Abstract
Background and Purpose:
Statins (3-hydroxy-3-methyl-glutaryl coenzyme A (HMG CoA) reductase inhibitors) have been demonstrated to reduce cardiovascular mortality. It is unclear how the expression level of HMG CoA reductase in cardiovascular tissues compares with that in cells derived from the liver. We hypothesized that this enzyme exists in different cardiovascular tissues, and simvastatin modulates the vascular iberiotoxin-sensitive Ca2+-activated K+ (BKCa) channels.
Experimental Approaches:
Expression of HMG CoA reductase in different cardiovascular preparations was measured. Effects of simvastatin on BKCa channel gatings of porcine coronary artery smooth muscle cells were evaluated.
Key Results:
Western immunoblots revealed the biochemical existence of HMG CoA reductase in human cardiovascular tissues and porcine coronary artery. In porcine coronary artery smooth muscle cells, extracellular simvastatin (1, 3 and 10 μM) (hydrophobic), but not simvastatin Na+ (hydrophilic), inhibited the BKCa channels with a minimal recovery upon washout. Isopimaric acid (10 μM)-mediated enhancement of the BKCa amplitude was reversed by external simvastatin. Simvastatin Na+ (10 μM, applied internally), markedly attenuated isopimaric acid (10 μM)-induced enhancement of the BKCa amplitude. Reduced glutathione (5 mM; in the pipette solution) abolished simvastatin -elicited inhibition. Mevalonolactone (500 μM) and geranylgeranyl pyrophosphate (20 μM) only prevented simvastatin (1 and 3 μM)-induced responses. simvastatin (10 μM) caused a rottlerin (1 μM)-sensitive (cycloheximide (10 μM)-insensitive) increase of PKC-δ protein expression.
Conclusions and Implications:
Our results demonstrated the biochemical presence of HMG CoA reductase in different cardiovascular tissues, and that simvastatin inhibited the BKCa channels of the arterial smooth muscle cells through multiple intracellular pathways.
Keywords: HMG CoA reductase, simvastatin, coronary artery, BKCa channels
Introduction
3-Hydroxy-3-methyl-glutaryl coenzyme A (HMG CoA) reductase is a 97 kDa glycoprotein embedded in the endoplasmic reticulum (ER) (Lange et al., 2002) responsible for the rate-limiting step of cholesterol synthesis in mammalian liver and intestine. It has been reported in large-scale clinical trials (Laufs et al., 1998; Futterman and Lemberg, 2004) that HMG CoA reductase inhibitors (the statins) reduced cardiovascular mortality. Recent evidence suggested that statins provide beneficial effects that are independent of the serum cholesterol reduction (pleiotropic effects) (Futterman and Lemberg, 2004), such as increasing the bioavailability of nitric oxide (NO) with an increased endothelial NO synthase (eNOS) protein, improving vascular relaxation (Laufs et al., 1998; Kalinowski et al., 2002) and promoting new vessel formation (Pourati et al., 2003).
Various statins have been reported altering the activities of different ion channels of blood vessels (Kajinami et al., 2000; Bergdahl et al., 2003; Terata et al., 2003; Sonmez Uydes-Dogan et al., 2005). In cultured rat aortic smooth muscle cells, simvastatin and atorvastatin inhibited the angiotensin II-induced mobilization of intracellular Ca2+ (Tesfamariam et al., 1999; Alvarez de Sotomayor et al., 2001). It has been demonstrated that simvastatin exists in two form, the lipophilic simvastatin (partition coefficient: 4.7) and the hydrophilic simvastatin salt (simvastatin Na+; partition coefficient: <2.1). The different inhibitory potencies provided by these two forms of simvastatin have been related to their lipophilicities (Yada et al., 1999; Bogman et al., 2001).
The large-conductance, Ca2+-activated K+ (BKCa) channels are abundant in different vascular tissues and activation of protein kinase C (PKC) inhibits the BKCa channel activities (Minami et al., 1993; Schubert et al., 1999). In the present study, we hypothesized that HMG CoA reductase exists in different cardiovascular tissues, and simvastatin has a direct modulation effects on the gating of the BKCa channels. Therefore, our study was designed to (1) provide biochemical evidence (protein expression) of HMG CoA reductase in human-isolated cardiovascular preparations, (2) evaluate the modulatory effect(s) of simvastatin on the BKCa channels and (3) examine the participation of PKC activation.
Materials and methods
Human tissue preparation
Fresh tissues were obtained from patients (Chinese, two males and five females, aged between 32 and 72 years) (with written consents obtained), who have undergone elective surgery at the Prince of Wales Hospital and the United Christian Hospital (Hong Kong SAR, PR of China). All procedures and guidelines for using human tissues for experiments were approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong (CREC ref. no. CRE-2006.313). Human umbilical vein endothelial cells (HUVECs) were obtained from American Type Culture Collection (Manassas, VA, USA). Owing to the irregular supply of human cardiovascular tissues for research purposes and medications that patients have taken before surgery may affect an accurate interpretation of our results, pig coronary artery smooth muscle cells were used in all functional studies. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and the principles outlined in the Declaration of Helsinki Principles.
Isolation of pig left anterior descending coronary arteries smooth muscle cells
Pig left anterior descending coronary artery smooth muscle cells were enzymatically dissociated, as reported previously by our group (Au et al., 2003, 2004) for single-cell, patch-clamp electrophysiology experiments.
Patch-clamp electrophysiology
Whole-cell, membrane-rupture recording of the macroscopic iberiotoxin-sensitive Ca2+-activated K+ (BKCa) channels gatings of single coronary artery smooth muscle cells were recorded, as described by our group previously (Au et al., 2003, 2004). External physiological solutions for recording the BKCa channel amplitude contained (in mM): NaCl 130, KCl 5, MgCl2 1.2, CaCl2 1.5, glucose 10 and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid) 10 (pH 7.4 with NaOH). Internal pipette solution containing ∼100 nM free [Ca2+] (estimated using the computer programme: Maxchelator, Stanford University, Stanford, CA USA) had the following composition (in mM): NaCl 10, KCl 110, MgCl2 5, CaCl2 2, ethylene glycol-bis [β-aminoethylether] N,N′,N′-tetraacetic acid 10, K2ATP 5 and HEPES 10 (pH 7.2 with KOH). For free [Ca2+] of ∼444 nM, the pipette solution contained 7 mM instead of 2 mM CaCl2, and 4 mM instead of 5 mM MgCl2, as described previously (Tammaro et al., 2004). Hence, the BKCa gating changes in response to a particular drug challenge were compared under the conditions of free intracellular calcium ion concentration [Ca2+]i of ∼100 and ∼444 nM in the pipette solutions. The range of free [Ca2+]i chosen in this study was similar to the previously measured global [Ca2+]i levels observed in a non-stimulated/resting (Schmidt et al., 2004; Shen et al., 2004) and vasoactive agonist-stimulated single porcine coronary arterial myocytes (Ndiaye et al., 2003), thus allowing the studying of BKCa gating changes in response to drug challenge under different [Ca2+]i (resting and elevated) conditions. To allow for an equilibration of the pipette solution with the cell interior, all recordings were started ∼5 min after the establishment of the whole-cell configuration. Most experiments were performed within 15 min of gaining access, during which time the macroscopic BKCa current amplitude remained stable. The BKCa current was elicited with test potentials between −80 and +80 mV (a 20-mV increment for a 500-ms duration) from a holding potential of −60 mV (resting membrane potential of porcine coronary artery) (Sirous et al., 2001; Ndiaye et al., 2003) and stimulated at 0.1 Hz. In this study, unless otherwise stated, a complete current–voltage relationship of BKCa channels (with and without drugs) was constructed. For comparison, however, the BKCa amplitude recorded at +80 mV was used as the magnitude of BKCa channels recorded at more negative potentials (0 and 20 mV) is relatively small and therefore it is difficult to make an accurate measurement.
To measure the rate of onset of block and recovery from the block in response to drug challenge, the BKCa current was elicited with a test potential to +80 mV (a 500-ms duration) from a holding potential of −60 mV and stimulated at 0.1 Hz. Cell membrane capacitance was estimated, as described previously (Au et al., 2003, 2004), and it was 19.2±2.3 pF (n=30). The amplitude of the BKCa current was recorded before (control), during (drug treated) and after (washout) the administration of a particular drug. Only one concentration of a particular drug was tested in each cell. External solution was delivered, through gravity, and controlled by solenoid valves coupled to a four-channel valve driver (General Valve, Brookshire, TX, USA). The solution change (∼5 ml, which was 10 times the volume of the recording chamber) could be completed in 15–20 s. Drugs (dissolved in the external recording solution) were applied to the external cell surface, unless otherwise stated.
Western immunoblot analysis
Fresh human-isolated cardiovascular tissues and pig-isolated coronary arteries (endothelial cells removed) were homogenized in the presence of protease inhibitors to obtain extracts of proteins. Protein concentrations were determined using BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Samples (25 μg of protein per lane) were loaded onto a 12% sodium dodecyl sulfate (SDS)–polyacrylamide electrophoresis gel. After electrophoresis (180 V, 60 min), the separated proteins were transferred (12 mA, 90 min) to polyvinylidene difluoride membrane (PerkinElmer Life Science, Waltham, MA, USA). Non-specific sites were blocked with 5% non-fat dry milk for 120 min, and the blots were then incubated with individual respective antibody: anti-caveolin-1, 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-HMG CoA reductase antibody, 1:1000 (Upstate, Chicago, IL, USA); anti-PKC-δ antibody, 1:1000 (Santa Cruz Biotechnology, USA) overnight at 4°C. Anti-rabbit horseradish peroxidase-conjugated immunoglobulin G (1:1000; DakoCytomation, Glostrup, Denmark) was used to detect binding of its correspondent antibody. Membranes were stripped and re-blotted with anti-β-actin antibody (1:10 000; Sigma-Aldrich, St Louis, MO, USA) to verify an equal loading of protein in each lane. The binding of the specific antibody was visualized by exposing to the photographic film after treating with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Science, USA). Blot density was quantified by densitometry using Scion Image Programme (version 1.63) (Scion Image, Frederick, MD, USA), and normalized to β-actin expression. For the determination of HMG CoA reductase protein expression, we used cultured human hepatocarcinoma (HepG2) cell line (provided by Professor HK Yeung, The Chinese University of Hong Kong) as the reference for comparison. Primary antibody omission controls were performed, and results showed that no non-specific binding was observed.
Tissue fractions
Pig coronary arteries (endothelium removed) were isolated, incubated with simvastatin (10 μM) for different periods (2, 15 and 30 min), and were rapidly transferred to ice-cold equilibrating buffer after incubation. Translocation/expression of PKC-δ in response to simvastatin was measured as described previously (Sirous et al., 2001), with a slight modification. Briefly, the tissue was homogenized in ice-cold lysis buffer (Tris–HCl 50 mM, NaCl 150 mM, ethylenediaminetetraacetic acid 1 mM, SDS 0.1%) with protease inhibitor, and centrifuged at 100 000 g for 60 min at 4°C (Optimax Max Ultra-Centrifuge; Beckman-Coulter, Fullerton, CA, USA). The supernatant after centrifugation was considered as the cytosolic fraction. The pellet was re-suspended in ice-cold lysis buffer containing Triton X-100 (1%) for 30 min and centrifuged at 100 000 g (60 min, 4°C). The supernatant was considered as the particulate (membrane) fraction. Both cytosolic and particulate fractions were subjected to Western immunoblot analysis. Band density was quantified by densitometry using Scion Image Programme (version 1.63) (Scion, USA), and normalized to the band intensity of β-actin expression.
Statistical analysis
In the whole-cell, patch-clamp electrophysiology experiments, n refers to the number of single vascular smooth muscle cells used, and results are expressed as mean±s.e.m. For western immunoblot experiments, results are expressed as mean±s.e.m. from three independent experiments. Statistical analysis was performed using analysis of variance and Student's t-test, where appropriate. Differences were considered significant when P<0.05.
Materials
Collagenase (type II) was obtained from Worthington Biochemicals Corp. (Lakewood, NJ, USA) and papain was purchased from Fluka (Buchs SG, Switzerland). Simvastatin was obtained from Tocris-Cookson (Bristol, UK) and dissolved in dimethyl sulphoxide (DMSO) as a stock solution (50 mM). Simvastatin Na+ (50 mM) was prepared from simvastatin using NaOH (in ethanol), as suggested by the manufacturer (Tocris-Cookson, UK). β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetra-sodium salt (NADPH), β-nicotinamide adenine dinucleotide phosphate di-sodium salt (NADH), indomethacin, L-arginine, reduced glutathione (GSH), oxidized glutathione (GSSG), 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS 1619), dithiothreitol (DTT), diphenyleneiodonium chloride (DPI), perillic acid, (±)-mevalonolactone, cylcoheximide and geranylgeranyl pyrophosphate ammonium salt (GGPP) were purchased from Sigma-Aldrich (USA). 4,5-Dihydroxy-1,3-benzenedisulphonic acid, disodium salt monohydrate (Tiron) was obtained from Arcos Organics (Morris Plains, NJ, USA). Wortmannin, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), 4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole (SB 203580), apocynin, 2′-amino-3′-methoxyflavone (PD 98059), phorbol-12-myristate-13-acetate (PMA) and rottlerin were purchased from Calbiochem-Novabiochem (San Diego, CA, USA). Iberiotoxin and isopimaric acid were purchased from Alomone Labs (Jerusalem, Israel).
Results
Expression of HMG CoA reductase in the cardiovascular tissues
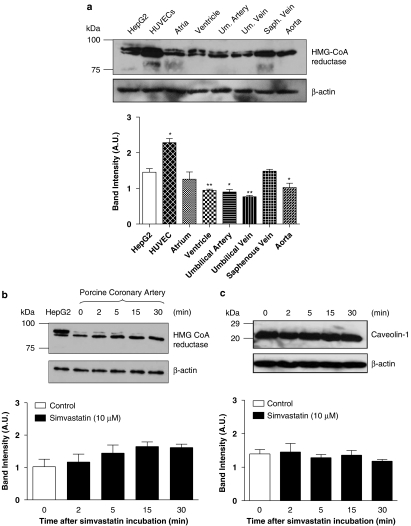
The protein expression of HMG CoA reductase in human-isolated cardiovascular tissues, pig-isolated coronary artery (endothelium denuded), cultured HUVECs and HepG2 cell lines (the reference for comparison) is illustrated in Figure 1a. The expression of HMG CoA reductase in cultured HUVEC was 51% higher (P<0.05) than that observed in the HepG2 cells. In the human tissues examined, a lower HMG CoA reductase protein expression was recorded (ventricular myocardium, 64%; aorta, 72%; umbilical artery, 67%; umbilical vein, 48%), compared to the HepG2 cells (Figure 1a). There was no apparent difference in HMG CoA reductase expression in the saphenous vein and left atrium, compared to the HepG2 cells (Figure 1a).
Figure 1.
(a) Western immunoblots analysis demonstrated the existence of HMG CoA reductase in heptocarcinoma (HepG2) cells, human umbilical vein endothelial cell (HUVEC) and human cardiovascular tissues. Results are expressed as mean±s.e.m. of three independent experiments (*P<0.05 and **P<0.01, compared to HepG2 cells). (b) Effects of simvastatin (10 μM) incubation on the protein expression of HMG CoA reductase of pig coronary artery using western immunoblots analysis. β-Actin was measured as a loading control. Results (blots) are normalized to β-actin expression and are expressed as mean (arbitrary units, AU)±s.e.m. of three independent experiments. (c) Effects of simvastatin (10 μM) incubation on the protein expression of caveolin-1 of pig coronary artery using western immunoblots analysis. β-Actin was measured as a loading control. Results (blots) are normalized to β-actin expression and are expressed as mean (arbitrary units, AU)±s.e.m. of three independent experiments. HMG CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A.
Effect of simvastatin on HMG CoA reductase and caveolin-1 expression
Effect of simvastatin (10 μM, hydrophobic) incubation on HMG CoA reductase (Figure 1b) and caveolin-1 (Figure 1c) protein expression in pig coronary artery (endothelium denuded) was evaluated. There was no apparent change in the protein expression of HMG CoA reductase (Figure 1b) and caveolin-1 (Figure 1c) after simvastatin (10 μM) incubation, compared to control (simvastatin-free, 0 min) conditions (P>0.05).
Mechanism(s) responsible for simvastatin-mediated inhibition of iberiotoxin-sensitive BKCa channels
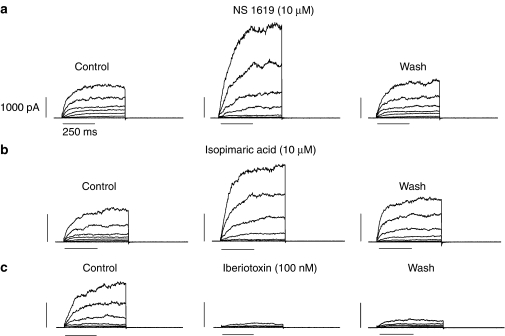
Macroscopic whole-cell, outward K+ currents of pig coronary artery smooth muscle cells recorded under our experimental conditions ([Ca2+]i ∼444 nM) were enhanced by NS 1619 (10 μM; n=6) and isopimaric acid (10 μM; n=4) (both are Ca2+-activated K+ (BKCa) channel openers), whereas the outward K+ current was inhibited by iberiotoxin (100 nM, a highly potent BKCa channel blocker; n=4; Figure 2). These results illustrate that the outward K+ currents recorded is the genuine iberiotoxin-sensitive BKCa channels.
Figure 2.
Effect of (a) NS 1619 (10 μM), (b) isopimaric acid (10 μM) and (c) iberiotoxin (100 nM) on the whole-cell, outward K+ currents of pig coronary artery smooth muscle cells. Representative whole-cell, outward K+ currents (pipette solution [Ca2+]i ∼444 nM) were elicited with test potentials between −80 mV and +80 mV (a 20-mV increment for a 500-ms duration, 0.1 Hz) from a holding potential of −60 mV. [Ca2+]i, intracellular calcium ion concentration; NS 1619, 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one.
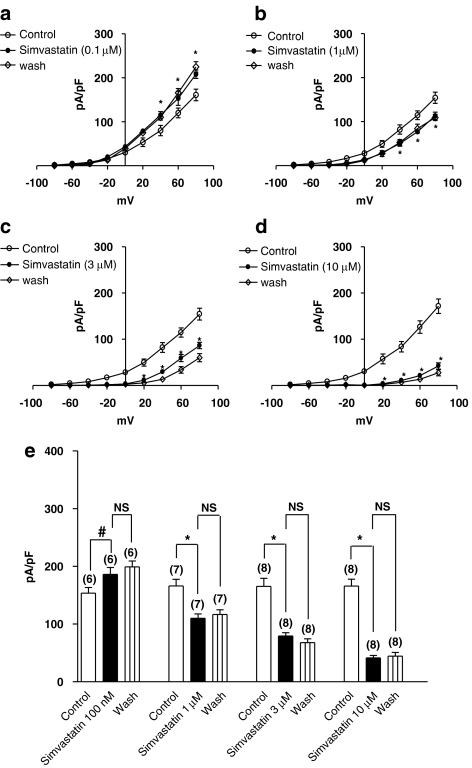
Acute simvastatin (1, 3 and 10 μM) caused a concentration-dependent inhibition of the BKCa amplitude ([Ca2+]i ∼444 nM) with no recovery after washout (n=7–8) (P<0.01) (Figures 3b–e). A similar inhibition of the BKCa amplitude by simvastatin was observed with [Ca2+]i of 100 nM (n=5–6, data not shown). However, simvastatin (100 nM) produced an enhancement of the BKCa amplitude ([Ca2+]i ∼444 nM) (n=6; P<0.05) (Figure 3a). No apparent effect on the BKCa amplitude was observed with simvastatin (10 and 30 nM) (BKCa amplitude measured at +80 mV: 152±24 pA/pF (control); 150±33 pA/pF (simvastatin, 10 nM); 148±26 pA/pF (simvastatin, 30 nM)) (n=5–6). Unlike simvastatin, the salt, simvastatin Na+ (0.1, 1, 3 and 10 μM) (hydrophilic), produced no effect on the BKCa amplitude, irrespective of the [Ca2+]i levels (n=5–6, data not shown). The phorbol ester (PMA) (100 nM) caused an inhibition of the BKCa amplitude ([Ca2+]i ∼444 nM) with no recovery after washout (BKCa amplitude measured at +80 mV: 150±27 pA/pF (control), 65±16 pA/pF (PMA, 100 nM) and 62±12 pA/pF (wash)) (n=5).
Figure 3.
Effects of simvastatin on the current–voltage relationship of the whole-cell BKCa channels of (a–d) pig coronary artery smooth muscle cells. Representative BKCa currents (expressed in pA/pF) (pipette solution [Ca2+]i ∼444 nM) were elicited with test potentials between −80 to +80 mV (a 20-mV increment for a 500-ms duration, 0.1 Hz) from a holding potential of −60 mV. Calibration bars: 400 pA and 250 ms. (e) Summary of the macroscopic BKCa current amplitude recorded (peak BKCa current (pA/pF) recorded at +80 mV from a holding potential of −60 mV for a 500-ms duration at 0.1 Hz) in response to simvastatin challenge. Mean±s.e.m. are indicated by columns and vertical bars, respectively (#P<0.05; *P<0.01, compared to control; NS, non-significant), and numbers in parentheses indicate the number of experiments performed. BKCa, [Ca2+]i, intracellular calcium ion concentration.
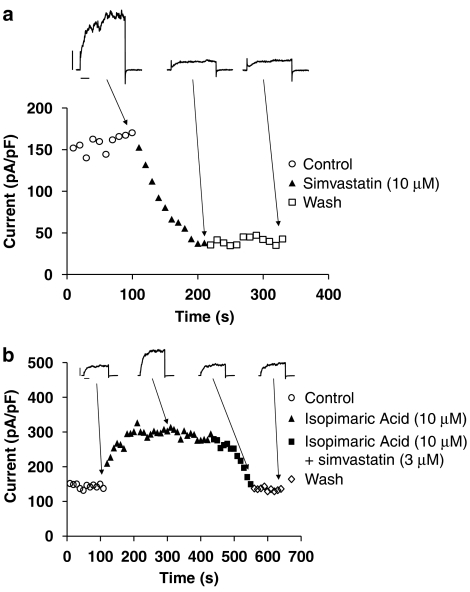
Simvastatin (10 μM) elicited a progressive inhibition of the BKCa amplitude and the maximum inhibition occurred at ∼2 min, with no recovery from block after washout (Figure 4a). Isopimaric acid (10 μM) produced a progressive enhancement of the BKCa current amplitude (Figure 4b) (BKCa amplitude measured at +80 mV: 153±24 pA/pF (control), 317±18 pA/pF (isopimaric acid, 10 μM)) (P<0.001), and the subsequent external application of simvastatin (1, 3 and 10 μM) (in the continuous presence of 10 μM isopimaric acid), but not simvastatin Na+ (1, 3 and 10 μM), ameliorated the enhanced BKCa amplitude and the BKCa amplitude returned to the baseline level (Figure 4b). In addition, simvastatin Na+ (10 μM, in the pipette solution) markedly attenuated isopimaric acid (10 μM)-induced enhancement of the BKCa amplitude (BKCa amplitude measured at +80 mV: 151±24 pA/pF (control), 203±20 pA/pF (isopimaric acid, 10 μM)) (P<0.05) (n=7).
Figure 4.
(a) Time course of the inhibitory effect of simvastatin (10 μM) on the BKCa amplitude. Calibration bars: 1000 pA and 100 ms. (b) Time course of the effect of isopimaric acid (10 μM), with and without simvastatin (3 μM), on the BKCa amplitude. The BKCa current was elicited using a train-pulse protocol with a test potential of +80 mV (a 500-ms pulse duration) from a holding potential of −60 mV at 0.1 Hz. Calibration bars: 1200 pA and 100 ms.
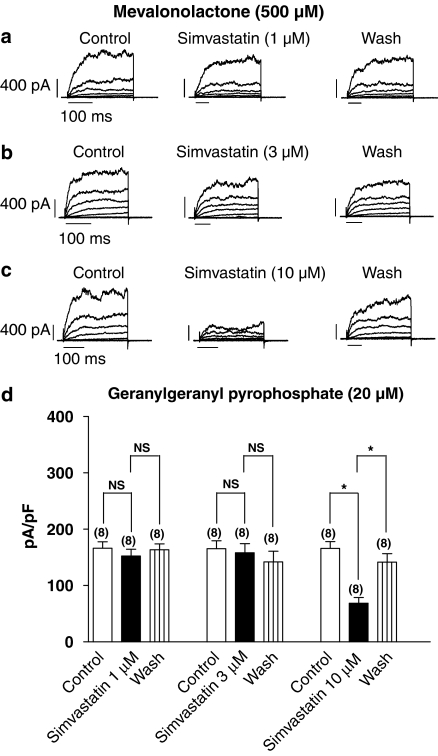
GSH (5 mM), but not GSSG (5 mM) or dithiothreitol (DTT, 5 mM), abolished simvastatin (1, 3 and 10 μM)-induced inhibition of the BKCa amplitude (data not shown). Mevalonolactone (500 μM; Figures 5a and b), GGPP (20 μM; Figure 5d), NADPH (500 μM, data not shown), NADH (500 μM, data not shown), applied alone, prevented simvastatin (1 and 3 μM)-mediated inhibition of the BKCa channels. Neither NADPH (500 μM), NADH (500 μM), mevalonolactone (500 μM; Figure 5c) nor GGPP (20 μM; Figure 5d) modified simvastatin (10 μM)-induced inhibition of the BKCa amplitude. However, there was a greater magnitude of recovery from the inhibition of the BKCa amplitude, compared to simvastatin (10 μM) alone, in the presence of NADPH (500 μM, data not shown), NADH (500 μM, data not shown), mevalonolactone (500 μM, data not shown) and GGPP (20 μM; Figure 5d).
Figure 5.
Effects of simvastatin on the current-voltage relationship of the BKCa channels (pipette solution contained: mevalonolactone, 500 μM; [Ca2+]i ∼444 nM) of (a–c) pig coronary artery smooth muscle cells. Representative BKCa currents were elicited with test potentials between −80 and +80 mV (a 20-mV increment for a 500-ms duration, 0.1 Hz) from a holding potential of −60 mV. Calibration bars: 400 pA and 100 ms. (d) Summary of the macroscopic BKCa current amplitude recorded (expressed as pA/pF) (pipette solution contained: geranylgeranyl pyrophosphate (GGPP), 20 μM; [Ca2+]i ∼444 nM) (peak BKCa current (pA/pF) recorded at +80 mV from a holding potential of −60 mV for a 500-ms duration at 0.1 Hz) recorded with and without simvastatin. Mean±s.e.m. are indicated by columns and vertical bars, respectively (*P<0.01, compared to control; NS, non-significant), and numbers in parentheses indicate the number of experiments performed. BKCa channels, iberiotoxin-sensitive Ca2+-activated K+ channels; [Ca2+]i, intracellular calcium ion concentration.
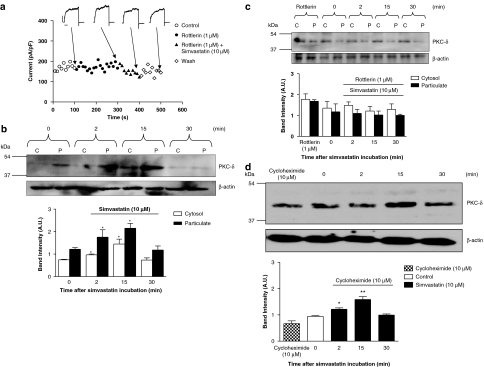
Rottlerin (1 μM, a selective inhibitor of the PKC-δ isoform) (Gschwendt et al., 1994) ameliorated simvastatin (10 μM)- (Figure 6a) and PMA (100 nM)-elicited inhibition of the BKCa channels. The participation of other intracellular signalling cascades was also explored but none of the tested agents affected simvastatin (10 μM)-induced inhibition of the BKCa channels (Table 1). These agents were apocynin (NAD(P)H oxidase inhibitor); indomethacin (cyclo-oxygenase inhibitor); PD 98095 (mitogen-activated protein kinase kinase inhibitor); SB 203580 (p38 mitogen-activated protein kinase inhibitor); (−)-perillic acid (p21ras inhibitor); L-arginine (substrate for NO synthesis); Tiron (cell-permeable superoxide scavenger); wortmannin (irreversible inhibitor of phosphatidylinositol 3-kinase (PI3K)); DPI (inhibitor of mitochondrial NADPH-ubiquinone oxido reductase) and PP2 (inhibitor of the Src family of protein tyrosine kinase). Other details of these experiments are given in Table 1.
Figure 6.
(a) Time course of the effect of simvastatin (10 μM), with and without rottlerin (1 μM), on the BKCa amplitude. The BKCa current was elicited using a train-pulse protocol with a test potential of +80 mV (a 500-ms pulse duration) from a holding potential of −60 mV at 0.1 Hz. Calibration bars: 250 pA and 100 ms. Western immunoblots analysis revealed the distribution of PKC-δ protein in the cytosolic (C) and particulate (P) fractions of pig-isolated coronary artery after incubation with simvastatin (10 μM) at different periods (0, 2, 15 and 30 min) as indicated, (b) with and (c) without 30 min pre-incubation of rottlerin (1 μM). (d) Western immunoblots analysis revealed PKC-δ protein expression of pig-isolated coronary artery in response to simvastatin (10 μM) at different periods (0, 2, 15 and 30 min), as indicated, after incubation with cycloheximide (10 μM, 2 h). β-Actin was measured as a loading control. Results (blots) are normalized to β-actin expression and are expressed as mean (arbitrary units, AU)±s.e.m. of three independent experiments (*P<0.05 and **P<0.01 compared to the respective controls at time 0 min). BKCa, iberiotoxin-sensitive Ca2+-activated K+ channels.
Table 1.
Effects of different intracellular signalling modulators on simvastatin (10 μM)-mediated BKCa channel currents
| Drug | Control (BKCa amplitude, pA/pF) | Simvastatin (10 μM) (BKCa amplitude, pA/pF) | Drug (BKCa amplitude, pA/pF) | Simvastatin (10 μM) plus drug (BKCa amplitude, pA/pF) | n | P-value |
|---|---|---|---|---|---|---|
| — | 152±27 | 47±11 | — | — | 7 | — |
| Apocynin (1 mM) | 151±22 | — | 150±20 | 49±14 | 7 | >0.05 |
| Indomethacin (10 μM) | 148±26 | — | 153±28 | 46±12 | 7 | >0.05 |
| PD 98059 (10 μM) | 154±29 | — | 150±21 | 55±11 | 4 | >0.05 |
| SB 203580 (10 μM) | 148±25 | — | 153±26 | 50±20 | 4 | >0.05 |
| (−)-Perillic acid (100 μM) | 154±27 | — | 150±25 | 52±12 | 5 | >0.05 |
| L-Arginine (500 μM) | 152±23 | — | 157±27 | 50±17 | 7 | >0.05 |
| Tiron (1 mM) | 154±27 | — | 150±25 | 52±21 | 5 | >0.05 |
| Wortmannin (100 μM) | 150±22 | — | 154±26 | 48±12 | 5 | >0.05 |
| DPI (10 μM) | 151±29 | — | 150±23 | 47±18 | 5 | >0.05 |
| PP2 (500 nM) | 154±21 | — | 156±27 | 57±12 | 4 | >0.05 |
Abbreviations: BKCa channel, iberiotoxin-sensitive Ca2+-activated K+ channels; DPI, diphenyleneiodonium chloride; PD 98059, 2′-amino-3′-methoxyflavone; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; SB 203580, 4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole; Tiron, 4,5-dihydroxy-1,3-benzenedisulphonic acid, disodium salt monohydrate.
All BKCa amplitudes were measured at +80 mV.
The significance levels of P-value refer to comparisons between simvastatin (10 μM)-treated and simvastatin (10 μM) plus individual drug-treated conditions.
Role of PKC-δ expression
The participation of PKC-δ in response to simvastatin (10 μM) was determined (incubation periods: 0, 2, 15 and 30 min). Simvastatin elicited a time-dependent (2–15 min) increase in the protein expression of PKC-δ in both the particulate and cytosol fractions (Figure 6b), with a relatively constant ratio (cytosol/particulate: 0 min, 0.67; 2 min, 0.56; 15 min, 0.67) (P>0.05) (Figure 6b). The PKC-δ level in the particulate and cytosol fractions returned to basal level after 30 min (cytosol/particulate: 0.62) (P>0.05). Pre-incubation with rottlerlin (1 μM) for 30 min before simvastatin application abolished the increase in PKC-δ expression by simvastatin (10 μM; Figure 6c). Cycloheximide (10 μM, a protein synthesis inhibitor) (2 h incubation) failed to alter simvastatin (10 μM)-induced increase in PKC-δ expression (Figure 6d), and a similar trend of increase in PKC-δ expression (in response to simvastatin (10 μM) after cycloheximide treatment), as observed in cycloheximide-free (Figure 6b) condition, was recorded.
Discussion
Herein we report the biochemical existence of HMG CoA reductase in human cardiovascular tissues and pig coronary artery. The mRNA expression of HMG CoA reductase has been described in hepatic cells and intestine (Feingold et al., 1994; Chen and Cheng, 2006). In addition, the existence of both hepatic and extrahepatic sterol synthesis in various internal organs has been demonstrated in different animal species (Turley et al., 1981; Andersen et al., 1982; Spady and Dietschy, 1983). It was generally believed that the cholesterol-lowering effects produced by statins reduced cardiovascular morbidity and mortality (Hernandez-Perera et al., 1998; Laufs et al., 1998; Kaesemeyer et al., 1999), but recent studies suggested otherwise (Byington et al., 1995; Sacks et al., 1996; O'Driscoll et al., 1997).
Fisslthaler et al. (2007) reported, for the first time, the expression of the extrahepatic HMG CoA reductase in HUVECs, as demonstrated in our present study. However, the protein expression of this enzyme in human-isolated cardiovascular tissues, as illustrated in this study, has never been reported. More importantly, the biochemical existence of the extrahepatic HMG CoA reductase in human cardiovascular tissues (Figure 1a) is probably responsible for the reported pleiotropic effects of statins in the cardiovascular systems. However, the physiological significance of the differential expression of HMG CoA reductase in different cardiovascular tissues remains to be determined.
On the other hand, an alteration of caveolin-1 expression in HUVECs has been attributed to the reported pleiotropic effect of statins (Feron et al., 2001). Hence, the expression of HMG CoA reductase after simvastatin incubation was studied. However, in our study using pig coronary arteries (endothelium removed), simvastatin caused no change in caveolin-1 and HMG CoA reductase expression (protein), questioning the role of changes in caveloin-1 and HMG CoA reductase expression in mediating the simvastatin-elicited responses.
It is interesting to point out that some statins (cerivastatin and atorvastatin) possess a biphasic dose-dependent effect on endothelial cell angiogenesis (Weis et al., 2002). In our study, simvastatin (100 nM) enhanced, whereas higher concentrations of simvastatin (1, 3 and 10 μM) inhibited the BKCa amplitude. In patients who are taking simvastatin alone, the peak plasma level of this agent is probably in the nanomolar (10–100 nM) ranges. However, it is not uncommon for patients with cardiovascular diseases receiving other drugs (for example, diltiazem and cyclosporin) which can change the CYP450 3A4 activity (a hepatic enzyme isoform responsible for statin metabolism), and an altered metabolism of statin has been suggested as a cause of various adverse effects (Worz and Bottorff, 2001; Williams and Feely, 2002).
Taking advantage of the existence of different forms of simvastatin – simvastatin itself, hydrophobic and membrane permeable; and the salt, simvastatin Na+, hydrophilic and membrane impermeable, we evaluated the site(s) of location of action (extracellular versus intracellular) of this statin. In our study, acute extracellular application of simvastatin (1, 3 and 10 μM), but not simvastatin Na+, caused a concentration-dependent inhibition of the BKCa amplitude with a maximum inhibition occurring at ∼2 min after drug administration and the inhibition persisting after washout. Therefore, our results suggest that the incorporation into/penetration of the plasma membrane by simvastatin is essential for the inhibition of BKCa channels. In addition, the enhancement of the BKCa amplitude by isopimaric acid (a novel activator of the BKCa channels α-subunit) (Imaizumi et al., 2002) was abolished by simvastatin, but not by simvastatin Na+ (applied externally). Interestingly, an intracellular application of simvastatin Na+ (10 μM, in the pipette solution) markedly suppressed isopimaric acid-induced enhancement of the BKCa amplitude. Taken together, our results suggest that the site of location is probably at the cytoplasmic side/cytosol (for example, sarco(endo)plasmic reticulum) (Hampton et al., 1996). A study is currently underway to evaluate, in detail, the modulation of BKCa channels by simvastatin (under the basal and isopimaric acid-stimulated conditions) at the single-channel level.
Simvastatin (1 and 3 μM)-mediated inhibition of BKCa amplitude was abolished by GSH, NADPH and NADH (all are physiological reductants), but not by DTT (a non-physiological reductant), arguing the simvastatin-induced response is merely a phenomenon of a simple reduction/oxidation of some BKCa channel components and/or HMG CoA reductase. In addition, simvastatin (1 and 3 μM) is acting through the classical HMG CoA reductase mevalonate cascade, as mevalonolactone and GGPP abolished the simvastatin-mediated inhibitory response. However, the participation of the cholesterol synthesis pathway could not be examined, as squalene (an intermediate in the biosynthesis of endogenous cholesterol) is immiscible (a triterpene with a specific gravity of 0.855) with all solutions (external recording solutions and internal pipette solution) used.
Bregestovski and co-workers (Bolotina et al., 1989; Bregestovski et al., 1989) reported that both 2-decenoic acid (increases membrane fluidity and cholesterol level) and mevinolin (an inhibitor of endogenous cholesterol synthesis) caused a marked increase in the open probability of the KCa channels in cultured human aortic smooth muscle cells and rabbit aortic smooth muscle cells, respectively. In our study, effects of simvastatin (1, 3 and 10 μM) on BKCa channels may not be simply due to a biophysical change of the plasma membrane fluidity/cholesterol, as our results clearly demonstrate the involvement of various cytosolic signalling (GSH, NADPH and PKC) pathways.
In most vascular smooth muscles, including coronary artery (Minami et al., 1993; Schubert et al., 1999; Barman et al., 2004), activation of PKC inhibits the BKCa channels. In human foreskin fibroblasts, PKC activation increased the level of ER cholesterol (Lange et al., 2002), and HMG CoA reductase is an integral membrane protein of ER (Hampton et al., 1996). In rat liver, PKC-mediated phosphorylation resulted in an inhibition of HMG CoA reductase activity (Beg et al., 1985). Simvastatin (2.5 μM for 24 h) caused cell growth arrest of rat C6 glioma cells with a significant increase of total PKC activity (Soma et al., 1994). In our study, both simvastatin and PMA (a well-known PKC activator) elicited a rottlerin (a potent PKC-δ isoform blocker)-sensitive inhibition of the BKCa channels suggesting the participation of the PKC-δ cascade. However, the involvement of PKC-δ translocation, as reported previously (Sirous et al., 2001), was not obvious in our study, as simvastatin (10 μM) caused an increase in PKC-δ expression in both cytosol and particulate fractions, with a relatively constant ratio (cytosol/particulate: 0.56–0.67). Our results suggested that the increased protein expression of PKC-δ in response to simvastatin (10 μM) challenge was not due to the newly synthesized PKC-δ protein, as a similar trend of increase of PKC-δ expression was recorded after cycloheximide (10 μM, a protein synthesis inhibitor) pre-treatment (for 2 h). Hence, the source(s) of the ‘newly recruited' PKC-δ protein in response to simvastatin challenge remains to be determined. Perhaps, the suppressive effect of rottlerin on simvastatin-mediated response (that is an increase in PKC-δ protein expression) is acting on constitutive or basal PKC-δ. In addition, the involvement of putative reactive oxygen species (ROS) pathways, that has been reported previously, in mediating the effects of statins are ruled out, as a range of ROS modulators (for example, apocynin, DPI and Tiron) failed to alter simvastatin-induced inhibition of the BKCa currents.
In conclusion, we have demonstrated the biochemical existence of extrahepatic HMG CoA reductase in different cardiovascular tissues of both human and pig. Acute simvastatin (100 nM) slightly enhanced, whereas simvastatin (⩾1 μM) inhibited the BKCa amplitude of pig coronary artery smooth muscle cells. The classical HMG CoA reductase mevalonate cascade is important in mediating the inhibitory effect of simvastatin observed at low concentrations (1 and 3 μM), whereas an increased PKC-δ protein expression is important in simvastatin (10 μM)-mediated inhibition of BKCa channels of the coronary artery.
Acknowledgments
We thank the excellent assistance provided by nurses of the operation rooms of The Prince of Wales Hospital and The United Christian Hospital (Hong Kong SAR, PR of China). Provision of the HepG2 cells by Professor John HK Yeung (Department of Pharmacology, The Chinese University of Hong Kong) is acknowledged. This work was financially supported (to YW Kwan) by the UGC Earmarked Grant of Hong Kong SAR, PR of China (Ref. nos.: 4107/01M and 4166/02M), the Croucher Foundation (Hong Kong) and Direct Grants for Research (Faculty of Medicine, The Chinese University of Hong Kong) (Ref no.: 2401149 and Project code: 2041296). SW Seto and TY Lam are recipients of post-graduate studentships of the Department of Pharmacology (The Chinese University of Hong Kong, Hong Kong SAR, PR of China).
Abbreviations
- BKCa channels
iberiotoxin-sensitive Ca2+-activated K+ channels
- [Ca2+]i
intracellular calcium ion concentration
- DPI
diphenyleneiodonium chloride
- DTT
dithiothreitol
- eNOS
endothelial NO synthase
- ER
endoplasmic reticulum
- GGPP
geranylgeranyl pyrophosphate
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HepG2
human hepatocarcinoma cells
- HMG CoA
3-hydroxy-3-methyl-glutaryl coenzyme A
- HUVECs
human umbilical vein endothelial cells
- ISOPA
isopimaric acid
- NADH
β-nicotinamide adenine dinucleotide phosphate disodium salt
- NADPH
β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetra-sodium salt; NS 1619, 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- PD 98059
2′-amino-3′-methoxyflavone
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- ROS
reactive oxygen species
- SB 203580
4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole
- Tiron
4,5-dihydroxy-1,3-benzenedisulphonic acid, disodium salt monohydrate
Conflict of interest
The authors state no conflict of interest.
References
- Alvarez de Sotomayor M, Perez-Guerrero C, Herrera MD, Marhuenda E. Effect of simvastatin on vascular smooth muscle responsiveness: involvement of Ca2+ homeostasis. Eur J Pharmacol. 2001;415:217–224. doi: 10.1016/s0014-2999(01)00819-6. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Turley SD, Dietschy JM. Relative rates of sterol synthesis in the liver and various extrahepatic tissues of normal and cholesterol-fed rabbits. Relationship to plasma lipoprotein and tissue cholesterol levels. Biochim Biophys Acta. 1982;711:421–430. doi: 10.1016/0005-2760(82)90056-x. [DOI] [PubMed] [Google Scholar]
- Au AL, Kwok CC, Lee AT, Kwan YW, Lee MM, Zhang RZ, et al. Activation of iberiotoxin-sensitive, Ca2+-activated K+ channels of porcine isolated left anterior descending coronary artery by diosgenin. Eur J Pharmacol. 2004;502:123–133. doi: 10.1016/j.ejphar.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Au ALS, Kwan YW, Kwok CC, Zhang RZ, He GW. Mechanisms responsible for the in vitro relaxation of ligustrazine on porcine left anterior descending coronary artery. Eur J Pharmacol. 2003;468:199–207. doi: 10.1016/s0014-2999(03)01691-1. [DOI] [PubMed] [Google Scholar]
- Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol. 2004;286:L1275–L1281. doi: 10.1152/ajplung.00259.2003. [DOI] [PubMed] [Google Scholar]
- Beg ZH, Stonik JA, Brewer HB., Jr Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and modulation of its enzymic activity by calcium-activated and phospholipid-dependent protein kinase. J Biol Chem. 1985;260:1682–1687. [PubMed] [Google Scholar]
- Bergdahl A, Persson E, Hellstrand P, Sward K. Lovastatin induces relaxation and inhibits L-type Ca2+ current in the rat basilar artery. Pharmacol Toxicol. 2003;93:128–134. doi: 10.1034/j.1600-0773.2003.930304.x. [DOI] [PubMed] [Google Scholar]
- Bogman K, Peyer AK, Torok M, Kusters E, Drewe J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br J Pharmacol. 2001;132:1183–1192. doi: 10.1038/sj.bjp.0703920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina V, Omelyanenko V, Heyes B, Ryan U, Bregestovski P. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 1989;415:262–268. doi: 10.1007/BF00370875. [DOI] [PubMed] [Google Scholar]
- Bregestovski PD, Bolotina VM, Serebryakov VN. Fatty acid modifies Ca2+-dependent potassium channel activity in smooth muscle cells from the human aorta. Proc Royal Soc Lond B. 1989;237:259–266. doi: 10.1098/rspb.1989.0048. [DOI] [PubMed] [Google Scholar]
- Byington RP, Jukema JW, Salonen JT, Pitt B, Bruschke AV, Hoen H, et al. Reduction in cardiovascular events during pravastatin therapy. Pooled analysis of clinical events of the Pravastatin Atherosclerosis Intervention Program. Circulation. 1995;92:2419–2425. doi: 10.1161/01.cir.92.9.2419. [DOI] [PubMed] [Google Scholar]
- Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J Nutr. 2006;136:1472–1476. doi: 10.1093/jn/136.6.1472. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Wilson DE, Wood LC, Kwong LK, Moser AH, Grunfeld C. Diabetes increases hepatic hydroxymethyl glutaryl coenzyme A reductase protein and mRNA levels in the small intestine. Metabolism. 1994;43:450–454. doi: 10.1016/0026-0495(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Feron O, Dessy C, Desager JP, Balligand JR. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I, Keseru B, Walsh K, Buss R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100:e12–e21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- Futterman LG, Lemberg L. Statin pleiotropy: fact or fiction. Am J Crit Care. 2004;13:244–249. [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Comm. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Hampton RY, Gardner RG, Rine J. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell. 1996;7:2029–2044. doi: 10.1091/mbc.7.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Sakamoto K, Yamada A, Hotta A, Ohya S, Muraki K. Molecular basis of pimarane compounds as novel activators of large-conductance Ca2+-activated K+ channel alpha-subunit. Mol Pharmacol. 2002;62:836–846. doi: 10.1124/mol.62.4.836. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999;33:234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- Kajinami K, Mabuchi H, Saito Y. NK-104: a novel synthetic HMG-CoA reductase inhibitor. Expert Opin Invest Drugs. 2000;9:2653–2661. doi: 10.1517/13543784.9.11.2653. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105:933–938. doi: 10.1161/hc0802.104283. [DOI] [PubMed] [Google Scholar]
- Lange Y, Ye J, Steck TL. Effect of protein kinase C on endoplasmic reticulum cholesterol. Biochem Biophys Res Commun. 2002;290:488–493. doi: 10.1006/bbrc.2001.6156. [DOI] [PubMed] [Google Scholar]
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Minami K, Fukuzawa K, Nakaya Y, Zeng XR, Inoue I. Mechanism of activation of the Ca2+-activated K+ channel by cyclic AMP in cultured porcine coronary artery smooth muscle cells. Life Sci. 1993;53:1129–1135. doi: 10.1016/0024-3205(93)90549-i. [DOI] [PubMed] [Google Scholar]
- Ndiaye M, Chataigneau T, Andriantsitohaina R, Stoclet JC, Schini-Kerth VB. Red wine polyphenols cause endothelium-dependent EDHF-mediated relaxations in porcine coronary arteries via a redox-sensitive mechanism. Biochem Biophys Res Commun. 2003;310:371–377. doi: 10.1016/j.bbrc.2003.09.028. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Taylor RR. Simvastatin, a HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Pourati I, Kimmelstiel C, Rand W, Karas RH. Statin use is associated with enhanced collateralization of severely diseased coronary arteries. Am Heart J. 2003;146:876–881. doi: 10.1016/S0002-8703(03)00413-7. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Zaib F, Samson SE, Kwan CY, Grover AK. Peroxynitrite resistance of sarco/endoplasmic reticulum Ca2+ pump in pig coronary artery endothelium and smooth muscle. Cell Calcium. 2004;36:77–82. doi: 10.1016/j.ceca.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66:1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Sirous ZN, Fleming JB, Khalil RA. Endothelin-1 enhances eicosanoids-induced coronary smooth muscle contraction by activating specific protein kinase C isoforms. Hypertension. 2001;37:497–504. doi: 10.1161/01.hyp.37.2.497. [DOI] [PubMed] [Google Scholar]
- Soma MR, Baetta R, Bergamaschi S, De Renzis MR, Davegna C, Battaini F, et al. PKC activity in rat C6 glioma cells: changes associated with cell cycle and simvastatin treatment. Biochem Biophys Res Commun. 1994;200:1143–1149. doi: 10.1006/bbrc.1994.1570. [DOI] [PubMed] [Google Scholar]
- Sonmez Uydes-Dogan B, Topal G, Takir S, Ilkay Alp F, Kaleli D, Ozdemir O. Relaxant effects of pravastatin, atorvastatin and cerivastatin on isolated rat aortic rings. Life Sci. 2005;76:1771–1786. doi: 10.1016/j.lfs.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Spady DK, Dietschy JM. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983;24:303–315. [PubMed] [Google Scholar]
- Tammaro P, Smith AL, Hutchings SR, Smirnov SV. Pharmacological evidence for a key role of voltage-gated K+ channels in the function of rat aortic smooth muscle cells. Br J Pharmacol. 2004;143:303–317. doi: 10.1038/sj.bjp.0705957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terata Y, Saito T, Fujiwara Y, Hasegawa H, Miura H, Watanabe H, et al. Pitavastatin inhibits upregulation of intermediate conductance calcium-activated potassium channels and coronary arteriolar remodeling induced by long-term blockade of nitric oxide synthesis. Pharmacology. 2003;68:169–176. doi: 10.1159/000070455. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Frohlich BH, Gregg RE. Differential effects of pravastatin, simvastatin, and atorvastatin on Ca2+ release and vascular reactivity. J Cardiovasc Pharmacol. 1999;34:95–101. doi: 10.1097/00005344-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Turley SD, Andersen JM, Dietschy JM. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981;22:551–569. [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Williams D, Feely J. Pharmacokinetic–pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–370. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- Worz CR, Bottorff M. The role of cytochrome P450-mediated drug–drug interactions in determining the safety of statins. Expert Opin Pharmacother. 2001;2:1119–1127. doi: 10.1517/14656566.2.7.1119. [DOI] [PubMed] [Google Scholar]
- Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol. 1999;126:1205–1213. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]