Abstract
Background and purpose:
Red blood cells (RBCs) are reservoirs of vasodilatory, antiaggregatory, and antiinflammatory lipid mediators—epoxyeicosatrienoic acids (EETs). This study addresses the formation and release of erythrocyte-derived EETs in response to ATP receptor stimulation that may represent an important mechanism regarding circulatory regulation.
Experimental approach:
Erythrocyte EET formation and release were investigated by incubating rat RBCs in physiological salt solution with agents that effected ATP release via P2 receptor stimulation of phospholipase A2 and epoxygenase-like activities with activation of the ATP secretory mechanism. EETs were analyzed by gas and liquid chromatography-mass spectrometry.
Key results:
EETs were released from rat RBCs: 14,15-, 11,12-, 8,9- and 5,6-EETs in a ratio of 1.2:1.0:0.9:0.8. EETs were produced by epoxidation of arachidonic acid catalyzed by hemoglobin. Spontaneous release of EETs, 0.66±0.14 ng per 109 RBCs, was dose-dependently increased by an ATP analog, BzATP, and inhibited by P2X7 receptor antagonists. 5 μM ATP increased release of EETs over 20% to 0.83±0.15 ng per 109 RBCs; 10 μM BzATP tripled the amount of EET release to 1.87±0.20 ng per 109 RBCs. EET release by ATP or BzATP was not associated with hemolysis. Carbenoxolone, a gap junction inhibitor that inhibits ATP release, and glibenclamide, an inhibitor of the cystic fibrosis transmembrane conductance regulator (CFTR), which is required for ATP release, inhibited the spontaneous and stimulated EET release from RBCs.
Conclusions and implications:
EETs are produced and released from RBCs via a mechanism that is mediated by ATP stimulation of P2X7 receptors coupled to ATP transporters, pannexin-1 and CFTR.
Keywords: ATP, purinoceptors, erythrocytes, haemoglobin, epoxyeicosatrienoic acids, CFTR, gap junction, pannexin-1, microcirculation
Introduction
We have reported that red blood cells (RBCs) are reservoirs for cis- and trans-epoxyeicosatrienoic acids (EETs) (Jiang et al., 2004, 2005). EETs are essential components of key vasoregulatory mechanisms and are candidate endothelium-derived hyperpolarizing factors (Quilley et al., 1997; Spector et al., 2004). As EETs mediate pleiotropic biological responses, including those affecting ion transport (Pascual et al., 1998; Watanabe et al., 2003), gene expression (Node et al., 2001; Michaelis and Fleming, 2006) vasorelaxation (Imig et al., 2001; Carroll et al., 2006), inflammation (Node et al., 1999; Campbell, 2000; Liu et al., 2005) and platelet aggregation (Fitzpatrick et al., 1987; Jiang et al., 2004), the release of EETs from RBCs is replete with potential contributions to the control of the microcirculation and the rheological properties of the circulating blood.
RBCs can regulate microvascular tone, which has been explored via mediators ranging from ATP (Ellsworth et al., 1995; Dietrich et al., 2000), nitric oxide (NO) (Jia et al., 1996; Stamler et al., 1997; McMahon et al., 2002) and haemoglobin (Jagger et al., 2001) to hypoxia-inducible factors (Hagen et al., 2003; Singel and Stamler, 2005). Through dynamic interactions between NO and haemoglobin, RBCs can elicit hypoxic vasodilation (Jia et al., 1996; McMahon et al., 2002). Coronary blood flow during exercise has been proposed to be regulated by ATP released from RBCs (Farias III et al., 2005). RBC deformation-induced ATP release requires the activity of the cystic fibrosis transmembrane conductance regulator (CFTR) (Sprague et al., 1998). In addition, pannexin-1 was recently identified as an ATP release channel in RBCs (Locovei et al., 2006). However, the release of vasoactive lipid mediators from RBCs has not been reported.
More than three decades ago, Parker and Snow (1972) demonstrated that external ATP influenced the permeability and metabolism of dog erythrocytes. ATP acts as an extracellular signalling molecule via interactions with specific P2 receptors to mediate a wide variety of processes (Burnstock et al., 1972; Burnstock and Knight, 2004). Extracellular ATP increases cation fluxes by activating the P2X7 receptor, which has been identified as the major form of P2X receptors in human erythrocytes (Sluyter et al., 2004). Activation of the P2X7 receptor has been associated with cytoskeletal rearrangement, membrane internalization, pore formation and permeability and trafficking changes (Kim et al., 2001; Kochukov and Ritchie, 2004). In response to deformation of RBCs and hypoxaemia on passage through the microcirculation (Bergfeld and Forrester, 1992; Sprague et al., 1998), ATP release from RBCs may represent an important mechanism involved in the regulation of the microcirculation.
ATP has the additional capability of activating secretion of EETs from RBCs, an effect that should greatly amplify the vascular response to ATP. EET release from rat RBCs was analysed by gas chromatography-mass spectrometry (GC-MS) as well as electrospray ionization liquid chromatography-mass spectrometry (ESI LC-MS). We have shown that (1) RBCs metabolized arachidonic acid (AA) to form EETs; (2) stimulation of the erythrocyte P2X7 receptors by ATP or an ATP analog, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP), increased EET release; (3) inhibition of phospholipase A2 (PLA2) prevented EET release from RBCs; (4) inhibition of ATP release by blocking either transporters or channels, CFTR or pannexin-1, reduced EET release from RBCs and (5) EET release from RBCs was not associated with haemolysis.
Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Eight-week-old male Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA). Rats were maintained at 22°C with alternating cycles of light and darkness and fed ad libitum with standard rat chow and water.
RBC preparation
Sprague–Dawley rats (9–12-week old) were anaesthetized with pentobarbital, 65 mg kg−1 intraperitoneal and 10 ml blood was drawn from the inferior vena cava after midline laparotomy using heparin rinsed syringes and transferred to Vacuette heparin tubes (Fisher). After inverting 4–6 times, the blood was cooled on ice and then centrifuged at 800 g at 4°C for 10 min. The supernate and the buffy layer were removed by aspiration. Packed RBCs were washed three times and resuspended in a physiological salt solution (PSS, in mM: 4.0 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 15.7 N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), 11.1 dextrose, and 2 mg ml−1 bovine serum albumin, pH adjusted to 7.35). The RBCs were examined and counted under the microscope, and the RBC solution was diluted with PSS to 2 × 109 RBCs ml−1.
RBC incubation and lipid extraction
RBCs (1–2 ml per sample) were incubated with or without treatment in a VWR incubating mini shaker at 37°C for 30 min with shaking around a 3 mm orbit at 600 r.p.m. Agents in the experiments were prepared in stock solutions at 100 to 200-fold of the targeted concentration and 1% of the solvents, including ethanol and dimethyl sulphoxide, were tested without significant effects. After incubation, the mixture was centrifuged at 2000 g for 10 min. Internal standards, EET-d8 (6 ng) and AA-d8 (10 ng), were added to aliquots of the incubation buffer and extracted with 2 ml ethyl acetate two times after adjusting pH to 4 with acetic acid. The combined organic phase was dried, reconstituted in acetonitrile for high-performance liquid chromatography (HPLC) separation and ESI LC-MS analysis. HPLC was carried out using Shimadzu LC-10AT Liquid Chromatograph with automatic sample injection and programmed fraction collection. A Beckman-Coulter Ultrasphere ODS column (25 cm × 4.6 mm × 5 μm) was used. Ultraviolet (UV) absorbance from 200 to 400 nm was monitored. A gradient from 75% acetonitrile–25% water–0.05% acetic acid to 100% acetonitrile in 20 min was used. Total EETs were collected from 11.8 to 14.8 min and AA was collected from 19.5 to 20.6 min.
Haemolysis measurements
The RBC incubation buffer, after centrifugation at 2000 g for 10 min, was diluted 1:4 with phosphate-buffered saline and the UV absorbances at 414 nm were measured with the subtraction of the average baselines at 380 and 450 nm (Malinauskas, 1997). The haemoglobin concentration was calculated from a standard curve using rat haemoglobin. Freeze–thawing of RBCs was carried out by freezing RBCs at −70°C for 10 min and warming up at 37°C. Haemoglobin of the freeze–thawed samples after centrifuging the ghosts at 2000 g for 20 min was measured by diluting 220-fold in 0.01% Na2CO3 and calculated according to Harboe (1959) method.
Quantitation of AA and total EETs
For quantitation of AA and total EETs using negative ion chemical ionization GC-MS, AA and EETs were derivatized to pentafluorobenzyl esters (Jiang et al., 2004). The GC column (DB-1 ms, 10 m length, 0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies Inc.) was temperature programmed from 150 to 250°C at a rate of 30°C min−1 for AA and EET detection. Methane was used as the reagent gas. The ions of m/z 303 and 311 for AA/AA-d8 and m/z 319 and 327 for EET/EETd8 were monitored. The total amounts of AA and EETs were calculated from their peak area ratios according to standard curves.
ESI LC-MS analyses
ESI LC-MS analyses of EETs were carried out as described (Jiang et al., 2005). Briefly, a Finnigan LCQ Advantage quadrupole ion-trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA) equipped with ESI source run by XCALIBUR software was used. HPLC was run with a Luna C18(2) 250 × 2.0 mm column (Phenomenex, Torrance, CA, USA) with an isocratic gradient of acetonitrile–water–methanol–acetic acid (60:30:10:0.05) at a flow rate of 0.25 ml min−1. ESI was carried out at an ion transfer tube temperature of 260°C, a spray voltage of 4.5 kV, a sheath gas flow of 34 units and an auxiliary gas flow of 20 units (units refer to arbitrary values set by the LCQ software).
Data analyses
Data were presented as mean±s.e. of n experiments. An unpaired Student's t-test and one-way analysis of variance were performed to test for differences between two groups. A value of P<0.05 was regarded as statistically significant.
Reagents
All chemicals including AA, ATP, BzATP, 2-methylthio-ATP (2MeSATP), adenosine 5′-O-(3-thiotriphosphate) (ATPγS), methyl arachidonyl flurophosphonate (MAFP), bromoenol lactone (BEL), S3319, HEPES, bovine serum albumin and rat haemoglobin were purchased from Sigma-Aldrich (St Louis, MO, USA). AA was used after further purification by HPLC. Methyl sulphonyl propargyloxyphenyl hexanamide (MSPPOH) and 17-octadecenoic acid (ODYA) were synthesized by Dr John R Falck. Acetonitrile, methanol and hexanes (all HPLC grade) were purchased from Fisher Scientific. EET-d8 standards were obtained from Biomol.
Results
EETs released from RBCs
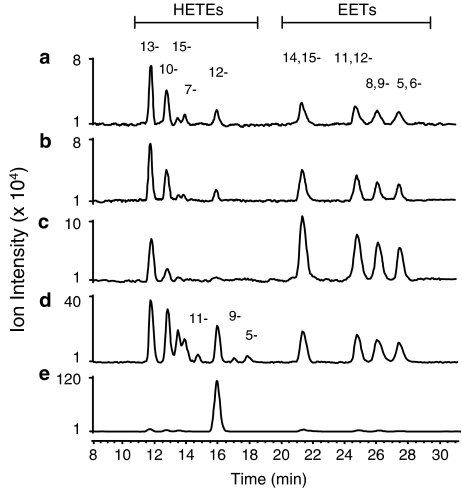
Analysis by ESI LC-MS of the basal release of lipid mediators from RBCs revealed hydroxyeicosatetraenoic acids (HETEs) and EETs (Figure 1a). The identification of HETEs and EETs was based on their HPLC retention times and tandem mass spectra (Brash et al., 1995; Jiang et al., 2004). RBCs released spontaneously 13-, 10-, 15-, 7- and 12-HETEs as well as four EETs. The EETs released were 14,15-, 11,12-, 8,9- and 5,6-cis-EETs in a ratio of 1.2:1.0:0.9:0.8, both in control and in response to stimulation with ATP, BzATP and AA (Figures 1b–d).
Figure 1.
Representative ESI LC-MS profiles (n=4–8, m/z 319) of the released HETEs and EETs from incubations of control (a), ATP 1 mM (b), BzATP 10 μM (c), AA 0.66 μM (d) and A23187 2 μM (e) with 4 × 109 rat RBCs in 2 ml buffer for 30 min at 37°C. Eicosanoid products from RBCs include 15-, 13-, 12-, 11-, 10-, 9-, 7- and 5-HETEs as well as 14,15-, 11,12-, 8,9- and 5,6-EETs. EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid.
Both ATP at 1 mM and BzATP at 10 μM increased the release of EETs and, although less apparent for ATP, they inhibited the release of HETEs (Figures1b and c). Exposure of RBCs with 2 μM calcium ionophore A23187 selectively stimulated the release of 12-HETE (Figure 1e), as has been reported (Kobayashi and Levine, 1983).
Addition of 0.66 μM AA to 4 × 109 RBCs in 2 ml PSS resulted in the synthesis and release of all of the HETEs and EETs (Figure 1d). When reacting the solution from the lysed RBCs or from purified rat haemoglobin with AA, various product profiles including formation of all cis- and trans-EETs were observed depending on the ratio of AA and haemoglobin concentrations (data not shown). The formation of total EETs from AA (1 μg ml−1) in the haemoglobin (1 mg ml−1) solution from lysed RBCs at 37°C for 30 min was inhibited by both the cytochrome P450 epoxygenase inhibitor, MSPPOH and the combined inhibitor of both the ω-hydroxylases and epoxygenases, ODYA (Table 1). The same level of EET formation and inhibition was also found for AA when reacting with purified rat haemoglobin (Sigma, St Louis, MO, USA).
Table 1.
Conversion of AA (1 μg ml−1) to EETs by haemoglobin (1 mg ml−1) from lysed rat erythrocytes and inhibition by MSPPOH or ODYA (n=4)
| Control | MSPPOH (24 μM) | ODYA (30 μM) | |
|---|---|---|---|
| EET (ng μg−1 AA) | 83.75±7.22 | 56.75±8.88* | 68.45±3.35* |
Abbreviations: AA, arachidonic acid; EET, epoxyeicosatrienoic acid; MSPPOH, methylsulphonylpropargyloxyphenyl hexanamide; ODYA, 17-octadecyenoic acid.
Data are expressed as mean±s.e.
P<0.05, as compared with control.
Extracellular ATP on EET release from RBCs
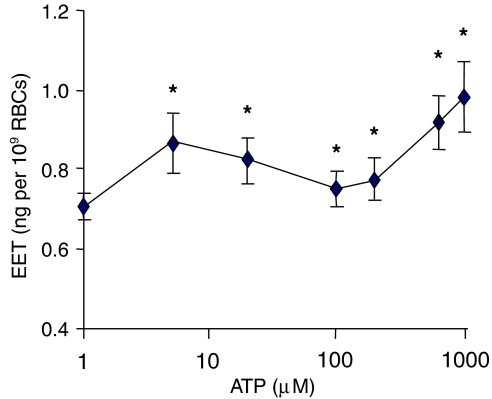
ATP from 1 μM to 1 mM produced a multiphasic response on EET release (Figure 2). A total of 1 mM ATP increased EET release over 40% relative to the spontaneous release (Figure 3). ATP at 5 μM, a concentration found in human plasma (Gonzalez-Alonso et al., 2002), increased EET release over 20% (0.83±0.15 ng/109 RBC). Suramin (100 μM), considered to be a specific P2Y2 receptor antagonist (Charlton et al., 1996), stimulated EET release (Table 2), while reactive blue-2 (RB-2, 50 μM), a non-specific ATP antagonist, inhibited EET release, suggesting a dual action of ATP on the EET releasing mechanisms in rat RBCs, which also explained the multiphasic effects of ATP on EET release from RBCs shown in Figure 2. Suramin, at 300 μM, inhibited EET release (data not shown) revealing non-specific P2 receptor inhibition at high concentrations.
Figure 2.
Total EET release from rat erythrocytes in response to extracellular ATP. ATP from 1 μM to 1 mM was incubated with rat erythrocytes for 30 min at 37°C. n=8–12, *P<0.05 vs spontaneous EET release at 0.66±0.14 ng per 109 RBCs. EET, epoxyeicosatrienoic acid.
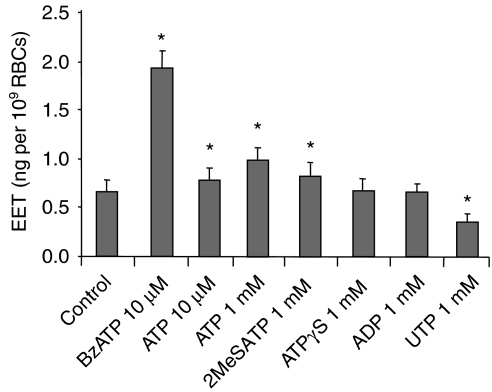
Figure 3.
Comparison of the potency of P2 receptor agonists on total EET release from rat erythrocytes. Rat RBCs were incubated with ATP, BzATP, 2MeSATP, ATPγS, ADP and UTP for 30 min at 37°C. EETs were analysed by GC-MS from the supernatent after centrifuging the RBCs. n=4–8, *P<0.05 vs control. BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; EET, epoxyeicosatrienoic acid; 2MeSATP, 2-methylthio-ATP.
Table 2.
Effects of P2 antagonists on EET release from rat erythrocytes (2 × 109 cells ml−1, n=4–8)
| Basal | Suramin (100 μM) | RB-2 (50 μM) | |
|---|---|---|---|
| Control | 0.66±0.14 | 0.97±0.15* | 0.44±0.08* |
| ATP (1 mM) | 0.98±0.16 | 1.49±0.28* | 0.68±0.12* |
Abbreviations: EET, epoxyeicosatrienoic acid; RB-2, reactive blue-2.
All values were expressed as ng per 109 RBCs.
Data are expressed as mean±s.e.
P<0.05 as compared with basal.
P2X7 receptor stimulation increased EET release from RBCs
Comparison of ATP with other P2 receptor agonists showed that stimulation of EET release was in the order of BzATP >2MeSATP >ATPγS (Figure 3), typical of the response of P2X7 receptors (Surprenant et al., 1996; Gargett et al., 1997). BzATP was most potent in releasing EETs from RBCs, consistent with P2X7 receptors serving as the predominant P2X receptor in human erythrocytes (Sluyter et al., 2004) and BzATP acting as a potent selective P2X7 receptor agonist (Baraldi et al., 2004). UTP (1 mM), mostly a P2Y receptor agonist (Kumari et al., 2003), inhibited EET release from RBCs (P<0.05 vs basal; Figure 3), which as indicated, can also explain the multiphasic effect of ATP on EET release from RBCs.
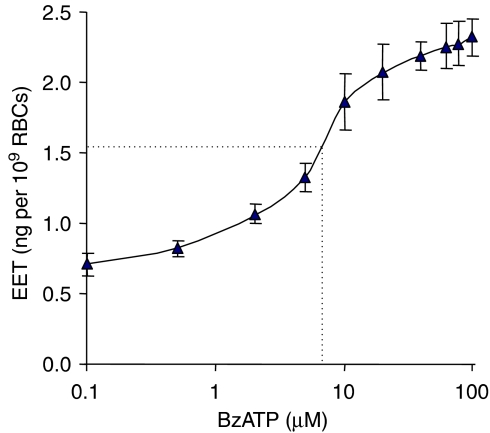
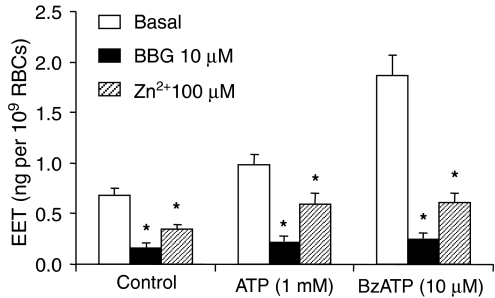
BzATP dose-dependently stimulated EET release with an EC50 of 6.6 μM (Figure 4). 100 μM BzATP more than tripled the spontaneous EET release. Although RBCs were routinely incubated with shaking at 37°C for 30 min after adding the reagents to the cold RBCs, the addition of 10 μM BzATP 2 min before finishing the incubation resulted in the same increase in EET release as produced by adding BzATP at the start of the 30 min incubation, indicating rapid formation and release of EETs by activating P2X7 receptors in RBCs. P2X7 antagonists, brilliant blue G (BBG, 10 μM) and Zn2+ (100 μM), inhibited ATP (1 mM) and BzATP (10 μM) stimulated EET release (Figure 5).
Figure 4.
Dose-dependent response of the P2X7 receptor agonist, BzATP, on EET release from rat erythrocytes. Incubations were carried out with BzATP from 0.1 to 100 μM. n= 6–10. BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; EET, epoxyeicosatrienoic acid.
Figure 5.
P2X7 antagonists, BBG and Zn2+, inhibit EET release from rat erythrocytes. n=6–10, *P<0.05 vs basal in the same group. BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; BBG, brilliant blue G; EET, epoxyeicosatrienoic acid.
Effects of ATP release inhibitors on EET releases from RBCs
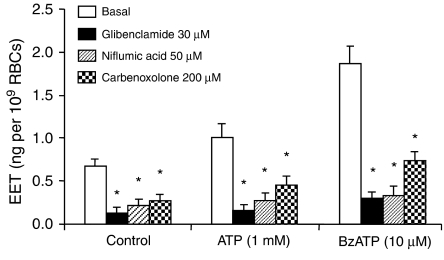
Both CFTR (Sprague et al., 1998) and pannexin-1 (Locovei et al., 2006) have been separately reported to mediate the release of ATP from RBCs although there are likely to be interactions between CFTR and pannexin (Kotsias and Peracchia, 2005). Glibenclamide and niflumic acid, inhibitors of CFTR activities, inhibited ATP release during erythrocyte deformation (Sprague et al., 1998). Carbenoxolone, an inhibitor of gap junctions, inhibited ATP release from RBCs induced by hypotonic stress (Locovei et al., 2006). These inhibitors, all blocked spontaneous, as well as ATP and BzATP stimulated EET release (Figure 6).
Figure 6.
Effects of ATP release inhibitors, glibenclamide, niflumic acid and carbenoxolone, on EET release from rat erythrocytes. n=6–12, *P<0.05 vs basal in the same group. BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; EET, epoxyeicosatrienoic acid.
Role of PLA2 on EET release from RBCs
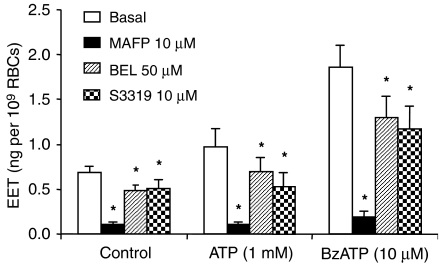
Effects of the inhibitors of Ca2+-dependent and independent cytosolic PLA2 (MAFP and BEL, respectively), and secretory PLA2 (S3319) on EET release from RBCs were tested (Figure 7). MAFP at 10 μM most effectively inhibited EET release. To a much lesser degree, BEL and S3319 inhibited EET release from RBCs.
Figure 7.
Effects of PLA2 inhibitors, MAFP, BEL and S3319, on EET release from rat erythrocytes. MAFP, a Ca2+-dependent cytosolic PLA2 inhibitor, BEL, a Ca2+-independent cytosolic PLA2 inhibitor, and S3319, a secretory PLA2 inhibitor were tested in the RBC incubations. n=6–8, *P<0.05 vs basal in the same group. BEL, bromoenol lactone; BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; EET, epoxyeicosatrienoic acid; MAFP, methyl arachidonyl flurophosphonate.
Haemolysis and AA release
Haemolysis and AA release were also monitored in the incubates to assess their effects on EET release from RBCs. Stimulated EET release by ATP and BzATP was not associated with increases in haemolysis or AA release into the buffer (Table 3). To induce haemolysis, a toxic concentration of Cu(NO3)2 (100 μM) was used in the incubation of RBCs. However, EET release was not increased by elevated haemolysis and AA release. Freezing and thawing of RBCs released lower amounts of EETs than basal EET release. The total AA in rat RBCs was analysed and found to be 18.07±0.68 μg per 109 RBCs, making the spontaneous AA release from RBCs (Table 3) account for about 0.1% of the total AA in RBCs.
Table 3.
Comparison of the total EET release with AA release and haemolysis from rat erythrocytes (2 × 109 cells ml−1, n=4–10)
| Basal | ATP (1 mM) | BzATP (10 μM) | Cu (NO3)2 (100 μM) | Freeze–thawing | |
|---|---|---|---|---|---|
| EET release | 0.66±0.14 | 0.98±0.16* | 1.87±0.20* | n.d. | 0.35±0.12* |
| AA release | 18.29±2.26 | 17.52±2.36 | 18.07±2.18 | 51.17±4.42* | 55.32±6.15* |
| Haemolysis (mg l−1) | 115±10 | 111±9 | 111±7 | 543±91* | 13480±430* |
Abbreviations: AA, arachidonic acid; BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; EET, epoxyeicosatrienoic acid; n.d., not detected.
Data are expressed as mean±s.e. EET and AA release are expressed as ng per109 RBCs.
P<0.05 as compared with basal in the same category.
Discussion
There is compelling evidence that the erythrocyte acts as an oxygen sensor; namely, in response to physiological declines in oxygen tension, ATP released from erythrocytes acts on microvascular endothelial cells to produce vasodilatation (Dietrich et al., 2000; Feigl, 2004). The present study expands the sphere of activity of ATP by showing that ATP, by stimulating erythrocyte P2X7 receptors, releases EETs. As EETs exhibit a wide range of actions within the circulation, viz., vasodilation as well as antithrombotic and anti-inflammatory activities (Spector et al., 2004), they can account for many of the vascular responses to ATP.
The sequence of events in RBCs that follow upon ATP/BzATP stimulation of P2X7 receptors is initiated by activation of PLA2, which releases AA and EETs from storage in phospholipids (Salgo et al., 1993; Jiang et al., 2005). As deacylated AA can be converted to EETs by the epoxygenase-like activity of haemoglobin acting as a catalyst in RBCs (Table 1), the EETs secreted from RBCs in response to ATP/BzATP stimulation represent both release from storage in RBC phospholipids and de novo synthesis (Figure 1d). The secretion of EETs from RBCs involves both CFTR and pannexin-1 acting as conductive, that is, secretory pathways, for ‘the selective delivery of nucleotides' and EETs ‘to the extracellular domain' (Reisin et al., 1994). This secretory mechanism is based on the functional duality of the P2X7 receptor that not only ‘acts as an ATP-activated ion channel but also forms a pore, gating passage of molecules up to 1000 Da' such as EETs ‘in response to ATP' (Lazarowski et al., 2003).
ATP at 5 μM and 1 mM increased EET release by ca. 20 and 40%, respectively (Figure 2), whereas at 10 μM, BzATP produced a threefold increase in EET release from RBCs (Figure 4). High concentrations of ATP (1 mM) were tested for EET release because of the relative insensitivity of RBC P2X7 receptors to extracellular ATP (Sluyter et al., 2004; Young et al., 2007). Additionally, a P2Y receptor has been identified in RBCs that represents a negative feedback mechanism regulating erythrocyte ATP secretion (Wang et al., 2005). The role of P2Y receptors was uncovered by inhibition of EET release from the rat erythrocyte in response to UTP, the prototypical P2Y agonist (Kochukov and Ritchie, 2004; Figure 3). Additionally, increased EET release from RBCs produced by suramin at 100 μM (Table 2) can be explained by selective inhibition of P2Y receptors (King et al., 1996; Bourke et al., 1999) that exert a tonic inhibitory effect on EET release. In contrast, inhibition of EET release by RB-2 (Table 2), a nonselective ATP antagonist, is a function of its capacity to block all P2 receptors.
The EC50 value, 6.6 μM of BzATP for EET release (Figure 4) is in accordance with the reported EC50 for the rat P2X7 receptor (Surprenant et al., 1996). The EC50 value for ATP on erythrocyte EET release could not be estimated because of the cited antagonism of P2Y receptors to P2X7 receptor-mediated EET release. Spontaneous EET release from RBCs was inhibited by both P2X7 antagonists, BBG and Zn2+ (Figure 5), suggesting a function for the RBC P2X7 receptor related to basal formation and release of EETs.
Haemoglobin catalysed conversion of AA into EETs was inhibited by MSPPOH or ODYA (Table 1), suggesting an epoxygenase-like activity of haemoglobin. Thus far, an epoxygenase has not been identified in RBCs, but ‘the mechanism and versatility of the monooxygenase activity of haemoglobin are qualitatively similar to those of the liver microsomal cytochrome P450' (Mieyal and Starke, 1994). In intact RBCs, the formation of EETs is regulated by P2X7 receptor responses such as increases in cytoskeleton movement (Kim et al., 2001; Kochukov and Ritchie, 2004), phospholipid signalling (Garcia-Marcos et al., 2006) and oxidative burst (Suh et al., 2001; Parvathenani et al., 2003). Further, P2X7 receptor stimulation by ATP and BzATP released EETs from RBCs without producing haemolysis, in contrast to the effects of A23187. Cu(NO3)2 and freeze–thawing produced haemolysis but had minimum or absent effect on EET release (Table 3).
CFTR activity in RBCs was linked to ATP release induced by deformation of erythrocytes (Sprague et al., 1998) as well as to ATP release produced by hypoxia (Bergfeld and Forrester, 1992). CFTR is a member of the ATP-binding cassette (ABC) family of proteins that ‘drive the transport of various molecules across all cell membranes' (Dean et al., 2001). Glibenclamide, a sulphonylurea inhibiting potassium and chloride channels, blocks activation of the CFTR channel (Sheppard and Welsh, 1993; Schwiebert et al., 1995) and ATP release in response to shear stress in blood vessels (Hassessian et al., 1993). Niflumic acid, a cyclooxygenase and chloride channel inhibitor, also inhibits CFTR activity (Sheppard and Welsh, 1993; Cuthbert et al., 1994); both glibenclamide and niflumic acid reduced basal as well as ATP- and BzATP-stimulated release of EETs from erythrocytes (Figure 6).
The ATP secretory mechanism in erythrocytes has been described as enigmatic. It has taken on added complexity with the finding that the gap junction protein, pannexin-1, also functions as an ATP release channel in erythrocytes (Locovei et al., 2006). Pannexin-1, in addition to forming gap junctions, can form membrane channels that are mechanosensitive and exhibit high ATP permeability. Inhibition of EET release by the gap junction inhibitor, carbenoxolone (Figure 6), came as no surprise since pannexin-1 is a hemichannel associated with the P2X7 receptor (Pelegrin and Surprenant, 2006). The common requirement of both CFTR and pannexin-1 activities for ATP and EET release from RBCs indicates an intrinsic association between the two transporters in erythrocytes that involves a regulatory ensemble including ABC transporters, a pannexin hemichannel and ATP-activated P2 receptors coupled to G proteins (P2Y receptors) and ATP-gated cation channels (P2X receptors).
There are several aspects of the contribution of erythrocytes to regulation of vascular function that bear emphasizing. First, its magnitude, given the RBC as the predominant formed element of the blood with ATP concentrations several 1000-fold higher than those in plasma (Gonzalez-Alonso et al., 2002). Second, the abundance of EETs in the phospholipids of RBCs (Jiang et al., 2005) that are subject to rapid release in response to stimulation of RBC P2X7 receptors with activation of PLA2. Cytosolic Ca2+-dependent PLA2 has been identified in human (Macdonald et al., 2004) and bovine RBCs (Shin et al., 2002). Third, ATP exhibits a duality of effects on EET release, inhibiting release by stimulating P2Y receptors and activating release by stimulating P2X7 receptors. The result is a multiphasic effect on EET release from RBCs in response to ascending concentration of ATP (Figure 2), whereas BzATP produced a curvilinear dose-dependent response on EET release (Figure 4).
In conclusion, the high levels of ATP in erythrocytes (Miseta et al., 1993) and the increasing recognition of the important role of ATP in regulating local blood flow (Dietrich et al., 2000) by stimulating endothelial purinoceptors (Stepp et al., 1996; Headrick et al., 2003), occasioned our studying the EET releasing mechanisms in RBCs. RBCs are reservoirs of EETs that are released by ATP activation of the P2X7 receptor. EETs participate in regulating vasomotion in the coronary (Gauthier et al., 2005; Larsen et al., 2006), cerebral (Medhora et al., 2001) and renal (Imig et al., 2001; Pomposiello et al., 2003) vasculatures. As ATP release from RBCs is increased in response to deformation (Sprague et al., 1998), exercise (Farias III et al., 2005) and hypoxia (Dietrich et al., 2000), the association of EET release with that of ATP points to a role of erythrocyte-derived EETs in regulating local blood flow.
Acknowledgments
Research described in this article was supported by the Philip Morris USA Inc. and Philip Morris International (HJ) and by NIH PPG 34300 (JCM) and HL-25394 (JCM).
Abbreviations
- ABC
ATP-binding cassette
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- BBG
brilliant blue G
- BEL
bromoenol lactone
- BzATP
2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- EET
epoxyeicosatrienoic acid
- ESI LC-MS
electrospray ionization liquid chromatography-mass spectrometry
- GC-MS
gas chromatography-mass spectrometry
- HEPES
N-2-hydroxyethylpiprazine-N′-2-ethanesulphonic acid
- HETE
hydroxyeicosatetraenoic acid
- MAFP
methyl arachidonyl flurophosphonate
- 2MeSATP
2-methylthio-ATP
- MSPPOH
methylsulphonyl propargyloxyphenyl hexanamide
- ODYA
17-octadecenoic acid
- PLA2
phospholipase A2
- PSS
physiological salt solution
- RBC
red blood cell
Conflict of interest
The authors state no conflict of interest.
References
- Baraldi PG, Di Virgilio F, Romagnoli R. Agonists and antagonists acting at P2X7 receptor. Curr Top Med Chem. 2004;4:1707–1717. doi: 10.2174/1568026043387223. [DOI] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Bourke J, Abel K, Huxham G, Cooper V, Manley S. UTP-preferring P2 receptor mediates inhibition of sodium transport in porcine thyroid epithelial cells. Br J Pharmacol. 1999;127:1787–1792. doi: 10.1038/sj.bjp.0702733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash AR, Boeglin WE, Capdevila JH, Yeola S, Blair IA. 7-HETE, 10-HETE, and 13-HETE are major products of NADPH-dependent arachidonic acid metabolism in rat liver microsomes: analysis of their stereochemistry, and the stereochemistry of their acid-catalyzed rearrangement. Arch Biochem Biophys. 1995;321:485–492. doi: 10.1006/abbi.1995.1421. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Doumad AB, Li J, Cheng MK, Falck J, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br J Pharmacol. 1996;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW, Evans MJ, Colledge WH, MacVinish LJ, Ratcliff R. Kinin-stimulated chloride secretion in mouse colon requires the participation of CFTR chloride channels. Braz J Med Biol Res. 1994;27:1905–1910. [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Farias M, III, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Berne's adenosine hypothesis of coronary blood flow control. Am J Physiol Heart Circ Physiol. 2004;287:H1891–H1894. doi: 10.1152/classicessays.00003.2004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FA, Ennis MD, Baze ME. Novel effects of epoxyeicosatrienoic acid isomers: inhibition of platelet aggregation and cyclooxygenase activity. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:109–114. [PubMed] [Google Scholar]
- Garcia-Marcos M, Pochet S, Marino A, Dehaye JP. P2X7 and phospholipid signalling: the search of the ‘missing link' in epithelial cells. Cell Signal. 2006;18:2098–2104. doi: 10.1016/j.cellsig.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Cornish JE, Wiley JS. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br J Pharmacol. 1997;122:911–917. doi: 10.1038/sj.bjp.0701447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest. 1959;11:66–70. doi: 10.3109/00365515909060410. [DOI] [PubMed] [Google Scholar]
- Hassessian H, Bodin P, Burnstock G. Blockade by glibenclamide of the flow-evoked endothelial release of ATP that contributes to vasodilatation in the pulmonary vascular bed of the rat. Br J Pharmacol. 1993;109:466–472. doi: 10.1111/j.1476-5381.1993.tb13592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headrick JP, Hack B, Ashton KJ. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2003;285:H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, et al. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem. 2004;279:36412–36418. doi: 10.1074/jbc.M403962200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Quilley J, Reddy LM, Falck JR, Wong PY, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2005;75:65–78. doi: 10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Wang S, Burnstock G.P2 purinoceptor-activated inward currents in follicular oocytes of Xenopus laevis J Physiol 199649417–28.Part 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Levine L. Arachidonic acid metabolism by erythrocytes. J Biol Chem. 1983;258:9116–9121. [PubMed] [Google Scholar]
- Kochukov MY, Ritchie AK. A P2X7 receptor stimulates plasma membrane trafficking in the FRTL rat thyrocyte cell line. Am J Physiol Cell Physiol. 2004;287:C992–C1002. doi: 10.1152/ajpcell.00538.2003. [DOI] [PubMed] [Google Scholar]
- Kotsias BA, Peracchia C. Functional interaction between CFTR and Cx45 gap junction channels expressed in oocytes. J Membr Biol. 2005;203:143–150. doi: 10.1007/s00232-005-0739-6. [DOI] [PubMed] [Google Scholar]
- Kumari R, Goh G, Ng LL, Boarder MR. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest. 2006;36:293–300. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald DJ, Boyle RM, Glen AC, Horrobin DF. Cytosolic phospholipase A2 type IVA is present in human red cells. Blood. 2004;103:3562–3564. doi: 10.1182/blood-2002-09-2698. [DOI] [PubMed] [Google Scholar]
- Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–1267. doi: 10.1111/j.1525-1594.1997.tb00486.x. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med. 2001;11:38–42. doi: 10.1016/s1050-1738(01)00082-2. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. doi: 10.1016/j.pharmthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Mieyal JJ, Starke DW. Hydroxylation and dealkylation reactions catalyzed by hemoglobin. Methods Enzymol. 1994;231:573–598. doi: 10.1016/0076-6879(94)31040-8. [DOI] [PubMed] [Google Scholar]
- Miseta A, Bogner P, Berenyi E, Kellermayer M, Galambos C, Wheatley DN, et al. Relationship between cellular ATP, potassium, sodium and magnesium concentrations in mammalian and avian erythrocytes. Biochim Biophys Acta. 1993;1175:133–139. doi: 10.1016/0167-4889(93)90015-h. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, et al. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- Parker JC, Snow RL. Influence of external ATP on permeability and metabolism of dog red blood cells. Am J Physiol. 1972;223:888–893. doi: 10.1152/ajplegacy.1972.223.4.888. [DOI] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Pascual JM, McKenzie A, Yankaskas JR, Falck JR, Zeldin DC. Epoxygenase metabolites of arachidonic acid affect electrophysiologic properties of rat tracheal epithelial cells1. J Pharmacol Exp Ther. 1998;286:772–779. [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension. 2003;42:548–554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol. 1997;54:1059–1070. doi: 10.1016/s0006-2952(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, et al. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Salgo MG, Corongiu FP, Sevanian A. Enhanced interfacial catalysis and hydrolytic specificity of phospholipase A2 toward peroxidized phosphatidylcholine vesicles. Arch Biochem Biophys. 1993;304:123–132. doi: 10.1006/abbi.1993.1330. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, et al. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Inhibition of the cystic fibrosis transmembrane conductance regulator by ATP-sensitive K+ channel regulators. Ann N Y Acad Sci. 1993;707:275–284. doi: 10.1111/j.1749-6632.1993.tb38058.x. [DOI] [PubMed] [Google Scholar]
- Shin HS, Chin MR, Kim JS, Chung JH, Ryu CK, Jung SY, et al. Purification and characterization of a cytosolic, 42-kDa and Ca2+-dependent phospholipase A2 from bovine red blood cells: its involvement in Ca2+-dependent release of arachidonic acid from mammalian red blood cells. J Biol Chem. 2002;277:21086–21094. doi: 10.1074/jbc.M200203200. [DOI] [PubMed] [Google Scholar]
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem. 2004;279:44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Van Bibber R, Kroll K, Feigl EO. Quantitative relation between interstitial adenosine concentration and coronary blood flow. Circ Res. 1996;79:601–610. doi: 10.1161/01.res.79.3.601. [DOI] [PubMed] [Google Scholar]
- Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Young MT, Pelegrin P, Surprenant A. Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol. 2007;71:92–100. doi: 10.1124/mol.106.030163. [DOI] [PubMed] [Google Scholar]