DRG sensory neurons was performed by following a slightly modified version of a previously described procedure (Steinhoff et al., 2000). Briefly, the spinal cord was extracted from rats and DRG were surgically removed under a binocular loop. DRG were rinsed in Hanks' balanced salt solution (HBSS) and minced in a Petri dish containing sterile HBSS. They were then incubated in HBSS containing 0.5% papain for 15 min at 37°C. DRG were washed once with Leibovitz's L-15 medium (supplemented with 2 mM glutamine, 0.2% glucose and 2.5% fetal bovine serum (FBS)) and further incubated in HBSS containing 1 mg ml−1 collagenase type I and 4 mg ml−1 dispase II for 10 min at 37°C. After titration and centrifugation at 900 g for 5 min, cells were resuspended in the complete culture medium (MEM supplemented with 2.5% FBS, 1% penicillin/streptomycin, 1% dextrose, 2 mM glutamine and 10 μM of arabinocytidine hydrochloride, floxuridine and uridine). The cells were finally plated in poly-L-orthinine-laminine-treated glass-bottom Petri dishes (35 mm diameter, MatTek Corporation, Ashland, MA, USA). Experiments were generally performed 24–36 h after isolation of the cells.
PAR4 detection by semi-quantitative duplex reverse transcription–polymerase chain reaction (RT-PCR) in rat DRG sensory neurons
mRNA from rat DRG was isolated using the TRIzol reagent, as indicated by the manufacturer. Samples were treated with DNase I to remove traces of genomic DNA, and a reverse transcription (RT) step was performed in order to obtain stable cDNA. Briefly, 1 μg of mRNA from each sample was submitted to a 50-min RT cycle at 42°C (after it had been on the bench for 10 min at room temperature) using 200 U of the superscript II RNase H reverse transcriptase, 40 U of RNasin, 1 mM of each dNTP and 90 nmol μl−1 of N6 random hexamer oligonucleotides, all in the appropriate buffer. The enzyme was then deactivated by heating it at 95°C for 10 min.
Following the RT step, a semiquantitative duplex polymerase chain reaction (PCR) was performed to evaluate the level of PAR4 expression in rat DRG. The housekeeping gene for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. An aliquot of cDNA (2 μl) was added to a PCR tube containing the appropriate buffer supplemented with 0.2 mM dNTPs and 0.2 μM of each of the oligonucleotides (four in total; two for the amplification of PAR4 and two for GAPDH). The sequences of the oligonucleotides used were as follows: GGAAGTCTTGAGAGAAAGGCAA (5′ primer of PAR4), GAACCAAGAGGCATCACCTATC (3′ primer of PAR4), CGGAGTCAACGGATTTGG-TCGTAT (5′ primer of GAPDH) and AGCCTTCTCCATGGTGGTGAAGAC (3′ primer of GAPDH). The primers for the amplification of PAR4 were selected on the basis of the rat sequence (Hoogerwerf et al., 2002; accession number: NM053808) and were designed to span only one exon. The Taq DNA polymerase (1 U μl−1) was added to the PCR mixture during the hot start of the first cycle. The samples were initially denatured at 95°C for 5 min. DNA amplification was then conducted under the following conditions: 34 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 30 s and extension at 72°C for 1 min. A final extension period of 10 min at 72°C completed the PCR.
The PCR products were separated on a 1% agarose gel containing 10 μg of ethidium bromide and a picture was taken under ultraviolet light for analysis.
Immunohistochemistry for the expression of PAR4 in sensory neurons
DRG neurons were isolated from rats and cultured as described above. Cultured neurons were fixed in 4% formaldehyde for 20 min and then washed before being incubated in phosphate-buffered saline (PBS) containing 5% normal goat serum and 0.1% saponin for 30 min. Neurons were then incubated in PBS with detergent and 2% normal goat as previously described (Steinhoff et al., 2000), in the presence of the primary antibody: the affinity purified goat polyclonal anti-PAR4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1/500 dilution) at 4°C for 24 h. In control experiments, the anti-PAR4 antibody was pre-incubated for 24–48 h at 4°C with 10 μM of the blocking peptide used for immunization (peptide mapping the C-terminal domain of mouse PAR4; Santa Cruz Biotechnologies). Neurons were washed in PBS and incubated with the secondary antibody (Cy3 conjugated AffiniPure donkey anti-goat; 1/500 dilution) for 2 h.
Colocalization of PAR4, calcitonin gene-related peptide (CGRP) and substance P (SP) in DRG neurons was performed with slight modifications from the previously described protocol of Steinhoff et al. (2000). Cells were plated in poly-L-ornithine-laminine glass-bottom Petri dishes (35 mm diameter; MatTek Corporation, Ashland, MA, USA) and covered with complete culture media (MEM, 2.5% FBS, 1% pen/strep, glutamine (200 mM), 1% dextrose and ARAC, FUDR, uridine, 10 μM each). Neurons were washed in PBS containing, 1% BSA and then incubated for 20 min in 4% paraformaldehyde. This step was followed by another incubation (5 min) with PBS, BSA 1% and 0.05% Triton X-100. After three washes of 10-min each (in PBS, BSA 1%), cells were incubated with primary antibodies against PAR4 (goat, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100), and CGRP (rabbit, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:500) or SP (rabbit, Serotec Inc., Raleigh, NC, USA; 1:750) overnight at 4°C. Neurons were washed and incubated with secondary antibodies conjugated to Alexa Fluor 555 or Alexa Fluor 488 (Molecular Probes, Invitrogen Canada Inc., Burlington, ON, Canada; 1:1000, room-temperature, 1 h). As a control for antibody specificity, the primary antiserum was pre-incubated with the peptide used for immunization (10 μM) for 24 h at 4°C before staining. The number of positively-stained neurons compared to the total number of neurons was counted in one field of the × 20 objective for each Petri dish; four Petri dishes were used for each condition. Confocal images were acquired using a Zeiss LSM-510 META confocal microscope using a × 20 objective in the inverted configuration. For all confocal images a regular phase transmission image was obtained. Images of stained and control cells were collected and processed identically.
Ca2+ imaging in sensory neurons
After visual inspection of the cells, DRG neurons were perfused at room temperature in NaCl-based extracellular solution containing (in mM): NaCl, 130; KCl, 3; MgCl2, 0.6; CaCl2, 2; NaHCO3, 1; HEPES, 10; glucose, 5. For calcium imaging, cells were loaded with Fluo-4 AM (0.3 μM for 15-min, Molecular Probes Invitrogen Corporation, Carlsbad, CA, USA). Following loading, neurons were perfused (approximately 2 ml min−1) for 20 min. Band limited excitation (420–495 nm) was provided by a mercury arc lamp and filter. Neurons were imaged using an inverted microscope (Nikon, Mississauga, ON, Canada) and a × 20 0.5 NA objective. Images were acquired using a CCD camera (Carl Zeiss, Canada Ltd, Toronto, ON, Canada) at an effective sampling rate of 1 Hz. Acquisition parameters were kept constant within each experiment.
Modulation of stimulated calcium influx was evaluated by application of NaCl-based extracellular solution containing 50 mM KCl. Application of KCl to cultured DRG neurons is reported to result in a depolarization that is proportional to the concentration of KCl used (Sutton et al., 2002). Capsaicin was used at 1 μM and PAR4 agonist (100 μM) was applied for 5 min before KCl or capsaicin challenge. Regions of interest (ROIs) were fitted around the perimeter of all cells, and intensity variations for each ROI were corrected for background levels and expressed in relation to a baseline fluorescence level preceding the stimulus resulting in ΔF/F fluorescence intensity values.
Intraplantar (i.pl.) injections
The PAR4-AP AYPGKF-NH2 (1, 10, 50 or 100 μg), the PAR4-inactive control peptide YAPGKF-NH2 (1, 10, 50 or 100 μg) or carrageenan (2% solution in sterile 0.9%. saline) was administered by i.pl. injection into the rat paw under light halothane anaesthesia. The total volume of injection was always 100 μl even in the cases of the co-administration of a peptide right after carrageenan.
Measurement of nociception
Nociceptive responses to a thermal stimulus were evaluated by measuring paw withdrawal latency in response to a radiant heat stimulus applied using a ‘plantar test' apparatus (Ugo Basile, Milan, Italy). Nociceptive responses to a mechanical stimulus were assessed using an Ugo Basile Analgesy Meter (Stoelting, Wood Dale, IL, USA), measuring mechanical nociceptive flexion reflex. Before any experiments, baseline measures (time 0) were recorded for both types of assays. The paw withdrawal latency and nociceptive threshold were then evaluated at different times, ranging from 30 min to 4 h after the i.pl. injections (either AYPGKF-NH2, YAPGKF-NH2 or sterile 0.9% saline). For dose–response curves, the parameters were measured 1 h after the injections.
Additional experiments, in which the nociceptive responses to mechanical stimulation were examined following the i.pl. injection of carrageenan (±AYPGKF-NH2, YAPGKF-NH2 or sterile 0.9% saline) were performed by using von Frey monofilaments with bending forces of 4, 15 and 60 g. Each rat was placed individually in a clear plastic testing box with a metal grid floor and was allowed to acclimatize to the new environment for a minimum of 10 min. The filaments were applied three times randomly among the tested rats for 1 to 2 s before (time=0) and after the i.pl. injections (45, 60, 90 and 120 min after). A score was assigned based on the animal's response: 0=no movement, 1=removal of the paw, 2=removal of the paw and vocalization, licking or holding of the paw. Mechanical nociceptive score was expressed as a percentage of the maximal score for the three applications.
For these assays, thermal and mechanical hyperalgesia were defined as a significant decrease in withdrawal latency and nociceptive threshold or score, respectively. The opposite, an increase, defines analgesia.
Assessment of inflammation
Inflammation in the rat paw was assessed by measuring oedema formation and granulocyte infiltration. A basal measurement of the paw volume of each rat was recorded using a hydroplethismometer (Ugo Basile, Milan, Italy). Six hours after the i.pl. administration of carrageenan, the PAR4-AP AYPGKF-NH2 or the PAR4-inactive control peptide YAPGKF-NH2, a second measurement was made in order to evaluate the formation of oedema induced by these compounds. Following this last measurement, samples of rat paw tissue were recovered in order to determine the myeloperoxidase (MPO) activity, an index of granulocyte recruitment, as previously described (Vergnolle et al., 2001a; Asfaha et al., 2002). Briefly, the samples were weighed and minced prior to homogenization in a 0.5% hexadecyltrimethylammonium bromide PBS (pH 6.0) using a polytron PT10-35 homogenizer (Kinematica, Lucerne, Switzerland). The homogenates were then centrifuged at 13 000 g for 3 min at 4°C in a micro-centrifuge. Five aliquots of each supernatant were then transferred into 96-well plates before the addition of a solution containing 3,3′-dimethoxybenzidine and 1% hydrogen peroxide. In parallel, a number of standard dilutions of pure myeloperoxidase were also tested for their activity to construct a standard curve (OD as a function of units of enzyme activity). Optical density readings at 450 nm were taken at 1 min (which corresponds to the linear portion of the enzymatic reaction) using a Spectra Max Plus plate reader linked to the SOFTmax Pro 3.0 software (Molecular Devices Corp., Sunnyvale, CA, USA). The MPO activity found in the paws was expressed as units of enzyme per milligrams of tissue.
Chemicals
The PAR4-AP AYPGKF-NH2 and the PAR4-inactive control peptide YAPGKF-NH2 were obtained from the Peptide Synthesis Facility of the University of Calgary (Calgary, Alberta, Canada; peplab@ucalgary.ca, Dr Dennis McMaster, Director). The composition and the purity of the peptides were confirmed by HPLC analysis. All peptides were dissolved in sterile 0.9% saline. The MPO, isolated from human neutrophils and used as a standard, was obtained from EMD Biosciences Inc. (San Diego, CA, USA). Papain and collagenase type I were purchased from Worthington (Cedarlane, Homby, Ontario, Canada) and dispase II from Roche (Laval, Québec, Canada). Media and common cell culture additives were generally obtained from Invitrogen (Burlington, Ontario, Canada). Reagents and enzymes used for the isolation of mRNA and the RT-PCR were purchased from either Invitrogen (Burlington, Ontario, Canada) or Qiagen (Mississauga, Ontario, Canada). Fluo-4-AM was obtained from Molecular Probes (Invitrogen, Burlington, Ontario, Canada). All other drugs and reagents were purchased from Sigma-Aldrich (St-Louis, MO, USA), most notably carrageenan, laminine, poly-L-orthinine, uridine, arabinocytidine hydrochloride and floxuridine.
Statistical analysis
Data are presented as mean±s.e.m. Paw oedema, MPO and calcium mobilization measurements were analysed by using Student's two-sided t-test with Bonferroni correction. The withdrawal latency and nociceptive score data were analysed by using a one-way analysis of variance followed by Dunnett's test. With all statistical analyses, P<0.05 was considered significant.
Results
PAR4 expression in rat DRG sensory neurons
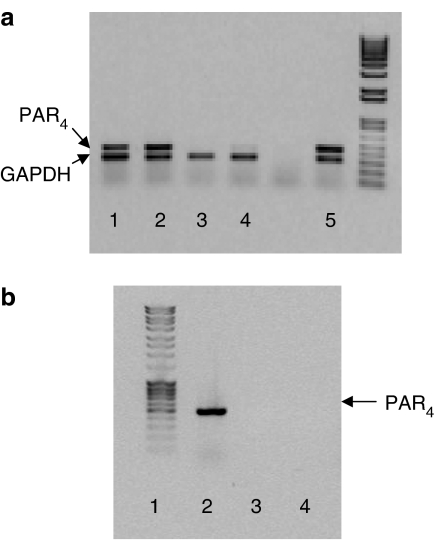
A PCR product of a predicted size of 463 bp was amplified from rat cultured DRG neurons, demonstrating the presence of PAR4 messenger in those tissues (lanes 1 and 2, panel a; lane 2, panel b; Figure 1). In the same tissues, a PCR product of a predicted size of 306 bp corresponding to GAPDH, was also detected. A PCR product of the size of PAR4, similar to that observed in DRG cultures, was observed in aorta tissues (lane 5, Figure 1a), showing a positive control for PAR4 expression. No band was detected at the predicted size of PAR4 in the absence of the PAR4 primer (panel a, lanes 3 and 4; panel b, lane 3; Figure 1), whereas the presence of GAPDH primers in the same preparation allowed the detection of a band at the predicted size of GAPDH (lanes 3 and 4, Figure 1). In order to exclude the possibility of genomic DNA amplification, DNAse was added, under these conditions and a PCR product at the predicted size of PAR4 was still detected (panel b, lane 2; Figure 1). When an RNA sample was added in place of the RT product no band was detected (panel b, lane 4; Figure 1).
Figure 1.
Detection of PAR4 in rat DRG and artery by RT-PCR. PAR4 was assessed by the amplification of a specific 463-bp PCR fragment, whereas GAPDH was a 306-bp fragment. PAR4 was detected in rat DRG cultures (a, lanes 1–2; b, lane 2), and aorta (a, lane 5), but not in DRG culture in the absence of PAR4 primer (a, lanes 3–4; b, lane 3). (b) Lanes 2 and 3: RNA was pre-treated with DNAse, PAR4 primers were added in lane 2, but not in lane 3. (b) Lane 4: RNA was added in place of the RT product. Experiments shown are representative of four.
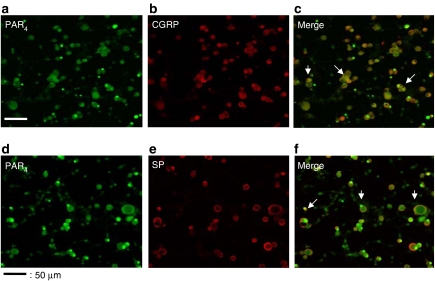
Immunohistochemistry with an anti-PAR4 antibody demonstrated the presence of the PAR4 protein in cultured DRG neurons (Figure 2a and d). No immunoreactivity was detected in the presence of the PAR4-blocking peptide previously used to raise the anti-PAR4 antibody (not shown). PAR4 was expressed by 62% of DRG neurons: over eight Petri dishes, 768 neurons were counted and 477 expressed PAR4. PAR4 was expressed in large (diameter>40 μM), medium (20 μM<diameter <40 μM) or small-diameter (diameter <20 μM) neurons (Figure 2a and d). The CGRP and SP were detected in 71 and 52% of DRG neurons, respectively (Figure 2b and e). Of 392 neurons, 243 expressed PAR4, 278 expressed CGRP and 223 expressed both PAR4 and CGRP. Of the 243 neurons that expressed PAR4, 92% (223 neurons) also expressed CGRP. Fifty-seven per cent of total DRG neurons express both PAR4 and CGRP (Figure 2c, merge of PAR4 and CGRP expression). In the SP staining experiments, of a total of 376 DRG neurons, 234 (62%) expressed PAR4, 196 (52%) expressed SP and 184 expressed both SP and PAR4. Of the 234 neurons that expressed PAR4, 184 (79%) also expressed SP. Forty-nine per cent of the DRG neurons expressed both PAR4 and SP (Figure 2f, merge of PAR4 and SP expression). Overall, these results show that the vast majority of PAR4-positive neurons also expressed the sensory neuropeptides SP and CGRP.
Figure 2.
Immunohistochemical detection of PAR4 and colocalization with CGRP and SP in cultured DRG neurons. Culture dishes were incubated in the presence of anti-PAR4 antibody (a, d), in addition to an incubation with anti-CGRP antibody (b), or an SP antibody (e). Panels c and f represent the merging of images from panels a and b, and panels d and e, respectively. Scale bar represents 115 μm.
Functional role for PAR4 in rat DRG sensory neurons
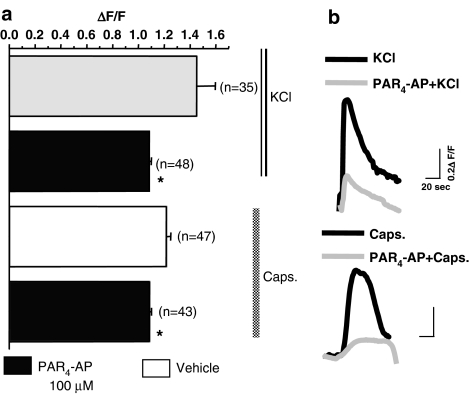
Although PAR4 is clearly expressed in DRG sensory neurons, it remains to be determined if PAR4 is a functional receptor that could modulate signalling pathways, and more specifically intracellular calcium mobilization. No calcium influx was observed in the rat cultured DRG sensory neurons in the presence of the PAR4 agonist AYPGKF-NH2 (100–200 μM) (data not shown). However, exposure of DRG neurons to the PAR4-AP (100 M) significantly reduced the amplitude of the response of the cells to KCl (50 mM) and capsaicin (1 μM) (Figure 3).
Figure 3.
Effects of PAR4-activating peptide (AYPGKF, 100 μM) or vehicle, in combination with KCl (50 mM) or capsaicin (1 μM), on calcium mobilization in cultured DRG neurons. (a) Mean values of relative fluorescence intensity (ΔF/F) evoked by KCl or capsaicin (caps) was significantly decreased in the presence (shaded columns) compared with the absence (open columns) of a PAR4 selective agonist (AYPGKF-NH2, 100 μM). Data are mean and horizontal lines show s.e.m.; *Significantly different from vehicle for P<0.05, n represents the number of neurons analyzed. (b) Representative traces of peak of relative fluorescence intensity values ΔF/F elicited by 50 mM KCl or 1 μM capsaicin in the presence (grey line) or absence (black line) of the PAR4 agonist AYPGKF-NH2 (100 μM).
PAR4 agonist modifies basal nociceptive response
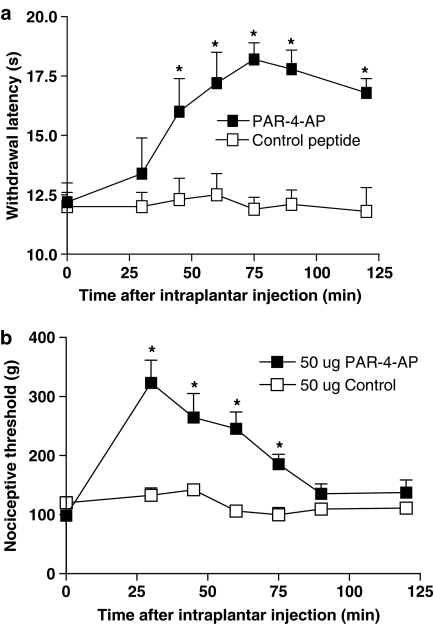
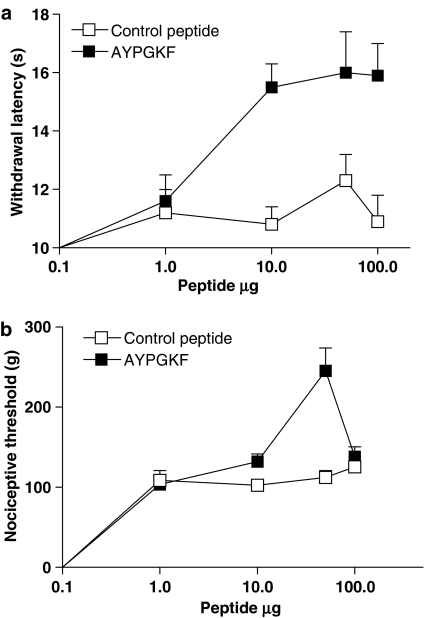
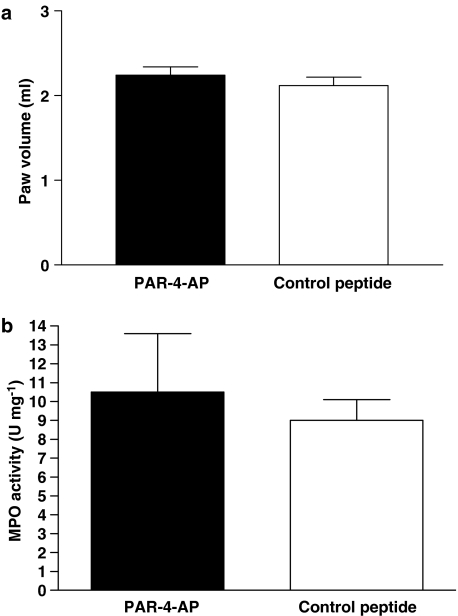
The i.pl. injection of 50 μg of the PAR4-inactive control peptide YAPGKF-NH2 did not modify withdrawal latency in response to a thermal stimulus or the nociceptive threshold in response to mechanical stimulation (Figure 4a and b). In contrast, the i.pl. injection of 50 μg of the PAR4 agonist AYPGKF-NH2 significantly increased the withdrawal latency, from 45 min to 120 min and nociceptive threshold, from 30 min to 75 min, in response to these stimuli (Figure 4a and b). The nociceptive threshold in response to the mechanical stimulus returned to baseline levels by 90 min, whereas the withdrawal latency in response to the thermal stimulus remained elevated for up to 120 min after i.pl. injection of the PAR4-AP. These effects of PAR4 activation on nociceptive pathways are probably mediated by a local effect on sensory neurons rather than a systemic effect, as an i.pl. injection of 50 μg of the PAR4 agonist AYPGKF-NH2 into the contralateral paw of the rats did not change the withdrawal latency or mechanical nociceptive threshold compared with basal measurements, when tested in the ipsilateral paw (data not shown). A dose–response curve for the effects of PAR4-AP in the nociceptive assays revealed that 50 μg was the optimal dose needed to observe an increase in withdrawal latency and nociceptive threshold (Figure 5a and b). Moreover, the i.pl. injection of 50 μg of AYPGKF-NH2, a dose that modulates nociceptive responses, did not induce an inflammatory response; no oedema formation (measured by the difference in paw volume) or granulocyte infiltration (measured by the MPO activity) was observed compared with a paw treated with the control peptide YAPGKF-NH2 (Figure 6a and b).
Figure 4.
Effects of PAR4-activating peptide (PAR4-AP) AYPGKF-NH2 or control peptide YAPGKF-NH2 (both 50 μg per paw), on withdrawal latency and nociceptive threshold in response to thermal stimulus (a) and to a mechanical stimulus (b). PAR4-AP caused a significant increase of thermal and mechanical nociceptive thresholds. Data represent mean and vertical lines show s.e.m. *Significantly different from basal (time 0) for P<0.05 (n=8 rat per group).
Figure 5.
Dose–response curves for the effects of i.pl. injection of PAR4-activating peptide (PAR4-AP) (AYPGKF) or control peptide (YAPGKF) on withdrawal latency in response to a thermal stimulus (a) and on nociceptive threshold in response to a mechanical stimulus (b) (observed at 60 min). Each symbol represents the mean and vertical lines show s.e.m. (n=8 rats per group).
Figure 6.
Effects of i.pl. injection of PAR4-activating peptide (PAR4-AP) or control peptide (50 μg per paw) observed at 6-h time point on inflammatory parameters: paw oedema (a) and MPO activity (b). Each column represents the mean and vertical lines show s.e.m. (n=8 rats per group).
PAR4 activation inhibits inflammatory hyperalgesia and allodynia
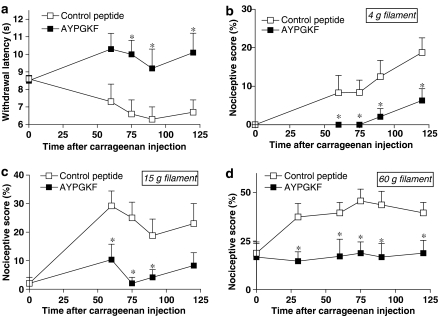
The i.pl. injection of carrageenan caused a decrease in withdrawal latency in response to a thermal stimulus, which is consistent with thermal hyperalgesia (Figure 7a). Co-injection of the PAR4-inactive control peptide YAPGKF-NH2 with carrageenan did not modify the decrease in withdrawal latency observed in saline-treated rats (data not shown). However, co-administration of the PAR4-AP, AYPGKF-NH2, with carrageenan significantly reversed the hyperalgesic response induced by carrageenan, which is consistent with an analgesic effect of the PAR4 agonist (Figure 7a). The i.pl. injection of carrageenan also modified the nociceptive responses to mechanical stimuli (Figure 7b–d). It caused a nociceptive response to a non-noxious stimulus (the 4 g von Frey filament) applied to the paw, which is characteristic of allodynia (Figure 7b), and provoked an increase in the nociceptive score in response to an intermediate stimulus (the 15 g von Frey filament) and a noxious stimulus (the 60 g von Frey filament), which is characteristic of hyperalgesia (Figure 7c and d). No difference was observed between rats treated with either saline or YAPGKF-NH2 (data not shown). The i.pl. injection of AYPGKF-NH2 significantly reduced the nociceptive score in response to both noxious and non-noxious mechanical stimuli, thus inhibiting carrageenan-induced mechanical hyperalgesia and allodynia (Figure 7b–d). Furthermore, the activation of PAR4 with the peptide agonist did not affect the inflammatory response, as carrageenan-evoked oedema and granulocyte infiltration (increased MPO activity) were not significantly different in AYPGKF-NH2-injected rats compared with YAPGKF-NH2 or saline-injected rats (data not shown). Thus, PAR4 activation can induce analgesia in inflammatory conditions, independently of the inflammatory reaction.
Figure 7.
Effects of i.pl. co-injection of carrageenan and selective PAR4-activating peptide (PAR4-AP) or control peptide (each 50 μg per paw) on carrageenan-induced inflammatory hyperalgesia and allodynia. Kinetics of nociceptive functions, in response to a thermal (a, withdrawal latency) or a mechanical (b–d, von Frey filament exposure) stimulus, were followed before (time 0) and at different times (in minutes) after i.pl. injection. For all, symbols represent the mean and vertical lines show s.e.m., n=8 rats per group, and *P<0.05 compared with the control peptide.
Discussion
Recent studies have implicated PARs in neural pathways signalling nociceptive responses in primary afferent neurons. Both PAR1 and PAR2 are able to signal to sensory neurons. Furthermore, in vivo, both PAR1 and PAR2 activation interfere with nociceptive pathways, PAR1 being analgesic (Asfaha et al., 2002; Fang et al., 2003), and PAR2 being pro-analgesic and hyperalgesic (Vergnolle et al., 2001a; Coelho et al., 2002). The presence and function of PAR4 on sensory neurons and nociceptive pathways has not been demonstrated previously. In the present study, we showed that PAR4 is expressed in neurons, and particularly in sensory neurons isolated from the DRG that express the sensory neuropeptides CGRP and SP. Although the PAR4 agonist used did not induce a calcium signal in DRG neurons, it was found to reduce the calcium signal of DRG neurons in response to KCl, suggesting that PAR4 activation could inhibit the nociceptive signal in DRG neurons. Further, we showed that i.pl. injection of the PAR4 agonist in vivo was able to increase nociceptive threshold to thermal and mechanical stimuli, and to reduce thermal and mechanical inflammatory hyperalgesia and allodynia. Thus, the present study identifies a previously unknown mechanism for the modulation of pain transmission, highlighting PAR4 as another potential receptor important for analgesia.
PAR4 has a number of physiological roles. In addition to platelet activation, PAR4 contributes to the relaxation of smooth muscle in the oesophagus (Kawabata et al., 2000). PAR4 is also expressed in human vascular smooth muscle cells, where it appears to be functionally active (Bretschneider et al., 2001), and on endothelial cells, where it contributes to the effects of thrombin (Kataoka et al., 2003). More recently, a pro-inflammatory role for PAR4 was demonstrated when PAR4 agonists were shown to induce leukocyte rolling and adherence, full granulocyte recruitment and oedema (Vergnolle et al., 2002; Kataoka et al., 2003; Hollenberg et al., 2004; Houle et al., 2005). In these studies, it was shown that, in contrast to PAR1 and PAR2, PAR4-induced oedema was not dependent on a neurogenic mechanism involving capsaicin sensitive neurons (Hollenberg et al., 2004), but was dependent on the activation of the kallikrein–kinin system (Kataoka et al., 2003; Houle et al., 2005). A study by D'Andrea et al. (2003) has shown the expression of PAR4 in peripheral nerve fibers and plexus cell bodies within detrusor muscle fibers of the mouse bladder. However, the effects of PAR4 activation on peripheral nerve functions have never been investigated. In the present study, we identified a previously unknown mechanism for the modulation of pain transmission, providing evidence for an inhibitory role for PAR4 on nociceptive functions. The finding that PAR4 is present on sensory neurons, and that in those neurons a PAR4 agonist inhibits KCl-induced calcium mobilization, suggest that a direct effect of PAR4 activation on sensory nerves inhibits the nociceptive signalling pathway. One possibility is that direct activation of DRG neurons by PAR4 agonists modulates ascending nociceptive transmission in a manner similar to that of opioid receptors, resulting in analgesia. However, such an effect needs to be confirmed; particularly, the effects of PAR4 agonists on electrical activity of sensory nerves need to be clearly defined. We cannot rule out the possibility that PAR4 agonists have a direct effect on cells adjacent to nerves, which would then release inhibitory signals for sensory neuron activation, thereby counteracting the transmission of nociceptive signals. In previous studies, it has been shown that PAR4 activation in transfected cells or in epithelial cells causes calcium mobilization (Hoogerwerf et al., 2002; Cottrell et al., 2004). However, in our study, we could not detect any calcium mobilization in response to PAR4 agonists in DRG neurons. In contrast, the PAR4 agonist used inhibited the calcium response evoked by KCl or capsaicin (see Figure 3). The signal transduction environment of a neuron can be totally different in epithelial from that in endothelial cells, and it is well known that a given GPCR can activate different sets of signalling pathways in different cell types, as observed for example for cannabinoid receptors (Demuth and Molleman, 2006). Therefore, PAR4 could induce a calcium response in one cell type and inhibit it in another.
In a previous study, we showed that the i.pl. injection of thrombin caused analgesia in response to a mechanical stimulus, but hyperalgesia in response to a thermal stimulus, whereas selective PAR1 activation caused analgesia in response to both thermal and mechanical stimuli (Asfaha et al., 2002). In the present study, it was shown that i.pl. injection of a PAR4 agonist induces an analgesic effect in the rat paw in response to both mechanical and thermal stimulation. These findings disprove the hypothesis that thrombin's hyperalgesic effect in response to a thermal stimulus (Asfaha et al., 2002) could be due to PAR4 activation. Rather, these findings are consistent with the idea that the analgesic effects of thrombin in response to mechanical stimulation are due to the activation of PAR1 alone or the combined activation of PAR1 and PAR4 (Asfaha et al., 2002). As for the hyperalgesic effects of thrombin in response to thermal stimulation, thrombin might act through a receptor other than PAR1 or PAR4, and/or exerts its effect independently of its catalytic site (Bar-Shavit et al., 1983; Bar-Shavit and Wilner, 1986; Herbert et al., 1994).
Although we show here that a PAR4 agonist exerts analgesia, higher concentrations of the PAR4-AP were required to produce this effect compared to the PAR1 agonist. For example, in this study, 50 μg PAR4-AP was required for analgesia to thermal and mechanical stimuli, compared with 10 μg of the PAR1 agonist in our previous study (Asfaha et al., 2002). This is consistent with the known pharmacology of the PARs, where high concentrations of PAR4-AP are required to observe an effect in all the systems tested, compared with the concentration needed to induce responses to PAR1 or PAR2 agonists (Hollenberg et al., 1999, 2004; Faruqi et al., 2000; Hollenberg and Compton, 2002). It is interesting to note that the dose of PAR4 agonist that is able to cause analgesia in rats (50 g per paw) is mainly smaller than the dose that has been shown to induce signs of inflammation (granulocyte infiltration and oedema) (200 g per paw) (see Figure 6 and Asfaha et al., 2002; Houle et al., 2005).
PAR1 and PAR4 activation have similar effects on nociception, they both caused analgesia in response to thermal and mechanical stimulation. One can hypothesize that they may have duplicate roles, with potentially different affinity, as has been described for platelet activation and endothelial cell functions (Kahn et al., 1998b; Kataoka et al., 2003). However, they might also be activated by different proteases. They can both be activated by thrombin, but PAR4 can also be activated by trypsin and the neutrophil granule protease cathepsin G (Sambrano et al., 2000). Therefore, the inhibitory effects of PAR1 and/or PAR4 activation on the transmission of nociceptive messages might depend on the type of proteases that are released in the vicinity of sensory nerves. In addition, although our study suggests that PAR4 could exert its inhibitory effect through a direct effect on sensory neurons, such evidence has never been raised for PAR1. We cannot rule out the possibility that the inhibitory effects of PAR1 activation on nociception are mediated by an indirect mechanism involving cell types other than sensory neurons. The fact that PAR1 activation in sensory neurons provokes the release of pro-nociceptive neuropeptides (CGRP and SP) would favour this last hypothesis.
In summary, this study identifies a novel potential endogenous analgesic mechanism: PAR4. Although activation of PAR4 has been shown to have coagulation and pro-inflammatory effects, this study is the first to provide evidence of a role for this receptor in pain pathways independent of its role in inflammation. We also provide evidence, that PAR4 is expressed in neurons, and particularly in sensory neurons. The finding that the PAR4 agonist alleviated the hyperalgesia and allodynia, associated with inflammatory responses to various stimuli, also suggests that PAR4 agonists may have potential as analgesic agents.
Acknowledgments
SA is the recipient of a Merck and Dohme Medical Award. NC is the recipient of a Canadian Association of Gastroenterology/Crohn's and Colitis Foundation of Canada fellowship. SH is the recipient of a post-doctoral fellowship from the Alberta Heritage Foundation for Medical Research and Neuroscience Canada. CA holds postdoctoral fellowships from the Alberta Heritage Foundation for Medical Research and the Heart and Stroke Foundation of Canada. GWZ is an Alberta Heritage Foundation for Medical Research Senior Scholar. NV is an Alberta Heritage Foundation for Medical Research Scholar and a Canadian Institute of Health Research New Investigator. This work was funded by a Canadian Institute of Health Research Operating grant (NV).
Abbreviations
- AP
activating peptide
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglia
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HBSS
Hanks' balanced salt solution
- MPO
myeloperoxydase
- PARs
protease-activated receptors
- PAR1
protease-activated receptor-1
- PAR2
protease-activated receptor-2
- PAR3
protease-activated receptor-3
- PAR4
protease-activated receptor-4
- RT-PCR
reverse transcription–polymerase chain reaction
Conflict of interest
The authors state no conflict of interest.
References
- Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R, Wilner GD. Biologic activities of nonenzymatic thrombin: elucidation of a macrophage interactive domain. Semin Thromb Hemost. 1986;12:244–249. doi: 10.1055/s-2007-1003561. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R, Kahn A, Wilner G, Fenton J. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Bretschneider E, Kaufmann R, Braun M, Nowak G, Glusa E, Schror K. Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol. 2001;132:1441–1446. doi: 10.1038/sj.bjp.0703947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Vergnolle N. Proteases and protease-activated receptors (PARs): novel signals for pain. Curr Top Med Chem. 2005;5:569–576. doi: 10.2174/1568026054367601. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV: a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Sabam MR, Nguyen NB, Andrade-Gordon P, Saban R. Overriding participation of protease activated receptor (PAR)s 1, 2, 3, and 4 in experimental bladder inflammation. Am J Pathol. 2003;162:907–923. doi: 10.1016/S0002-9440(10)63886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, et al. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Fang M, Kovacs KJ, Fisher LL, Larson AA. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J Physiol. 2003;549:903–917. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruqi TR, Weiss EJ, Shapiro MJ, Huang W, Coughlin SR. Structure function analysis of protease activated receptor 4 tethered ligand peptides: determinants of specificity and utility in assays of receptor function. J Biol Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- Gao CY, Liu SM, Hu HZ, Gao NA, Kim GY, Xia Y, et al. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- Herbert J, Dupuy E, Laplace M, Zini J, Bar-Shavit R, Tobelem G. Thrombin induces endothelial cell growth via both a proteolytic and non-proteolytic pathway. Biochem J. 1994;303:227–231. doi: 10.1042/bj3030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Al Ani B, Gui Y. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can J Physiol Pharmacol. 1999;77:458–464. [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Sandhu S, Houle S, Vergnolle N. Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br J Pharmacol. 2004;143:443–454. doi: 10.1038/sj.bjp.0705946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf WA, Hellmich HL, Micci M, Winston JH, Zou L, Pasricha PJ. Molecular cloning of the rat proteinase-activated receptor 4 (PAR4) BMC Mol Biol. 2002;3:2. doi: 10.1186/1471-2199-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle S, Papez P, Ferazzini M, Hollenberg MD, Vergnolle N. Neutrophils and the kallikrein–kinin system in proteinase-activated receptor 4-mediated inflammation. Br J Pharmacol. 2005;146:670–678. doi: 10.1038/sj.bjp.0706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Connolly A, Zeng D, Kahn M, Zheng Y, Timmons C, et al. Protease-activated receptor-3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- Kahn M, Hammes S, Botka C, Coughlin S. Gene and locus structure and chromosomal localization of the protease-activated receptor gene family. J Biol Chem. 1998a;273:23290–23296. doi: 10.1074/jbc.273.36.23290. [DOI] [PubMed] [Google Scholar]
- Kahn M, Zheng Y, Huang C, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet activation. Nature. 1998b;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, et al. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kuroda R, Kuroki N, Nishikawa H, Kawai K. Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br J Pharmacol. 2000;131:578–584. doi: 10.1038/sj.bjp.0703590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Mule F, Pizzuti R, Capparelli A, Vergnolle N. Evidence for the presence of functional protease activated receptor 4 (PAR(4)) in the rat colon. Gut. 2004;53:229–234. doi: 10.1136/gut.2003.021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shutoff with distinct kinetics after activation by thrombin. J Biol Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young S, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001a;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Derian CK, D'Andrea MR, Steinhoff M, Andrade-Gordon P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential pro-inflammatory role for proteinase-activated receptor-4 (PAR-4) J Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001b;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Vu T, Hung D, Wheaton V, Coughlin S. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Xu W, Andersen H, Whitmore T, Presnell S, Yee D, Ching A, et al. Cloning and characterization of human protease-activated receptor-4. Proc Natl Acad Sci USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]