Abstract
Background and purpose:
Beta-lactam antibiotics are the first practical pharmaceuticals capable of increasing the expression and activity of the glutamate transporter, GLT-1, in the CNS. However, the functional impact of beta-lactam antibiotics on specific drugs which produce their pharmacological effects by increasing glutamatergic transmission is unknown. One such drug is morphine, which causes hyperthermia in rats, mediated by an increase in glutamatergic transmission. Since drugs (e.g. antibiotics) that enhance glutamate uptake also decrease glutamatergic transmission, we tested the hypothesis that ceftriaxone, a beta-lactam antibiotic, would block the glutamate-dependent portion of morphine-evoked hyperthermia.
Experimental approach:
A body temperature assay was used to determine if ceftriaxone decreased morphine-induced hyperthermia in rats by increasing glutamate uptake.
Key results:
Body temperatures of rats treated with ceftriaxone (200 mg kg−1, i.p. × 7 days) did not differ from rats receiving saline. Morphine (1, 4, 8 and 15 mg kg−1, s.c.) caused significant hyperthermia. Pre-treatment with ceftriaxone, as described above, decreased the hyperthermic response to these doses of morphine. The effects of ceftriaxone were prevented by TBOA (0.2 μmol, i.c.v.), an inhibitor of glutamate transport.
Conclusions and implications:
Ceftriaxone attenuated the hyperthermia caused by morphine, an effect prevented by inhibiting glutamate transport. Thus this effect of ceftriaxone was most likely mediated by increased glutamate uptake. These data revealed a functional interaction between ceftriaxone and morphine and indicated that a beta-lactam antibiotic decreased the efficacy of morphine in conscious rats.
Keywords: antibiotics, morphine, glutamate, GLT-1, hyperthermia, ceftriaxone, TBOA
Introduction
Common antibiotics may do more than just kill bacteria. A recent screening of over 1000 clinically approved drugs revealed that the β-lactam antibiotics were the only agents capable of increasing both the expression and activity of the glutamate transporter subtype 1 (GLT-1) (Beghi et al., 2005; Miller and Cleveland, 2005; Rothstein et al., 2005; Secko, 2005). The GLT-1 transporter protein is expressed in rats and humans (excitatory amino-acid transporter 2, EAAT2) and is responsible for 90% of glutamate uptake in the central nervous system (CNS) (Rothstein, 1996; Danbolt, 2001). Earlier work reveals that extracellular glutamate increases in animals lacking the GLT-1 transporter (Mitani and Tanaka, 2003). Animal models demonstrate that GLT-1 dysfunction contributes to a number of clinical disorders, including amyotrophic lateral sclerosis, neurotoxicity, stroke, Parkinson's disease, adult motor neuron disease, opioid dependence and opioid withdrawal (Tanaka et al., 1997; Ye et al., 1999; Rao et al., 2001; Nakagawa and Satoh, 2004; Ozawa et al., 2001, 2004; Fujio et al., 2005).
In spite of the importance of GLT-1 in physiological and pathophysiological conditions, no practical pharmaceuticals were known to modulate its expression and activity until recently. This situation changed when Rothstein et al. (2005) discovered that the β-lactam class of antibiotics caused the following effects: increased GLT-1 expression in the rat brain; increased functional and biochemical activity of GLT-1 in the rat brain; protection against ischaemic injury and motor neuron degeneration in vitro; and delayed loss of neurons and muscle strength in a mouse model of ALS. Because β-lactam antibiotics are the most widely used antibiotics in the world, cause no known side effects at antibacterial doses, and increase GLT-1 transcription, they may be useful in the clinical management of glutamate-mediated conditions (Goodman et al., 2001; Rothstein et al., 2005).
The hyperthermic effect of morphine in rats is one end point mediated by glutamate (Geller et al., 1983; Rawls et al., 2003). Low doses of morphine cause hyperthermia in rats by stimulating μ-opioid receptors, whereas higher doses of morphine produce hypothermia by activating κ-opioid receptors (Spencer et al., 1988, 1990; Adler and Geller, 1993; Chen et al., 1996, 2001). Because glutamate increases body temperature and heat production, an enhancement in glutamatergic transmission is thought to cause hyperthermia (Singh and Gupta, 1997; Yasumatsu et al., 1998). Several lines of evidence suggest that glutamate is a key factor in morphine-induced hyperthermia. For example, pharmacological antagonism of NMDA receptors blocks the hyperthermia caused by systemically administered morphine (Rawls et al., 2003). In addition, a hyperthermic dose (10 mg kg−1) of morphine causes a downregulation of NMDA receptor subunit mRNAs in the hypothalamus, the major thermoregulatory centre in the brain (Boulant, 1981; Le Greves et al., 1998). An in vivo microdialysis study showed that extracellular glutamate levels in the striatum, a structure that plays an indirect role in thermoregulation, are elevated following the systemic injection of morphine (Lin et al., 1992; Huang et al., 1997). Collectively, these results suggest that morphine-evoked hyperthermia is mediated in part by an increase in glutamatergic transmission at NMDA receptors located in regions of the brain that regulate body temperature. A number of other behavioural and neuro-adaptive effects of morphine, including neural plasticity, dependence, withdrawal and antinociception, are also mediated by an increase in glutamatergic transmission at NMDA and non-NMDA receptors (Rasmussen and Aghajanian, 1989; Akaoka and Aston-Jones, 1991; Rasmussen et al., 1991; Aghajanian et al., 1994; Herman et al., 1995; Inturrisi, 1997; Larcher et al., 1998; Trujillo, 2000; Allen and Dykstra, 2001).
In the present study, we used a body temperature assay to test the hypothesis that ceftriaxone would block morphine-evoked hyperthermia. Ceftriaxone was chosen because it is water-soluble and penetrates the brain more effectively than penicillin. To test our hypothesis, rats treated with ceftriaxone for 7 days were injected with one of four doses of morphine and body temperatures were determined. To determine if an enhancement of glutamate uptake was the mechanism of ceftriaxone, we used DL-threo-β-benzyloxyaspartic acid (TBOA), a glutamate uptake inhibitor, in a second set of experiments. To confirm that glutamatergic transmission mediates morphine-evoked hyperthermia, we tested the effects of 2-amino 6-trifluoromethoxy-benzothiazole (riluzole), a glutamate release inhibitor, and morphine on body temperature (Rawls et al., 2003).
Methods
Animals
Animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Temple University Animal Care and Use Committee. Male Sprague–Dawley rats (Zivic-Miller, Pittsburgh, PA, USA) weighing 200–250 g (age, 60–80 days) were housed two per cage for a minimum of 5 days before experimental use. Rats were maintained on a 12-h light/dark cycle and fed rat chow and water ad libitum. Individual rats were used in one experiment and then killed humanely. A total of 134 rats were used in the entire study.
Cannula implantation
Rats were anaesthetized with an intraperitoneal (i.p.) injection of ketamine hydrochloride (100–150 mg kg−1) and acepromazine maleate (0.2 mg kg−1). A polyethylene cannula was implanted stereotaxically into the right lateral ventricle (Rawls et al., 2005). Dental acrylic cement was used to secure the cannula to the cranium. The route of TBOA administration was intracerebroventricular (i.c.v.) and it was given by inserting the needle tip of a 10-μl syringe into the polyethylene cannula. TBOA (0.2 μmol) was administered in a volume of 5 μl. The dose of TBOA was determined from a previous in vivo study (Cechova and Zuo, 2006). Following i.c.v. experiments, injection sites were verified with an injection of 0.1% Evan's blue (5 μl). If the ventricles of a rat were not labelled with dye, the injections were considered as misplaced and those rats were omitted from the data analysis.
Dosing schedule
Rats were randomly divided into two groups. One group of rats received a single injection of ceftriaxone (200 mg kg−1, i.p.) daily for 7 consecutive days. The second group received injections of saline for 7 days. The ceftriaxone and saline injections were made each day between 0700 and 0800 hours. On day 8, rats from both groups were injected with morphine between 1100 and 1200 hours Ceftriaxone was not administered to rats on day 8.
Body temperature experiments
Body temperature experiments were always started between 0800 and 0900 hours on day 8 to minimize the effects of circadian variation. Rats were placed individually into an environmental room maintained at a constant temperature of 21±0.3°C and relative humidity of 52±2%. The animals were allowed to acclimatize for 60 min before taking the first temperature reading. Before drug administration, baseline temperatures were taken every 30 min for 90 min using a thermistor probe (YSI series 400, Yellow Springs Instrument Co., Yellow Springs, OH, USA; sensitivity of 0.10°C), which was lubricated and inserted approximately 7 cm into the colon. A digital thermometer (Model 49 TA, YSI) was used to record body temperature. Rats were unrestrained throughout the experiment, with only the tail being held gently between two fingers. Following the baseline interval, rats were injected with saline or morphine (1, 4, 8, or 15 mg kg−1, subcutaneously (s.c.)). Body temperatures were recorded at 30, 60, 90, 120 and 150 min following injection. Doses of ceftriaxone and morphine were based on previous studies in conscious rats (Benamar et al., 2001; Rawls et al., 2003; Rothstein et al., 2005). To determine if ceftriaxone affected morphine-evoked hyperthermia by decreasing glutamatergic transmission, a separate set of experiments tested the effects of morphine and TBOA on the body temperatures of rats pretreated for 7 days with ceftriaxone (200 mg kg−1, i.p.) or saline. Rats treated with ceftriaxone (200 mg kg−1, i.p.) or saline for 7 days were given TBOA (0.2 μmol/ rat, i.c.v.) or vehicle (5 μl, 20% dimethylsulphoxide (DMSO)/water) on day 8, followed by either morphine (4 mg kg−1, s.c.) or saline 15 min later.
As a positive control, we investigated the effect of riluzole on the hyperthermic response to a single dose of morphine (4 mg kg−1, s.c.). This experiment was performed to confirm that an increase in glutamatergic transmission is necessary for morphine to produce its full hyperthermic effect (Rawls et al., 2003). Following a 90-min baseline interval, drug-naïve rats were given riluzole (2.5 or 5 mg kg−1, s.c.) or vehicle (0.1 N hydrochloric acid). Thirty minutes later, rats were administered saline or morphine (4 mg kg−1, s.c.). Body temperatures were recorded 30, 60, 90, 120 and 150 min post-injection. The riluzole dose range was based on work showing that riluzole (2, 4 and 8 mg kg−1, s.c.) attenuated the formation of conditioned place aversion induced by naloxone in rats undergoing a single morphine exposure (Jin et al., 2006).
Data analysis
Three consecutive temperature readings were measured and averaged to establish a baseline temperature before drug injection. Data were calculated as the mean±s.e.m. of the change in body temperature. Before analysis, all data were transformed into ‘normalized ranks' to address non-normality. Transformed data were analysed using either a Student's t-test or two-way (group, time) mixed-model analysis of variance (ANOVA) with repeated measures on time followed by pairwise multiple comparisons incorporating the Bonferroni correction. Area under the body temperature time curve (AUC) values were calculated from 15 to 150 min using the difference score from 0 min (trapezoidal rule) and differences between individual groups were determined by Tukey's post hoc analysis. In all cases, values of P<0.05 were considered to be statistically significant.
Drug preparation and administration
Morphine sulphate was obtained from the National Institute on Drug Abuse (Rockville, MD, USA). Ceftriaxone hydrochloride was purchased from Apotex Corporation (Weston, FL, USA). Riluzole (2-amino 6-trifluoromethoxy-benzothiazole), a glutamate release inhibitor, and TBOA, a glutamate uptake inhibitor, were purchased from Tocris Bioscience (St Louis, MO, USA). Morphine and ceftriaxone were dissolved in pyrogen-free saline. TBOA was dissolved in a 20% dimethyl sulphoxide (DMSO)/ water solution. Riluzole was dissolved in 0.1 N hydrochloric acid. Morphine and riluzole were injected s.c. and ceftriaxone was injected i.p.. TBOA was administered centrally. Systemically injected drugs were given in a volume of 1 ml kg−1, whereas TBOA was administered in a volume of 5 μl (intracerebroventricular, i.c.v.).
Results
Ceftriaxone attenuates morphine-evoked hyperthermia
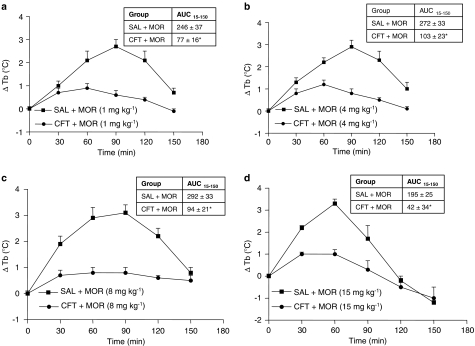
The body temperatures of rats receiving repeated administrations of ceftriaxone (200 mg kg−1, i. p.) for 7 days did not differ significantly from rats receiving saline for 7 days (P>0.05) (data not shown). Morphine (1, 4, 8 and 15 mg kg−1, s.c.) produced hyperthermia in saline pretreated rats (Figure 1). For combined administration, the hyperthermic responses to graded doses of morphine (1, 4, 8 and 15 mg kg−1, s.c.) were attenuated in rats that received a pretreatment of ceftriaxone (200 mg kg−1, i.p.) for 7 days (Figures 1a–d) (P<0.05). Morphine did not produce any other overt behavioural effects over the duration of our experiments. The acute injection of ceftriaxone (200 mg kg−1, i.p.) did not affect the hyperthermia caused by a single dose of morphine (P>0.05) (data not shown). This result indicates that repeated administration of the antibiotic is required for the attenuation of morphine-induced hyperthermia (Rothstein et al., 2005).
Figure 1.
Repeated ceftriaxone attenuated the hyperthermia caused by morphine. (a–d) Time courses, separated according to dose of morphine: ceftriaxone (CFT) (200 mg kg−1, intraperitoneally (i.p.)) or saline (SAL, i.p.) was injected for 7 consecutive days. On day 8, all rats received an injection of morphine (MOR) (1, 4, 8, or 15 mg kg−1, subcutaneous (s.c.)) or SAL. Data are expressed as the mean±s.e.m. of the change in body temperature (ΔTb) from baseline (time 0). Inserted table, AUC15−150 profile: Area under the body temperature time curve (AUC) was calculated from 15 to 150 min using the difference score from 0 min (trapezoidal rule). *P<0.05, Student's t-test.
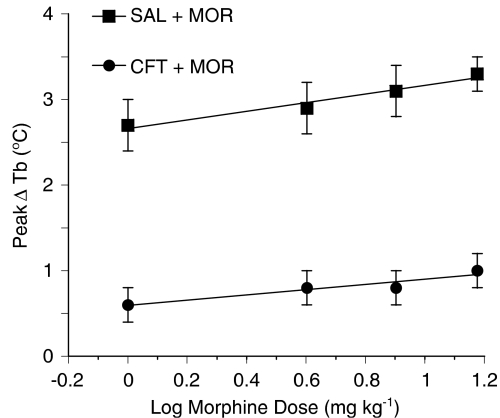
To quantitate more effectively the time course data in Figures 1a–d, we compared the dose–response relation of the active agent (morphine) and the dose–response relation of that agent (four doses) in combination with the inactive agent (ceftriaxone). A simply additive interaction would lead to the same dose–response relation, whereas a significant shift in the combination curve means that an interaction has occurred (Tallarida, 2001). The regression lines for these two dose–response data sets, using the peak elevation in body temperature (60 or 90 min following morphine administration) are shown in Figure 2. The dose–response data of both morphine (1, 4, 8 and 15 mg kg−1, s.c.) by itself and morphine (1, 4, 8 and 15 mg kg−1, s.c.) with a fixed dose (200 mg kg−1, i.p.) of ceftriaxone are linear (r=0.98 and 0.94, respectively) and do not differ significantly in slope (P>0.05). There is a pronounced downward shift in the combination's regression line, indicating antagonism of the morphine hyperthermia that resulted in a mean temperature decrease of 2.2±0.06°C (ANOVA, F=1240; P<0.001). The downward shift in the regression line of the combination means that morphine is less efficacious when given with ceftriaxone.
Figure 2.
Combination of ceftriaxone and morphine produced subadditive hypothermia. Using the data from Figure 1, regression lines for morphine (MOR) (1, 4, 8 and 15 mg kg−1, subcutaneous (s.c.)) by itself and in combination with an inactive dose of ceftriaxone (CFT) (200 mg kg−1, intraperitoneal (i.p.) were constructed. The effect was a decrease in the peak body temperature. The dose–response data of both MOR (1, 4, 8, and 15 mg kg−1, s.c.) by itself and MOR (1, 4, 8, and 15 mg kg−1, s.c.) with a fixed dose (200 mg kg−1, s.c.) of ceftriaxone are linear (r=0.98 and r=0.94, respectively) and do not differ significantly in slope (P>0.05). There is a pronounced downward shift in the regression line for the combination, indicating antagonism of the MOR hyperthermia that resulted in a mean temperature decrease of 2.2±0.06°C (analysis of variance (ANOVA), F=1240; P<0.001). The significant downward shift in the regression line of the combination indicates an interaction between the two drugs and reveals that morphine is less efficacious in the presence of ceftriaxone.
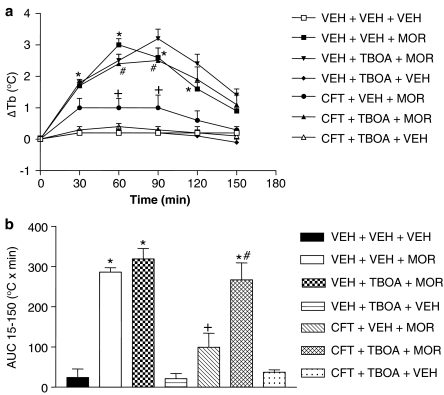
Glutamate uptake inhibitor (TBOA) prevents the inhibition of morphine-evoked hyperthermia by ceftriaxone
The effects of TBOA (0.2 μmol/ rat, i.c.v.) on the inhibition of morphine-induced hyperthermia by ceftriaxone are shown in Figure 3. Two-way ANOVA revealed a significant drug interaction (F (6,36)=46.09, P<0.0001), time interaction (F (4,124)=21.09, P<0.0001) and drug × time interaction (F (10,124)=15.34, P<0.0001). In rats pretreated with saline for 7 days, morphine (4 mg kg−1, s.c.) produced its usual hyperthermia compared to the injection of an equivalent volume of saline, with significant increases in body temperature occurring 30, 60, 90 and 120 min post-administration (P<0.05). In rats pretreated with saline for 7 days, TBOA (0.2 μmol/ rat, i.c.v.) did not produce a change in body temperature that was significantly different compared to saline (P>0.05). Consistent with results presented in Figure 1, morphine (4 mg kg−1, s.c.) produced significantly less hyperthermia in rats pretreated for 7 days with ceftriaxone (200 mg kg−1, i.p.) than in rats that received saline for 7 days (P<0.05). This inhibition of morphine-induced hyperthermia by repeated injections of ceftriaxone (200 mg kg−1, i.p.) was prevented when TBOA (0.2 μmol/ rat, i.c.v.) was administered 15 min before morphine (4 mg kg−1, s.c.) (P<0.05). A one-way ANOVA on AUC means followed by a Tukey's post hoc analysis confirmed the effects of TBOA (Figure 3b).
Figure 3.
The glutamate uptake blocker, DL-threo-β-benzyloxyaspartic acid (TBOA), prevented the inhibition of morphine-evoked hyperthermia by ceftriaxone. (a) Time course: rats were divided into two groups and given either ceftriaxone (CFT) (200 mg kg−1, i.p.) or saline for 7 consecutive days. On day 8, rats were injected with either TBOA (0.2 μmol/ rat, i.c.v.) or vehicle (VEH), followed by the administration of either morphine (MOR) (4 mg kg−1, s.c.) or VEH 30 min later. Data are expressed as the mean±s.e.m. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05, compared to VEH+VEH+VEH; +P<0.05, compared to VEH+VEH+MOR; and #P<0.05, compared to CFT+VEH+MOR using a two-way analysis of variance (ANOVA) followed by the Bonferroni correction at the different time points. (b) AUC15−150 profile: area under the body temperature time curve (AUC) was calculated from 15 to 150 min using the difference score from 0 min (F 6,36=28.60, P<0.0001). *P<0.05, compared to VEH+VEH+VEH; +P<0.05, compared to VEH+VEH+MOR; and #P<0.05, compared to CFT+VEH+MOR using a one-way ANOVA followed by Tukey's post hoc analysis.
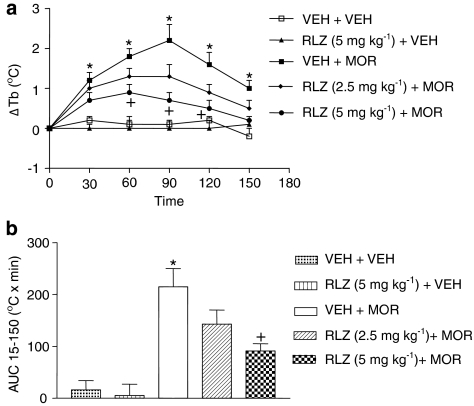
Glutamate release inhibitor attenuates morphine-evoked hyperthermia
The effects of riluzole (2.5 and 5 mg kg−1, i.p.) on the hyperthermia caused by a single dose of morphine (4 mg kg−1, s.c.) are presented in Figure 4. Two-way ANOVA revealed a significant drug interaction (F (4,26)=39.05, P<0.0001), time interaction (F (4,104)=17.03, P<0.0001) and drug × time interaction (F (8,104)=12.00, P<0.0001). Rats given a pretreatment of riluzole (5 mg kg−1, i.p.) and injected with saline displayed body temperatures that were not significantly different than riluzole-naïve rats injected with saline (P>0.05). Morphine (4 mg kg−1, s.c.) produced its usual hyperthermia compared to vehicle/ saline-treated rats, with significant increases in body temperature occurring at 30, 60, 90, 120 and 150 min following morphine administration (P<0.05). For combined administration, rats given a pretreatment of riluzole (5 mg kg−1, i.p.) and then injected with morphine (4 mg kg−1, s.c.) displayed significantly lower body temperatures than riluzole-naïve rats injected with morphine (4 mg kg−1, s.c.) 60, 90 and 120 min post-injection (P<0.05). A lower dose (2.5 mg kg−1, i.p.) did not affect morphine-evoked hyperthermia (P>0.05). A one-way ANOVA on AUC means followed by a Tukey's post hoc analysis confirmed our results with riluzole (Figure 4b).
Figure 4.
Riluzole (RLZ), an inhibitor of glutamate release, attenuated morphine-evoked hyperthermia. (a) Time course: rats were injected with riluzole (2.5 or 5 mg kg−1, i.p.) or vehicle (VEH). Thirty minutes later, morphine (MOR) (4 mg kg−1, s.c.) or VEH was injected. Data are expressed as the mean±s.e.m. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05, compared to VEH+VEH+VEH and +P<0.05, compared to VEH+VEH+MOR using a two-way ANOVA followed by the Bonferroni correction at the different time points. (b) AUC15−150 profile: area under the body temperature time curve (AUC) was calculated from 15 to 150 min using the difference score from 0 min (F5,26=8.874, P=0.0001). *P<0.05, compared to VEH+VEH+VEH and +P<0.05, compared to VEH+VEH+MOR using a one-way (ANOVA) analysis of variance followed by Tukey's post hoc analysis.
Discussion
The present study investigated the effect of ceftriaxone, a representative β-lactam antibiotic, on the hyperthermia caused by morphine. We hypothesized that repeated ceftriaxone administration would block morphine-induced hyperthermia. This is, in fact, what was found. Ceftriaxone blocked a significant proportion of the hyperthermia caused by morphine and these effects were prevented by a broad spectrum glutamate transport inhibitor (TBOA). The effects of ceftriaxone on morphine-induced hyperthermia were similar to the effects of a glutamate release inhibitor and a NMDA antagonist (Rawls et al., 2003), which both significantly inhibit the hyperthermic response to morphine. These data suggest that morphine-evoked hyperthermia is controlled by the endogenous glutamate system and can be inhibited by drugs that increase glutamate uptake (β-lactam antibiotics), decrease glutamate release (riluzole) and block glutamatergic transmission at NMDA receptors (dextromethorphan) (Rawls et al., 2003).
The attenuation of morphine-induced hyperthermia by riluzole supports our finding that NMDA receptor blockade reduces the hyperthermic response to morphine and extends the finding to include a role for glutamate release in the hyperthermia caused by morphine (Rawls et al., 2003). The major mechanism of action of riluzole is the inhibition of glutamate release from presynaptic terminals in the CNS (Malgouris et al., 1989; Martin et al., 1993; Prakriya and Mennerick, 2000). Riluzole affects a number of ion channels that regulate glutamate release, including voltage-activated calcium channels (Huang et al., 1997), voltage-dependent sodium channels (Stefani et al., 1997) and potassium channels (Duprat et al., 2000). Riluzole also increases glutamate uptake in synaptosomal preparations and blocks some of the post-synaptic effects of glutamate by blocking NMDA receptors (Doble, 1996; Frizzo et al., 2004). Regardless of the exact mechanism, the outcome is that riluzole decreases glutamatergic transmission.
The major finding of the present study is that ceftriaxone decreased a significant proportion of the hyperthermic response to morphine. The effect was observed after 7 days of repeated ceftriaxone administration, but not after a single, acute injection of ceftriaxone. These data demonstrate an interaction between morphine and ceftriaxone and reveal that ceftriaxone reduces the efficacy of morphine. One explanation is that increased glutamatergic transmission mediates a component of morphine-evoked hyperthermia that is suppressed by ceftriaxone. In this model, morphine administration to ceftriaxone-naïve rats increases glutamate release in CNS regions that regulate body temperature (Boulant, 1981). The elevation in extracellular glutamate leads to the activation of NMDA receptors and enhancement of the hyperthermic response to morphine (Rawls et al., 2003). In rats pretreated with ceftriaxone, both the expression and activity of GLT-1 transporters in the rat brain are elevated before morphine administration (Rawls et al., 2003; Beghi et al., 2005; Ji et al., 2005; Miller and Cleveland, 2005; Rothstein et al., 2005; Secko, 2005). The resultant increase in glutamate uptake accelerates glutamate clearance from the extracellular compartment, leading to a reduction in glutamatergic transmission at NMDA receptors. Thus, the usual increase in glutamatergic transmission caused by morphine does not occur in rats treated with ceftriaxone. This means that the β-lactam antibiotic blocks the component of morphine-evoked hyperthermia, which is dependent on enhanced glutamatergic transmission.
A glutamate uptake inhibitor, TBOA, prevented the inhibition of morphine-evoked hyperthermia by ceftriaxone (Shimamoto et al., 1998). TBOA is a potent, competitive, non-transportable blocker of excitatory amino acid transporters (EAATs) with high selectivity for EAATs versus ionotropic and metabotropic glutamate receptors (Shimamoto et al., 1998, 2000). Although the mechanism of ceftriaxone is not entirely clear, the lack of an interaction between ceftriaxone and morphine in the presence of TBOA supports a role for the glutamate transport system. The most probable explanation and the explanation best supported by the current literature, is that TBOA counteracted the increase in glutamate uptake caused by ceftriaxone. In this event, the repeated administration of ceftriaxone increased GLT-1 expression before TBOA administration, but the actual uptake of glutamate normally caused by ceftriaxone is prevented by the uptake block. The decline in extracellular glutamate and subsequent reduction in glutamatergic transmission expected to arise following ceftriaxone administration is prevented by TBOA (Rawls and McGinty, 1997; Xi et al., 2002). Therefore, when morphine is administered with ceftriaxone and TBOA, the component of morphine-induced hyperthermia that depends on an increase in glutamatergic transmission is apparent. This results in morphine producing its full hyperthermic response.
The effect of centrally administered TBOA suggests that ceftriaxone acted in the brain to antagonize morphine-evoked hyperthermia. One possible locus for the interaction between ceftriaxone and morphine is the hypothalamus, the major thermoregulatory centre in the brain and a primary site of opioid-induced effects on body temperature (Boulant, 1981). For example, μ-opioid agonists microdialysed into the preoptic anterior hypothalamus increase body temperature (Geller et al., 1986; Chen et al., 1996; Xin et al., 1997). Future studies will investigate a role for the hypothalamus and other brain regions in the ceftriaxone–morphine interaction.
The dose of ceftriaxone that attenuated the hyperthermic response to morphine is equivalent to a dose of 13 g day−1 for a typical adult patient. The maximal dose of ceftriaxone administered to humans as an antibiotic is 2 g day−1. Assuming a linear, allometric relationship in ceftriaxone dose for a rat-to-human scale-up, plasma levels of ceftriaxone are clearly greater under our conditions than those levels achieved by a therapeutic dose in humans. Future studies will determine if lower doses of ceftriaxone attenuate the hyperthermic response to morphine. Because it is likely that ceftriaxone inhibited morphine-evoked hyperthermia by increasing the expression and activity of GLT-1 transporters in the brain, a more important consideration in the present study was the concentration of ceftriaxone achieved in the brain (Rothstein et al., 2005). Pharmacokinetic results suggest that the i.p. administration of 200 mg kg−1 of ceftriaxone yields CNS concentrations of the antibiotic comparable to those CNS levels required to increase GLT-1 expression (3.5 μM), and attained with therapy for meningitis (0.3–6 μM) (Chandrasekar et al., 1984; Nau et al., 1993; Granero et al., 1995; Rebuelto et al., 2003; Rothstein et al., 2005). A dose of 200 mg kg−1 of ceftriaxone administered to rats and mice displays neuroprotective and antidepressant properties, both effects that are thought to be mediated by GLT-1 activation (Rothstein et al., 2005; Chu et al., 2007; Mineur et al., 2007).
Although a pharmacokinetic interaction may have contributed to the attenuation of morphine-evoked hyperthermia by ceftriaxone, two lines of evidence argue against this possibility. First, TBOA administered into the brain abolished the effects of ceftriaxone. Given that both ceftriaxone and TBOA directly affect glutamate uptake by acting at the GLT-1 transporter protein, the most probable explanation for our findings is that ceftriaxone altered the glutamate transport system, not the morphine concentration, to attenuate morphine-evoked hyperthermia (Shimamoto et al., 1998, 2000; Miller and Cleveland, 2005; Rothstein et al., 2005). Second, our data show that ceftriaxone blocks a significant proportion of the hyperthermia caused by each dose (1, 4, 8, or 15 mg kg−1) of morphine. An earlier work from our laboratory demonstrates that this dose range of morphine produces significant hyperthermia in rats (Rawls et al., 2003). Given that the lowest dose (1 mg kg−1) of morphine produced a significant hyperthermia when administered by itself, a hyperthermic response would still be predicted even if plasma, or brain, concentrations of morphine were lower than normal following the concurrent administration of ceftriaxone and a dose of morphine such as 8 mg kg−1.
In summary, the data presented here show that a common antibiotic, ceftriaxone, blocked a significant proportion of the hyperthermia caused by morphine. These data reveal a functional interaction between ceftriaxone and morphine and indicate that morphine is less efficacious in the presence of a β-lactam antibiotic. A glutamate uptake blocker, TBOA, antagonized the effects of ceftriaxone, suggesting that an increase in glutamate transport mediated the effects of ceftriaxone. It is possible that the effects of ceftriaxone extend to other morphine-evoked effects, such as analgesia, constipation, tolerance and dependence, which depend on increased glutamatergic transmission. In this event, β-lactam antibiotics may prove to be a useful clinical alternative to treat some of the adverse effects that accompany morphine therapy.
Acknowledgments
This work is supported in part by National Institutes on Drug Abuse (NIH) grant DA 09793-03. We thank Dr Martin W Adler for invaluable advice on body temperature experiments and Dr Jim Gallo for his advice on the pharmacokinetics of ceftriaxone.
Abbreviations
- EAAT2
excitatory amino-acid transporter 2
- GLT-1
glutamate transporter subtype 1
- riluzole
2-amino 6-trifluoromethoxy-benzothiazole
- TBOA
DL-threo-β-benzyloxyaspartic acid
Conflict of interest
The authors state no conflict of interest.
References
- Adler MW, Geller EB.Physiological functions of opioids: temperature regulation Handbook of Experimental Pharmacology 1993Springer-Verlag: Berlin, Germany; 205–238.In: Herz A, Akil H, Simon EJ (eds).Vol. 104/II, Opioids II. [Google Scholar]
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Dykstra LA. N-methyl-D-aspartate receptor antagonists potentiate the antinociceptive effects of morphine in squirrel monkeys. J Pharmacol Exp Ther. 2001;298:288–297. [PubMed] [Google Scholar]
- Beghi E, Bendotti C, Mennini T. Merits of a new drug trial for ALS. Science. 2005;308:632–633. doi: 10.1126/science.308.5722.632b. [DOI] [PubMed] [Google Scholar]
- Benamar K, Xin L, Geller EB, Adler MW. Effect of central and peripheral administration of a nitric oxide synthase inhibitor on morphine hyperthermia in rats. Brain Res. 2001;894:266–273. doi: 10.1016/s0006-8993(01)02025-x. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981;40:2843–2850. [PubMed] [Google Scholar]
- Cechova S, Zuo Z. Inhibition of glutamate transporters increases the minimum alveolar concentration for isoflurane in rats. Br J Anaesth. 2006;97:192–195. doi: 10.1093/bja/ael152. [DOI] [PubMed] [Google Scholar]
- Chandrasekar PH, Rolston KV, Smith BR, LeFrock JL. Diffusion of ceftriaxone into the cerebrospinal fluid of adults. J Antimicrob Chemother. 1984;14:427–430. doi: 10.1093/jac/14.4.427. [DOI] [PubMed] [Google Scholar]
- Chen X, McClatchy DB, Geller EB, Liu-Chen L, Tallarida RJ, Adler MW. Possible mechanism of hypothermia induced by intracerebroventricular injection of orphanin FQ/nociceptin. Brain Res. 2001;904:252–258. doi: 10.1016/s0006-8993(01)02467-2. [DOI] [PubMed] [Google Scholar]
- Chen XH, Geller EB, DeRiel JK, Liu-Chen LY, Adler MW. Antisense confirmation of mu- and kappa-opioid receptor mediation of morphine's effects on body temperature in rats. Drug Alcohol Depend. 1996;43:119–124. doi: 10.1016/s0376-8716(96)01295-1. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Frizzo ME, Dall'Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–128. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, et al. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Geller EB, Hawk C, Keinath SH, Tallarida RJ, Adler MW. Subclasses of opioids based on body temperature change in rats: acute subcutaneous administration. J Pharmacol Exp Ther. 1983;225:391–398. [PubMed] [Google Scholar]
- Geller EB, Rowan CH, Adler MW. Body temperature effects of opioids in rats: intracerebroventricular administration. Pharmacol Biochem Behav. 1986;24:1761–1765. doi: 10.1016/0091-3057(86)90517-4. [DOI] [PubMed] [Google Scholar]
- Goodman LS, Hardman JG, Limbird LE, Gilman AG. The Pharmacological Basis of Therapeutics. McGraw-Hill Medical Pub. Division: New York; 2001. Goodman & Gilman's. [Google Scholar]
- Granero L, Santiago M, Cano J, Machado A, Peris JE. Analysis of ceftriaxone and ceftazidime distribution in cerebrospinal fluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob Agents Chemother. 1995;39:2728–2731. doi: 10.1128/aac.39.12.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology. 1995;13:269–293. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;282:1280–1290. [PubMed] [Google Scholar]
- Inturrisi CE. Preclinical evidence for a role of glutamatergic systems in opioid tolerance and dependence. Semin Neurosci. 1997;9:110–119. [Google Scholar]
- Ji HF, Shen L, Zhang HY. Beta-lactam antibiotics are multipotent agents to combat neurological diseases. Biochem Biophys Res Commun. 2005;333:661–663. doi: 10.1016/j.bbrc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Jin C, Araki H, Kawasaki Y, Nagata M, Suemaru K, Shibata K, et al. The glutamate release inhibitor riluzole attenuates the formation of conditioned place aversion induced by naloxone in rats undergoing a single morphine exposure. Brain Res. 2006;1069:120–126. doi: 10.1016/j.brainres.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- Le Greves P, Huang W, Zhou Q, Thornwall M, Nyberg F. Acute effects of morphine on the expression of mRNAs for NMDA receptor subunits in the rat hippocampus, hypothalamus and spinal cord. Eur J Pharmacol. 1998;341:161–164. doi: 10.1016/s0014-2999(97)01400-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Ho MT, Young MS. Stimulation of the nigrostriatal dopamine system inhibits both heat production and heat loss mechanisms in rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:504–510. doi: 10.1007/BF00169004. [DOI] [PubMed] [Google Scholar]
- Malgouris C, Bardot F, Daniel M, Pellis F, Rataud J, Uzan A, et al. Riluzole, a novel antiglutamate, prevents memory loss and hippocampal neuronal damage in ischemic gerbils. J Neurosci. 1989;9:3720–3727. doi: 10.1523/JNEUROSCI.09-11-03720.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Thompson MA, Nadler JV. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- Miller TM, Cleveland DW. Medicine. Treating neurodegenerative diseases with antibiotics. Science. 2005;307:361–362. doi: 10.1126/science.1109027. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, Sanacora G. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry. 2007;61:250–252. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Satoh M. Involvement of glial glutamate transporters in morphine dependence. Ann N Y Acad Sci. 2004;1025:383–388. doi: 10.1196/annals.1307.047. [DOI] [PubMed] [Google Scholar]
- Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, et al. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37:1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Sekiya Y, Minami M, Satoh M. Effect of gene transfer of GLT-1, a glutamate transporter, into the locus coeruleus by recombinant adenoviruses on morphine physical dependence in rats. Eur J Neurosci. 2004;19:221–226. doi: 10.1111/j.1460-9568.2004.03101.x. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001;905:254–258. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Rao VL, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochem Res. 2001;26:497–502. doi: 10.1023/a:1010956711295. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Fuller RW, Stockton ME, Perry KW, Swinford RM, Ornstein PL. NMDA receptor antagonists suppress behaviors but not norepinephrine turnover or locus coeruleus unit activity induced by opiate withdrawal. Eur J Pharmacol. 1991;197:9–16. doi: 10.1016/0014-2999(91)90358-w. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Gaughan JP, Geller EB, Adler MW, Cowan A. NMDA antagonists attenuate morphine-induced hyperthermia. Brain Res. 2003;984:76–83. doi: 10.1016/s0006-8993(03)03093-2. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Hewson JM, Inan S, Cowan A. Brain delta2 opioid receptors mediate SNC-80-evoked hypothermia in rats. Brain Res. 2005;1049:61–69. doi: 10.1016/j.brainres.2005.04.074. [DOI] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF. L-trans-pyrrolidine-2,4-dicarboxylic acid-evoked striatal glutamate levels are attenuated by calcium reduction, tetrodotoxin, and glutamate receptor blockade. J Neurochem. 1997;68:1553–1563. doi: 10.1046/j.1471-4159.1997.68041553.x. [DOI] [PubMed] [Google Scholar]
- Rebuelto M, Ambros L, Rubio M. Daily variations in ceftriaxone pharmacokinetics in rats. Antimicrob Agents Chemother. 2003;47:809–812. doi: 10.1128/AAC.47.2.809-812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin Neurosci. 1996;3:348–359. [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Secko D. Antibiotics that protect the brain. CMAJ. 2005;172:467–468. doi: 10.1503/cmaj.050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, et al. -threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Shigeri Y, Yasuda-Kamatani Y, Lebrun B, Yumoto N, Nakajima T. Syntheses of optically pure beta-hydroxyaspartate derivatives as glutamate transporter blockers. Bioorg Med Chem Lett. 2000;10:2407–2410. doi: 10.1016/s0960-894x(00)00487-x. [DOI] [PubMed] [Google Scholar]
- Singh J, Gupta MC. Effect of aspartate and glutamate on nociception, catalepsy and core temperature in rats. Indian J Physiol Pharmacol. 1997;41:123–128. [PubMed] [Google Scholar]
- Spencer RL, Hruby VJ, Burks TF. Body temperature response profiles for selective mu, delta and kappa opioid agonists in restrained and unrestrained rats. J Pharmacol Exp Ther. 1988;246:92–101. [PubMed] [Google Scholar]
- Spencer RL, Hruby VJ, Burks TF. Alteration of thermoregulatory set point with opioid agonists. J Pharmacol Exp Ther. 1990;252:696–705. [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: implications for neuroprotective strategies. Exp Neurol. 1997;147:115–122. doi: 10.1006/exnr.1997.6554. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xin L, Geller EB, Adler MW. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J Pharmacol Exp Ther. 1997;281:499–507. [PubMed] [Google Scholar]
- Yasumatsu M, Yazawa T, Otokawa M, Kuwasawa K, Hasegawa H, Aihara Y. Monoamines, amino acids and acetylcholine in the preoptic area and anterior hypothalamus of rats: measurements of tissue extracts and in vivo microdialysates. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:13–23. doi: 10.1016/s1095-6433(98)10096-x. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]