Abstract
Background and purpose:
We investigated expression of cannabinoid receptors and the effects of the endogenous cannabinoid virodhamine and the synthetic agonist CP55,940 on cAMP accumulation and interleukin-8 (IL-8) release in human bronchial epithelial cells.
Experimental approach:
Human bronchial epithelial (16HBE14o-) cells were used. Total mRNA was isolated and cannabinoid receptor mRNAs were detected by RT-PCR. Expression of CB1 and CB2 receptor proteins was detected with Western blotting using receptor-specific antibodies. cAMP accumulation was measured by competitive radioligand binding assay. IL-8 release was measured by ELISA.
Key results:
CB1 and CB2 receptor mRNAs and proteins were found. Both agonists concentration-dependently decreased forskolin-induced cAMP accumulation. This effect was inhibited by the CB2 receptor antagonist SR144528, and was sensitive to Pertussis toxin (PTX), suggesting the involvement of CB2 receptors and Gi/o-proteins. Cell pretreatment with PTX unmasked a stimulatory component, which was blocked by the CB1 receptor antagonist SR141716A. CB2 receptor-mediated inhibition of cAMP production by virodhamine and CP55,940 was paralleled by inhibition of tumor necrosis factor-α (TNF-α) induced IL-8 release. This inhibition was insensitive to SR141716A. In the absence of agonist, SR144528 by itself reduced TNF-α induced IL-8 release.
Conclusions and implications:
Our results show for the first time that 16HBE14o− cells respond to virodhamine and CP55,940. CB1 and CB2 receptor subtypes mediated activation and inhibition of adenylyl cyclase, respectively. Stimulation of the dominant CB2 receptor signalling pathway diminished cAMP accumulation and TNF-α-induced IL-8 release. These observations may imply that cannabinoids exert anti-inflammatory properties in airways by modulating cytokine release.
Keywords: cannabinoids; virodhamine; CP55,940; human bronchial epithelium; interleukin-8; cAMP; tumour necrosis factor-α
Introduction
Up till now, two cannabinoid receptors have been identified, responding to the active principle of marijuana, 9Δ-tetrahydrocannabinol (9ΔTHC). The CB1 receptor and CB2 receptor both belong to the 7-transmembrane G-protein-coupled receptor superfamily (Howlett et al., 2002). The CB1 receptor has primarily been observed in central and peripheral nervous structures, while the CB2 receptor is found predominantly in immune cells (Galiegue et al., 1995; Berdyshev, 2000).
A major signalling pathway used by CB receptors is modulation of adenylyl cyclase activity (Pertwee, 1999; Howlett et al., 2002). Activation of CB1 and CB2 receptors was found to decrease forskolin-induced cyclic adenosine monophosphate (cAMP) accumulation (Felder et al., 1995). Both CB receptors are Pertussis toxin (PTX)-sensitive and coupled to Gi/o-proteins (Howlett et al., 2002). However, in some systems, cannabinoids have been found to enhance cAMP production through linkage to Gs-proteins (Glass and Felder, 1997; Bonhaus et al., 1998).
Virodhamine is a recently identified endogenous cannabinoid, the ester of arachidonic acid and ethanolamine, and closely related to the first recognized endogenous cannabinoid, anandamide, which is the amide of arachidonic acid and ethanolamine. In skin, spleen, kidney and heart tissues, which express the CB2 receptor, two- to ninefold higher concentrations of virodhamine compared to anandamide have been found (Porter et al., 2002). Virodhamine acts as a partial agonist on the CB1 receptor and as a full agonist on the CB2 receptor (Porter et al., 2002).
Cannabinoids are able to modulate the expression and secretion of cytokines and exert anti-inflammatory properties (Berdyshev, 2000; Klein, 2005). In a murine model of allergic asthma cannabinol and 9ΔTHC significantly attenuated the ovalbumin-induced gene expression of interleukin-2 (IL-2), IL-4, IL-5 and IL-13. In addition, elevation of ovalbumin-specific serum immunoglobulin (Ig) E and mucus production was also markedly attenuated by pretreatment with these agonists (Jan and Kaminski, 2001; Jan et al., 2003). Immunohistological data from marijuana smokers suggest a dysregulation of the bronchial epithelium, showing hyperplasia of surface epithelial (goblet) cells and of non-ciliated reserve (basal) cells (Tashkin et al., 2002). The pro-inflammatory cytokine IL-8 is an important chemoattractant and activator of neutrophils and has been implicated in the pathophysiology of chronic obstructive pulmonary disease and severe asthma. IL-8 is produced by various cells of the immune system as well as by bronchial epithelial cells (Barnes et al., 1998). Recently, release of IL-8 induced by tumour necrosis factor-α (TNF-α) was shown to be inhibited by the non-selective cannabinoid agonist WIN55212-2 in human colonic epithelial cells (Ihenetu et al., 2003; Mormina et al., 2006).
In this study, we provide the first evidence that human bronchial epithelial (16HBE14o−) cells respond to the endogenous cannabinoid virodhamine and the synthetic non-selective receptor agonist CP55,940. Both CB1 and CB2 receptor subtypes were present in these cells and coupled to stimulatory and inhibitory adenylyl cyclase pathways, respectively. Importantly, we report here that CB receptor signalling triggered by virodhamine and CP55,940 diminished IL-8 release induced by TNF-α, from these cells.
Methods
Cell culture
The immortal human bronchial epithelial cell line 16HBE14o− was kindly donated by Dr DC Gruenert, University of Vermont, Burlington, VT, USA (Cozens et al., 1994). Cells were grown at 37°C in minimum essential medium (MEM), supplemented with 10% foetal bovine serum, penicillin (50 μg ml−1), streptomycin (50 μg ml−1) and L-glutamine (2 mM) in fibronectin/collagen-coated flasks to near confluency and in an atmosphere of 5% CO2/95% O2. All culture media and supplements were obtained from Life Technologies (Breda, The Netherlands). Cell culture dishes were from Costar (Badhoevedorp, The Netherlands) and flasks from Greiner (Alphen a/d Rijn, The Netherlands).
RNA isolation and reverse transcription-PCR
RNA extraction was carried out using the RNeasy minikit (Qiagen, Venlo, The Netherlands) according to the manufacturer's instructions. RNA (1 μg) was used for cDNA synthesis. cDNA synthesis and PCR reactions were performed using the RT system kit, RQ1 RNase-free DNase and the PCR Core system kit from Promega (Leiden, The Netherlands), according the manufacturer's instruction, using a Mastercycler Gradient (Eppendorf, Hamburg, Germany).
The following primer pairs specific for the CB receptors were obtained from Invitrogen (Leek, The Netherlands): CB1 (forward): 5′-TGG AGA ACC TAC TGG TGC TGT G-3′; CB1 (reverse): 5′-GTG GAT GAT GCT CTT CTG G-3′; CB2 (forward): 5′-CTC CGC CGG AGG CCC TCA TA-3′; CB2 (reverse): 5′-AGG CAC AGC ATG GAG CAG AAA GCA-3′. With these primers, the amplified gene fragments were 566 and 677 bp, respectively.
PCR reactions were performed as follows: a denaturing step at 94°C for 5 min was followed by 30 cycles of 94°C, 1 min; 58°C, 1 min; 72°C, 1 min. These cycles were concluded by 10 min at 72°C. The amplified product (2 μl) was loaded on a 2% agarose gel and the DNA visualized by ethidium bromide. A 100 bp ladder (MBI Fermentas, St Leon-Rot, Germany) was used as DNA marker. Restriction analysis was performed with BstEII (Sigma, Zwijndrecht, The Netherlands) for 2 h at 60°C according to the manufacturer's protocol.
Western blotting
Cells were lysed using homogenization buffer (Tris-HCl 50 mM, NaCl 150 mM, ethylenediaminetetraacetic acid (EDTA) 1 mM, phenylmethanesulphonyl fluoride 1 mM, Na3VO4 1 mM, NaF 1 mM, leupeptin 10 μg ml−1, aprotinin 10 μg ml−1, pepstatin 10 μg ml−1, Na-deoxycholate 0.25% and Igepal 1%; pH 7.4). Cell lysate (corresponding to 20 μg protein per lane) was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, using 10% gel at 100 V. Proteins in the gel were transferred onto nitrocellulose membranes, which were subsequently blocked with blocking buffer (Tris-HCl 50 mM, NaCl 150 mM, Tween-20 0.1%, dried milk powder 5%) for 90 min. Membranes were incubated overnight at 4°C using either polyclonal rabbit anti-CB1 receptor (1:1000) (Twitchell et al., 1997) or polyclonal rabbit anti-CB2 receptor R (1:500) (Walter et al., 2003), respectively. Antibodies were kindly provided by Dr K Mackie, University of Washington, Seattle, WA 98195, USA. After three washes of 10 min each, membranes were incubated with horseradish peroxidase-labelled secondary antibodies (1:3000 in blocking buffer) for 90 min at room temperature, followed by another three washes. Antibodies were visualized by enhanced chemiluminescence.
cAMP measurement
Cells were preincubated in MEM for 20 h in the absence or presence of 100 ng ml−1 PTX. Subsequently, cells were trypsinized and suspended at 106 cells ml−1 in cell stimulation buffer solution (118 mM NaCl, 4.7 mM KCl, 3 mM CaCl2, 1.2 mM MgSO4, 0.5 mM EDTA, 10 mM glucose, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid, 1.2 mM KH2PO4; pH 7.4), including 100 μM 3-isobutyl-1-methyl-2,6(1H,3H)-purinedione (IBMX) to prevent cAMP degradation, and plated in 24-well plates (2 × 105 cells well−1). The experiment was started by adding forskolin (0.1 μM, 10 min), followed by agonist (10 min). Antagonists (1 μM) were added 10 min before the agonist. After stopping the reaction with perchloric acid (3.5%), the amount of cAMP produced was measured as described previously, using a competitive [3H]cAMP radioligand binding assay (Oostendorp et al., 2005).
IL-8 measurement
TNF-α-induced IL-8 release was determined by enzyme-linked immunosorbent assay (Ihenetu et al., 2003). Confluent 16HBE14o− cells grown in 24-well plates were activated with 100 ng ml−1 TNF-α for 24 h in the presence or absence of appropriate concentrations of cannabinoid agonist and/or antagonists. At the end of the incubation time, supernatants were collected and stored at −20°C until the assay was performed, using a Biocarta kit (Bio-Connect, Huissen, The Netherlands), according to the manufacturer's instructions. Immunoplates (96-well) were coated overnight with 5 μg ml−1 antihuman IL-8 monoclonal capture antibody. Plates were subsequently washed three times with washing buffer (phosphate-buffered saline plus 0.05% Tween 20) and blocked with assay buffer (phosphate-buffered saline plus 0.05% Tween 20, 0.5% bovine serum albumin and 0.1% Triton X-100) for 2 h at room temperature. After three washes, standards and samples (supernatants diluted 1:10 in assay buffer) were added and incubated at room temperature for 2 h. Subsequently, plates were washed five times with washing buffer. Detection was performed in a single step by incubating the plates in the presence of a biotinylated antihuman IL-8 antibody and streptavidin-linked peroxidase conjugate (each at 0.5 μg ml−1, respectively). After incubation for 1 h plates were washed seven times and tetramethylbenzidine (125 μg ml−1) was used as substrate to quantify the amount of bound conjugate by colorimetric measurement (450 nm, Biorad 680 plate reader).
Data analysis
Values are expressed as mean values±s.e.m. Comparison of treatments was made using the paired or unpaired Student's t-test, when appropriate. All curves were fitted using a logistic fit four-parameter model (Sigma Plot version 10, SYSTAT Software Inc., Chicago IL, USA).
Chemicals
All compounds and chemicals, including 5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol (CP55,940), were obtained from Sigma (Zwijndrecht, The Netherlands), unless stated otherwise. The stock solution of IBMX was prepared in dimethyl sulphoxide and stored at −20°C. Virodhamine [O-(2-aminoethyl)-5Z,8Z,11Z,14Z-eicosatetraenoate] was obtained from Tocris-Cookson (Avonmouth, UK). SR 141716 [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide] and SR 144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were kind gifts from Sanofi (Montpellier, France). Stock solutions of virodhamine were prepared in ethanol, shielded from light and stored at −20°C, SR141716 and SR144528 were dissolved in dimethyl sulphoxide and stored at room temperature. [3H]cAMP (30 Ci mmol−1) was obtained from Du Pont-NEN (Wilmington, DE, USA).
Results
In 16HBE14o− cells, the mRNA of both CB1 and CB2 receptors was present, as revealed by reverse transcription-PCR (Figure 1a). Following treatment with the restriction enzyme BstEII, the 566 bp fragment of CB1 receptor cDNA was split into the expected 449 and 117 bp fragments (Figure 1a, left panel) and the 677 bp fragment of CB2 receptor cDNA into the expected 413 and 246 bp fragments (Figure 1a, right panel). Using receptor-specific antibodies, endogenous protein expression of CB1 (Figure 1b, left panel) and CB2 receptors (Figure 1b, right panel) in 16HBE14o− cells was detected as well.
Figure 1.
mRNA and protein expression of CB1 and CB2 receptors in 16HBE14o− cells. (a) Total RNA was reverse-transcribed into cDNA and specific fragments amplified by PCR, separated on 2% agarose gel and visualized with ethidium bromide. Restriction analysis was performed with BstEII. (b) Western blot analysis of CB1 and CB2 receptor expression (20 μg protein per lane) using receptor-specific antibodies.
Modulation of cAMP accumulation after stimulation of CB1 and CB2 receptors by virodhamine or the synthetic CB receptor agonist CP55,940 was studied following activation of adenylyl cyclase by forskolin (0.1 μM); basal cAMP levels were enhanced 3.5-fold by this treatment (Figure 2c). Virodhamine and CP55,940 did not affect basal cAMP accumulation (not shown). In contrast, both virodhamine and CP55,940 inhibited forskolin-induced cAMP accumulation in a concentration-dependent manner (Figures 2a and b; Table 1). This inhibition was prevented by preincubation with the CB2 receptor antagonist SR144528 (1 μM), but remained largely unaffected by the CB1 receptor antagonist SR141716A (1 μM) (Figures 3a and b, Table 1). Both antagonists did not affect basal or forskolin-induced cAMP accumulation in the absence of CB receptor agonist (Figure 2c).
Figure 2.
Concentration–effect curves of virodhamine (a) and CP55,940 (b) on forskolin-induced cAMP accumulation. Data are presented as mean values±s.e.m. from six experiments. *P<0.05 vs control. (c) The lack of effect of the CB receptor antagonists SR141716A (1 μM) and SR144528 (1 μM) on basal cAMP accumulation and that induced by forskolin (0.1 μM). Values of pIC50/pEC50 and ΔEmax are summarized in Table 1.
Table 1.
Modulation by virodhamine and CP55,940 of forskolin-induced cAMP accumulation in 16HBE14o− cells
|
Virodhamine |
CP55,940 |
|||
|---|---|---|---|---|
| Treatment | pIC50 | ΔEmax | pIC50 | ΔEmax |
| Control | 7.24±0.45 | −26.9±3.1 | 6.20±0.25 | −14.8±7.4 |
| +SR141716A | 7.74±0.50 | −25.2±1.2 | 6.96±0.46 | −9.7±1.8 |
| +SR144528 | NE | 0 | NE | 0 |
| |
pEC50 |
ΔEmax |
pEC50 |
ΔEmax |
| PTX | 5.72±0.30 | 23.5±2.2 | 5.51±0.20 | 15.1±1.7 |
| +SR144528 | 6.67±0.19* | 18.4±10.3 | — | — |
| +SR141716A | NE | 0 | — | — |
Abbreviations: cAMP, cyclic adenosine monophosphate; NE, no effect; PTX, Pertussis toxin.
Summarized values for half-maximal concentration (pIC50 or pEC50 (−log M)) and change of maximal effect (ΔEmax (% forskolin)) as obtained from data presented in figures 2, 3 and 4, and representing means±s.e.m.
P<0.05 compared to PTX-treatment.
Figure 3.
Effect of CB receptor antagonists on virodhamine and CP55,940-induced inhibition of forskolin induced cAMP accumulation. (a) Virodhamine-induced inhibition in the presence of SR141716A (1 μM) or SR144528 (1 μM). (b) CP55,940-induced inhibition in the presence of SR141716A (1 μM) or SR144528 (1 μM). Virodhamine- and CP55,940-induced inhibition in the absence of SR141716A or SR144528, see Figure 2. Data are presented as mean values±s.e.m. from six experiments. *P<0.05 vs control. Values for pIC50/pEC50 and ΔEmax are summarized in Table 1.
To investigate whether CB2 receptors-mediated inhibition of cAMP formation involves Gi/o-proteins, the cells were preincubated with PTX (100 ng ml−1, 20 h). Following this treatment, stimulation of cAMP accumulation was unmasked, both with virodhamine and CP55,940 (Figures 4a and c). This increase was abolished by the CB1 receptor selective antagonist SR141716A (1 μM) (Figure 4b, Table 1); interestingly, both in the absence and presence of virodhamine, SR141617A diminished cAMP accumulation by about 20% (Figure 4b). In contrast, the CB2 receptor antagonist SR144528 (1 μM) did not significantly alter the maximal virodhamine-induced cAMP increase in the PTX-pretreated cells, but did induce some leftward shift of the concentration–response curve (Figure 4d; Table 1).
Figure 4.
Effect of PTX pretreatment on virodhamine and CP55,940-induced modulation of forskolin induced cAMP accumulation. Cells were pretreated with PTX (100 ng ml−1; 20 h). Effect on concentration–response curve of virodhamine alone (a), in the presence of SR141716A (1 μM; b) or in the presence of SR144528 (1 μM; d). Effect on concentration–response curve of CP55,940 alone (c). Data are presented as mean values±s.e.m. from six (a–c) or three experiments (d). *P<0.05 vs control. Values of pIC50/pEC50 and ΔEmax are summarized in Table 1.
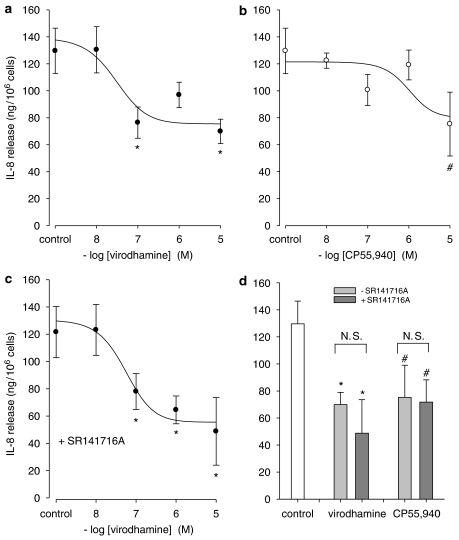
Modulation of cytokine release was studied to investigate whether the observed CB2 receptor-mediated inhibition of cAMP formation might alter physiological responses of human bronchial epithelial cells. Virodhamine significantly inhibited the TNF-α-induced IL-8 release, while a strong tendency towards inhibition was observed for CP55,940 (Figures 5a and b; Table 2). Indirect evidence that this response is mediated via CB2 receptors is presented in Figure 5c and Table 2, showing that virodhamine inhibited, concentration dependently, the IL-8 release induced by TNF-α with the same potency in the presence of 1 μM of the CB1 receptor-selective antagonist, SR141716A. The response induced by CP55,940 (10 μM) was not affected by SR141716A either (Figure 5d). Interestingly, pretreatment with SR144528 (1 μM) alone greatly diminished TNF-α-induced IL-8 release, a phenomenon not observed with SR141716A (Figure 6). Basal IL-8 release remained unchanged in the presence of both antagonists.
Figure 5.
Effect of CB receptor stimulation on IL-8 release induced by TNF-α. Accumulation of IL-8 was measured 24 h after stimulation with 100 ng ml−1 TNF-α in the presence of virodhamine (a) or CP55,940 (b). Effect of virodhamine in the presence of SR141716A (1 μM, c). Lack of effect of SR141716A (1 μM) on inhibition induced by virodhamine (10 μM) or CP55,940 (10 μM) (d). Data are presented as mean values±s.e.m. from three to five experiments. #P<0.10 and *P<0.05 vs control, respectively. NS, not significantly different. Values for pIC50 and ΔEmax are summarized in Table 2.
Table 2.
Inhibition by virodhamine and CP55,940 of TNF-α-induced IL-8 release in 16HBE14o− cells
|
Virodhamine |
CP55,940 |
|||
|---|---|---|---|---|
| Treatment | pIC50 | ΔEmax | pIC50 | ΔEmax |
| Control | 7.53±0.88 | −60±9 | 6.00±1.90 | −54±23 |
| +SR141716A | 7.26±0.19 | −73±14 | — | — |
Abbreviations: IL, interleukin; TNF-α, tumour necrosis factor-α.
Summarized values for half-maximal effects (pIC50 (−log M)) and maximal change of effect (ΔEmax; ng per 106 cells) were obtained from data as presented in figure 5 and represent means±s.e.m.
Figure 6.
Effect of selective CB receptor antagonists on IL-8 release. Basal and 100 ng ml−1 TNF-α-induced accumulation of IL-8 was measured after 24 h in the absence or presence of SR141716A (1 μM) or SR144528 (1 μM). Data are presented as mean values±s.e.m. from 13 experiments. *P<0.05 vs control.
Discussion
Here, we report for the first time that the recently identified endocannabinoid virodhamine, which is present in peripheral tissues in relatively large quantities (Porter et al., 2002), interacts with cannabinoid receptors in human airway epithelium (16HBE14o−) cells. In these cells CB1 and CB2 receptors were found to be expressed, both at the level of mRNA and as proteins. As expected, both receptor types are coupled to adenylyl cyclase. Virodhamine leads to a concentration-dependent inhibition of forskolin-induced cAMP accumulation. This finding was confirmed with the potent synthetic and highly efficacious non-selective CB receptor agonist CP55,940 (Burkey et al., 1997; Thomas et al., 2007), although, unexpectedly, the action of CP55,940 was less pronounced compared to virodhamine. However, by studying Gi-specific guanine-5′-triphosphate binding in insect Sf9 cell membranes expressing mammalian CB2 receptor, virodhamine has been shown to be a full agonist at this receptor subtype (Porter et al., 2002). The inhibitory responses induced by both agonists were totally prevented by SR144528, strongly suggesting that the effect was mediated by CB2 receptors. As this inhibition was PTX sensitive, Gi/o-proteins are probably involved.
Interestingly, PTX pretreatment unmasked a stimulatory component for both agonists, enhancing forskolin-induced cAMP formation. This stimulatory response remained largely unaffected by SR144528, although some leftward shift of the concentration–response curve for virodhamine was observed. In contrast, the stimulatory response could be prevented by SR141716A, indicating that this effect is mediated by CB1 receptors coupled to Gs-proteins. Remarkably, treatment with the CB1 receptor antagonist alone decreased the forskolin-induced cAMP accumulation below the control value, suggesting that some of the CB1 receptors in 16HBE14o− cells are constitutively activated. Indeed, it has been reported that SR141716A exhibits inverse agonistic properties (Pertwee, 1999). The fact that in non-PTX-pretreated cells, this inverse agonistic effect is not observed suggests a dominant role of Gi proteins, whether or not coupled in part to CB2 or other inhibitory receptors, dampening constitutive CB1 receptor activity.
Although CB1 receptors are abundant in nervous tissue, their presence on human bronchial epithelial cells is not unexpected in view of recent reports showing the expression of this subtype in peripheral tissues. We have reported on CB1 receptor-mediated capacitative and non-capacitative Ca2+ entry in DDT1 MF-2 smooth muscle cells (Filipeanu et al., 1997; Demuth et al., 2005). In peripheral blood mononuclear cells, basal CB1 receptor expression was found, which decreased following short-term phytohemagglutinin stimulation, while the expression of CB2 receptor mRNA remained unchanged (Nong et al., 2002). In RBL 2H3 mast cells both subtypes are co-expressed as well. CB1 receptor stimulation can suppress IgE-mediated secretory responses, while the CB2 receptor is the predominant receptor phosphorylating the intracellular signalling kinases, AKT and ERK (Samson et al., 2003). Interestingly, while both CB1 and CB2 ligands acutely decrease cytosolic cAMP levels in these cells, long-term stimulation (1–2 h) with a CB1 or CB2 ligand causes an elevation or a suppression of cAMP levels, respectively, the former being attributed to the modulation of cAMP-responsive genes (Small-Howard et al., 2005).
Importantly, we found in the present study that virodhamine and CP55,940, at concentrations similar to those causing CB2 receptor-mediated adenylyl cyclase inhibition (Tables 1 and 2), inhibited TNF-α induced IL-8 release from 16HBE14o− cells. This inhibition was resistant to selective CB1 receptor blockade indeed, strongly suggesting that attenuation of cAMP accumulation through stimulation of CB2 receptors was involved. This is in accordance with the observation that, in human intestinal epithelial HT-29 cells, the cannabinoid-induced inhibition of IL-8 release is mediated via CB2 receptors (Ihenetu et al., 2003; Mormina et al., 2006). Furthermore, elevation of cAMP in human bronchial epithelial cells by β-adrenoceptor agonists is known to enhance IL-8 release (Linden, 1996; Sasaki and Manabe, 2004). The selective CB2 receptor antagonist SR144528 could not be used in the present experiments, because this compound, when given alone, markedly reduced TNF-α-induced IL-8 release from 16HBE14o− cells by an unknown mechanism – possibly through TNFα-receptor antagonism, although, to our knowledge, no evidence for it has been reported yet – while the basal IL-8 release remained unaffected.
In conclusion, as depicted in the hypothetical scheme (Figure 7), our results showed that both CB1 and CB2 receptors were present in 16HBE14o− cells, and were differentially coupled to adenylyl cyclase. Virodhamine and CP55,940 predominantly stimulated CB2 receptors, leading, via activation of PTX sensitive Gi/o-proteins, to decreased cAMP levels and to inhibition of TNF-α induced IL-8 release from these cells. These findings suggest that the endocannabinoid virodhamine via stimulation of CB2 receptors may exert anti-inflammatory effects in the airways by modulation of cytokine release from the bronchial epithelium.
Figure 7.
Hypothetical scheme of virodhamine- and CP55,940-induced cannabinoid signalling in 16HBE14o− cells. Stimulation of CB receptors with the non-selective endocannabinoid virodhamine or the non-selective synthetic agonist CP55,940 predominantly inhibits cAMP formation via CB2 receptor coupling to PTX-sensitive Gi/o-proteins. Stimulation of cAMP formation is mediated by CB1 receptors and only observed after removing the Gi/o response by PTX pretreatment. The CB2 receptor-induced decrease in cAMP formation might be responsible for the observed inhibition of TNF-α-induced IL-8 release.
Acknowledgments
We thank K Mackie (Grant NIH DA11322) for providing the CB receptor-specific antibodies.
Abbreviations
- 9ΔTHC
9Δ-tetrahydrocannabinol
- IL
interleukin
- MEM
minimum essential medium
- PTX
Pertussis toxin
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- Burkey TH, Quock RM, Consroe P, Ehlert FJ, Hosohata Y, Roeske WR, et al. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur J Pharmacol. 1997;336:295–298. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Gkoumassi E, Dröge MJ, Dekkers BG, Esselink HJ, van Ree RM, et al. Arachidonic acid mediates non-capacitative calcium entry evoked by CB(1)-cannabinoid receptor activation in DDT(1) MF-2 smooth muscle cells. J Cell Physiol. 2005;205:58–67. doi: 10.1002/jcp.20390. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Filipeanu CM, de Zeeuw D, Nelemans SA. Delta9-tetrahydrocannabinol activates [Ca2+]i increases partly sensitive to capacitative store refilling. Eur J Pharmacol. 1997;336:R1–R3. doi: 10.1016/s0014-2999(97)01254-5. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ihenetu K, Molleman A, Parsons ME, Whelan CJ. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003;458:207–215. doi: 10.1016/s0014-2999(02)02698-5. [DOI] [PubMed] [Google Scholar]
- Jan TR, Farraj AK, Harkema JR, Kaminski NE. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol Appl Pharmacol. 2003;188:24–35. doi: 10.1016/s0041-008x(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Jan TR, Kaminski NE. Role of mitogen-activated protein kinases in the differential regulation of interleukin-2 by cannabinol. J Leukoc Biol. 2001;69:841–849. [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Linden A. Increased interleukin-8 release by beta-adrenoceptor activation in human transformed bronchial epithelial cells. Br J Pharmacol. 1996;119:402–406. doi: 10.1111/j.1476-5381.1996.tb16000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormina ME, Thakur S, Molleman A, Whelan CJ, Baydoun AR. Cannabinoid signalling in TNF-alpha induced IL-8 release. Eur J Pharmacol. 2006;540:183–190. doi: 10.1016/j.ejphar.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol. 2002;127:169–176. doi: 10.1016/s0165-5728(02)00113-3. [DOI] [PubMed] [Google Scholar]
- Oostendorp J, Postma DS, Volders H, Jongepier H, Kauffman HF, Boezen HM, et al. Differential desensitization of homozygous haplotypes of the {beta}2-adrenergic receptor in lymphocytes. Am J Respir Crit Care Med. 2005;172:322–328. doi: 10.1164/rccm.200409-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Samson MT, Small-Howard A, Shimoda LM, Koblan-Huberson M, Stokes AJ, Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol. 2003;170:4953–4962. doi: 10.4049/jimmunol.170.10.4953. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Manabe H. KF19514, a phosphodiesterase 4 and 1 inhibitor, inhibits TNF-alpha-induced GM-CSF production by a human bronchial epithelial cell line via inhibition of PDE4. Inflamm Res. 2004;53:31–37. doi: 10.1007/s00011-003-1217-1. [DOI] [PubMed] [Google Scholar]
- Small-Howard AL, Shimoda LM, Adra CN, Turner H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem J. 2005;388:465–473. doi: 10.1042/BJ20041682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol. 2002;42:71S–81S. doi: 10.1002/j.1552-4604.2002.tb06006.x. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB(1) and CB(2) receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]