Abstract
Background and purpose:
Retinal complications may be encountered during the development of hypertension as a response to oxidative stress. Statins may reduce the risk of developing hypertension and ocular diseases. We evaluate the effects of rosuvastatin (ROSU) on retinal functionality and oxidative stress levels in normotensive and spontaneously hypertensive rats (SHR).
Experimental approach:
Wistar Kyoto (WKY) and SHR were treated for 3 weeks with rosuvastatin (10 mg kg−1 day−1). Electroretinograms (ERG) were recorded before and after rosuvastatin treatment. Reactive oxygen species (ROS) were determined in the retina with dihydroethidium staining and NAD(P)H oxidase activity was evaluated.
Key results:
Retinal ganglion cell ROS and retinal NAD(P)H oxidase activity were higher in SHR than in WKY rats, respectively (17.1±1.1 vs 10.2±1.2 AU, P<0.01; 38095±8900 vs 14081±5820 RLU mg−1; P<0.05). The ERG b-wave amplitude in SHR was significantly lower than that in WKY rats. Rosuvastatin reduced SBP in SHR but did not change plasma lipid levels. Rosuvastatin treatment in SHR significantly decreased ROS levels (11.2±1.3, P<0.01), NAD(P)H activity in retinal ganglion cells (9889±4290; P<0.05), and increased retinal plasmalogen content in SHR, but did not modify the ERG response.
Conclusions and implications:
Rosuvastatin, beyond lowering cholesterol levels, was able to lower ROS in the retina induced by hypertension, but without improving retinal function in SHR. These findings point to a complex relationship between ROS in the pathogenesis of retinal disease and hypertension.
Keywords: oxidative stress, electroretinogram, retina, hypertension, HMGCoA reductase, NAD(P)H oxidase, statin
Introduction
Oxidative stress underlies the development and the persistence of several cardiovascular and ocular diseases such as hypertension and retinopathies (Sagar et al., 1992; Du et al., 2003; Moreno et al., 2004; Touyz, 2004). Superoxide anion, produced by mitochondria and oxidases, is of particular interest because it can react with nitric oxide (NO) to produce peroxynitrite, a toxic oxidative species known to have deleterious effects (Halliwell et al., 1999), including lipid peroxidation and cytotoxicity (Chemtob et al., 1995). Moreover, in the major models of hypertension, including spontaneously hypertensive rats (SHR), reduced NO availability has been implicated as a major cause of endothelial dysfunction in hypertension (Schiffrin et al., 2000) and an increase in arterial blood pressure is associated with production of free radicals in many organs (Touyz, 2004).
The development of eye diseases, associated with increased oxidative stress, is relatively common in patients with elevated blood pressure (Ciulla et al., 2003). For instance, signs of hypertensive retinopathy, from arteriolar narrowing to isolated microaneurysms and haemorrhages are strongly related to blood pressure, but inconsistently associated with plasma cholesterol levels. Indeed, the retina is constantly exposed to several sources of oxygen species (UV radiation, high mitochondrial activity) and contains a high level of polyunsaturated fatty acids, making it more susceptible to lipid peroxidation (SanGiovanni and Chew, 2005). It has been suggested that, in the eye, oxygen-derived free radicals play a role in diseases such as cataracts, uveitis, age-related macular degeneration (AMD) (Austin and Mamdani, 2005) or glaucoma (Moreno et al., 2004). Retinal cells are equipped with multiple reactive oxygen species (ROS) scavengers, including enzymes such as endogenous peroxidases (Kortuem et al., 2000). Statins are inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase, and are widely used for their potent properties to reduce cholesterol levels in humans. However, their beneficial effects go beyond this pharmacological definition, as it has been shown that, among many other effects, they are able to exert anti-inflammatory and antioxidant properties (Davignon, 2004). For instance, in experimental models, rosuvastatin at 10 mg kg−1 day−1 is able to reduce the development of hypertension (Susic et al., 2003) by increasing NO bioavailability and decreasing oxidative stress (Bayorh et al., 2005). Moreover, recent papers suggest that statins may reduce the risk of developing ocular diseases such as AMD (McGwin et al., 2005) and primary open-angle glaucoma (McGwin et al., 2004). Therefore, statins should be of use in the treatment of hypertension-associated retinal dysfunction. The aim of this study was to assess the role of rosuvastatin, a new hydrophilic HMGCoA reductase inhibitor, on retinal function, lipid composition and oxidative stress level in normotensive and hypertensive rats. In this paper, we are able to show for the first time that retinal functionality in SHR is impaired, relative to that in WKY rats. We also provide data showing increased retinal oxidative stress in SHR can be lowered by treatment with rosuvastatin.
Methods
Animals and treatment
All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and with the French legislation and animal quarters (agreement number A21 231010EA). The animals were housed under controlled temperature (21±1°C) and light conditions (12-h light/12-h dark cycle). The light intensity measured at various locations of the animal quarters was less than 20 lux. Eleven-week-old male adult SHR (200–220 g, n=10) and WKY rats (200–230 g, n=10) purchased from Charles River Laboratories (L'Arbresle, France) were used. They received either vehicle (water) or rosuvastatin (10 mg kg−1 day−1, AstraZeneca, Rueil-Malmaison, France) by gavage for 3 weeks. The rosuvastatin dose was chosen according to previous studies in rats (Susic et al., 2003; Mooradian et al., 2005; Otto et al., 2005; Sicard et al., 2005).
Standard laboratory rat chow (Purina) and tap water were available ad libitum. All animals were allowed to acclimatize for at least 7 days before experimental manipulations.
Systolic blood pressure and heart rate measurement
Systolic blood pressure (SBP) and heart rate (HR) were measured in conscious rats every week using a non-invasive tail-cuff method (Bioseb, Chaville, France). The rats were handled repeatedly and allowed to adapt to the restraint chamber for 3 days before the actual measurements started. A mean of six measurements was considered as one individual SBP.
Electroretinography
The electroretinogram (ERG) was recorded in vivo before rosuvastatin treatment (11 weeks of age) and at the end of the gavage period (14 weeks of age). The following ERG measurement procedures were adapted from those described by Doly et al. (1993). Before ERG recordings, the rats were dark-adapted for at least 3 h. All further procedures were carried out under dim red light (λ>650 nm) and at a constant temperature of 25°C. The animals were anaesthetized with an intramuscular injection of ketamine (120 mg kg−1 body weight) and xylazine (6 mg kg−1 body weight) in a saline solution. The pupils were dilated with 0.5% tropicamide (Ciba Vision Ophthalmics, Blagnac, France). An irrigating solution (BSS, Alcon Laboratories, Rueil Malmaison, France) was used to prevent corneal desiccation. After 10 min, the corneal electrode was put in place. The ERG was recorded via the corneal electrode (thin Ag/AgCl wire with a 3-mm ring end) and a reference Ag/AgCl electrode placed on the rat's tongue. The retina was stimulated by a photostimulator (model PS33 PLUS, Grass Telefactor, Astro-Med Inc., West Warwick, RI, USA) delivering light flashes (white light, 6500 mcds m−2) to the eye through fibre optics and a white sphere that mimics a Ganzfeld dome. One flash was delivered every minute and the average of ten individual ERGs was considered as one measurement. The ERG response was amplified using a low-pass filter setting of 1 Hz and a high-pass filter of 1000 Hz. After amplification, the signal was digitized and processed. The amplitudes were determined for each recording and were measured from the baseline (a-wave) or from the peak of the a-wave (b-wave).
Tissue collection and processing
At the end of the 3-week rosuvastatin treatment, the animals were anaesthetized with sodium thiopental (60 mg kg−1, intraperitoneal) and heparin was intravenously injected (500 IU kg−1). Blood samples were collected from the aorta and immediately centrifuged at 4°C. The plasma was removed, aliquoted, instantaneously frozen in liquid nitrogen and stored at −80°C until assessed. The animals were killed by decapitation. One eye from each animal was enucleated and the retina was removed, immediately frozen in liquid nitrogen and stored at −80°C for lipid analyses and chemiluminescence experiments. The other eye was embedded on OCT (Dako, Trappes, France) for 10 μm-thick cryosections.
In situ detection of superoxide anion
The oxidative fluorescent probe dihydroethidium (DHE, Molecular Probe, Cergy Pontoise, France) was used to localize superoxide anion (Oudot et al., 2006). The freshly frozen eye sections were incubated in a light-protected humidified chamber at room temperature with DHE (5 μM) for 5 min, and then counterstained with a nuclear tracer: 4′,6-diamidino-2-phenylindole (DAPI, 30 μg ml−1, Sigma, Lyon, France). The slides were fixed for 10 min in acetone and were immediately analysed with a computer-based digitizing image system (Microvision, Evry, France) using a fluorescent microscope (Eclipse 600, Nikon, Champigny-Sur-Marne, France) connected to a video camera (Tri CCD, Sony, Paris, France). Fluorescence was detected with 510–560 nm excitation and 590 nm emission filters. Automatic computer-based analysis was performed with the same threshold for all sections (× 500 magnification). Three cell layers were delimited (ganglion cells, bipolar cells and photoreceptor cells). Nucleus quantification was performed in each layer. Results are expressed as DHE/DAPI ratio. To verify the specific detection of superoxide anion with DHE, cryosections from fresh-frozen retinas of WKY and SHR were incubated with superoxide dismutase (SOD, 300 U ml−1, Dako) or apocyanin (APO, 100 μM) for 10 min before DHE incubation.
NAD(P)H oxidase activity measured by lucigenin-enhanced chemiluminescence
The capacity of the retina to produce superoxide anion in a nicotinamide adenine dinucleotide phosphate (NADPH)-dependent way was assessed using an LB 9507 luminometer (Berthold Systems, Aliquippa, PA, USA) by measuring superoxide-enhanced lucigenin (0.5 μM, Sigma) chemiluminescence in the presence or absence of NAD(P)H (30 μM, Sigma) (Sicard et al., 2006). The results are expressed in relative light units per gram of dry tissue. To verify the specific effect of NAD(P)H oxidase and superoxide anion, WKY and SHR retinas were incubated with apocynin (100 μM, Sigma) or superoxide dismutase (300 U ml−1, Dako) before NADPH incubation.
Retinal lipid analyses
Total lipids from the retinas were extracted according to the Folch procedure (Folch et al., 1957) using 2:1 chloroform-methanol (v : v) and 0.73% NaCl in water. One aliquot of total lipids was used to determine the proportions of lipid classes (phospholipids, cholesterol, cholesteryl esters, diacylglycerols, triacylglycerols) using a combination of thin layer chromatography on silica gel-coated quartz rods and a flame ionization detector (Iatroscan System, Iatron, Tokyo, Japan) according to the technique first published by Ackman (1981). The remaining part of total lipids was used to determine retinal fatty acid composition. Total lipids were transesterified with boron trifluoride in methanol (7% w/v) according to Morrison and Smith (Grech et al., 1996). The fatty acid methyl esters (from acyl radicals) and dimethyacetals (DMA, from alkenyl radicals as an index of plasmalogen levels) were analysed on a Hewlett-Packard (Palo Alto, CA, USA) 5890 series II gas chromatograph equipped with a split/splitless injector, a flame ionization detector, and a BPX 70-silica capillary column (120 m × 0.5 mm internal diameter film thickness 0.25 μm; SGE, Melbourne, Australia). The injector and the detector were maintained at 250 and 280°C, respectively. Hydrogen was used as the carrier gas (inlet pressure 300 kPa). The oven temperature was fixed at 60°C for 1 min, then increased from 60 to 200°C at a rate of 20°C min−1 and left at this temperature until the end of the analysis. DMA were identified by comparison with commercial or synthetic standards and data were computed using DIAMIR software (JMBS Ins., Portage, MI, USA).
Determination of plasma cholesterol levels
Total and high-density lipoprotein (HDL) cholesterol as well as triglyceride plasma concentrations were measured in a Cobas Fara analyser (Roche, Basel, Switzerland). Low-density lipoprotein (LDL) cholesterol was calculated with Friedwall methods.
Statistical analysis
All data are expressed as means±s.e.m. Statistical analyses were performed with a two-factor analysis of variance (ANOVA) test (SigmaStat); the two factors were rat strain (SHR vs WKY) and rosuvastatin treatment. ANOVA was followed by inter-group pair-wise comparisons with Tukey HSD multiple comparisons.
Results
Physiological and hemodynamic parameters
Body weights were the same for WKY and SHR rats (Table 1) whether they received rosuvastatin treatment or not. HDL, LDL and total cholesterol levels in plasma were higher in WKY than in SHR (P<0.05). No difference was observed between WKY and SHR for triglyceride plasma levels. Rosuvastatin treatment did not modify plasma cholesterol and triglyceride levels in WKY or SHR. SBPs were dramatically higher in SHR than in WKY (175 mm Hg±2 vs 131±1 mm Hg, P<0.001). In SHR, 3 weeks of rosuvastatin treatment decreased SBP by 18 mm Hg (P<0.01). HR was similar for SHR and WKY animals, and was not modified by rosuvastatin treatment.
Table 1.
Body weight and plasma lipid levels of WKY and SHR after 3 weeks of treatment with rosuvastatin
| Rats treatments |
WKY |
SHR |
||
|---|---|---|---|---|
| Control | ROSU | Control | ROSU | |
| Rat weight (g) | 301±6 | 290±5 | 298±3 | 296±6 |
| Total cholesterol (mg l−1) | 710±30 | 690±20 | 520±20* | 480±10 |
| HDL (mg l−1) | 360±10 | 350±10 | 280±10* | 280±10 |
| LDL (mg l−1) | 260±20 | 230±10 | 150±20* | 130±10 |
| Triglycerides (mg l−1) | 450±60 | 530±70 | 480±60 | 580±50 |
Abbreviations: WKY, Wistar Kyoto; SHR, spontaneously hypertensive rats; HDL, high-density lipoprotein; LDL, low-density lipoprotein, ROSU, rosuvastatin.
Results are presented as means±s.e.m.
P<0.05 SHR control vs WKY control; n=10 for each group.
Retinal function
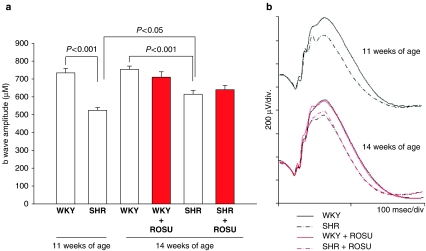
Before any treatment (11 weeks of age), the ERG b-wave amplitude was significantly lower in SHR than in WKY (Figure 1). This was maintained at 14 weeks, even though a significant increase in the ERG b-wave amplitude was observed in SHR rats between 11 and 14 weeks of age (P<0.05). Treatment with rosuvastatin did not modify the ERG b-wave amplitudes in SHR or WKY groups. No changes were observed in the oscillatory potentials (OPs) in the recorded ERGs. No variations were observed in the ERG a-wave amplitude whatever the animal strain, the age and the gavage administered (data not shown).
Figure 1.
(a) ERG b-wave amplitudes of WKY and SHR at 11 and 14 weeks of age treated with vehicle (water) or ROSU (10 mg kg−1 day−1 for 3 weeks). Results are expressed as means±s.e.m. (n=10 for each group). (b) Representative ERG traces of WKY and SHR at 11 and 14 weeks of age treated with vehicle (water) or rosuvastatin. ERG, electroretinogram; ROSU, rosuvastatin; WKY, Wistar Kyoto; SHR, spontaneously hypertensive rats.
Retinal oxidative stress
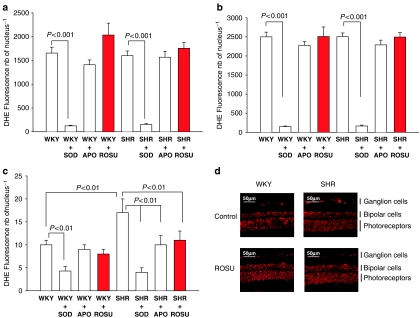
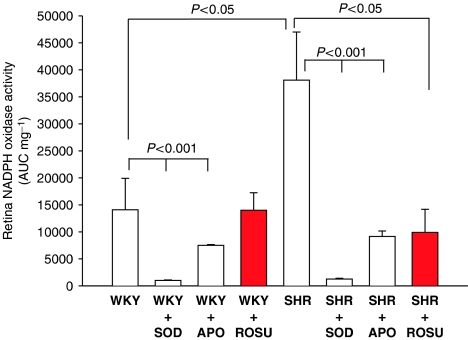
Superoxide anion production was analysed in the three retinal cell layers: photoreceptors (Figure 2a), bipolar cells (Figure 2b) and in ganglion cells (Figure 2c). No differences were observed in photoreceptors and in bipolar cells in SHR, WKY with or without rosuvastatin treatment. However, markers of superoxide anion production were 40% higher in SHR ganglion cells than in those from WKY (P<0.01). Rosuvastatin treatment for 3 weeks decreased superoxide anion production in SHR ganglion cells (P<0.01). No differences were observed in the other retinal layers of SHR and WKY rats. Retinal NAD(P)H oxidase activity (Figure 3) in SHR was more than two-fold higher in WKY rats. Rosuvastatin treatment for 3 weeks significantly decreased retinal NAD(P)H oxidase activity in SHR, without affecting that of WKY rats.
Figure 2.
Effect of ROSU (10 mg kg−1 day−1 for 3 weeks) on DHE fluorescence intensity (a) in photoreceptor cells (b) bipolar cells and (c) ganglion cells (d). Localization of this fluorescence on frozen eye sections. The slices were incubated in vitro with or without SOD or APO. Results are expressed as means±s.e.m. (n=10 for each group). ROSU, rosuvastatin; DHE, dihydroethidium; SOD, superoxide dismutase; APO, apocyanin.
Figure 3.
Effect of ROSU (10 mg kg−1 day−1 for 3 weeks) on retinal NAD(P)H oxidase activity evaluated with lucigenin in WKY and SHR. The retinas were incubated in vitro with or without SOD or APO. Results are expressed as means±s.e.m. (n=10 for each group). ROSU, rosuvastatin; WKY, Wistar Kyoto; SHR, spontaneously hypertensive rats; SOD, superoxide dismutase; APO, apocyanin.
Retinal lipid composition
Proportions of retinal lipid classes in rosuvastatin-treated and non-treated WKY and SHR rats are presented in Table 2. In retinas, phospholipids were the major lipid class with approximately 87% of total lipids. The remaining lipids were exclusively cholesterol in the free and esterified forms; no diacylglycerols or free fatty acids were detected. No differences were observed between treated and non-treated WKY and SHR for total saturated, total monounsaturated and total polyunsaturated fatty acid levels (data not shown). Among the polyunsaturated fatty acids, the levels of docosahexaenoic acid (DHA; 22:6 n−3) were found to be unaffected by the animal strain and by the rosuvastatin treatment (data not shown).
Table 2.
Retinal phospholipid, free cholesterol and esterified cholesterol levels of SHR and WKY treated or not with rosuvastatin for 3 weeks (% of total lipids)
| Rats treatments |
WKY |
SHR |
||
|---|---|---|---|---|
| Control | ROSU | Control | ROSU | |
| Phospholipids | 88.7±0.8 | 86.2±1.2 | 86.9±0.6 | 87.2±1.3 |
| Free cholesterol | 7.9±0.7 | 9.4±0.6 | 8.7±0.6 | 8.1±0.6 |
| Esterified cholesterol | 3.3±0.4 | 4.5±0.7 | 4.3±0.9 | 4.8±1.0 |
| Phospholipids/total cholesterol | 7.6±0.6 | 6.3±0.5 | 6.9±0.4 | 6.4±0.7 |
Abbreviations: SHR, spontaneously hypertensive rats; WKY, Wistar Kyoto; ROSU, rosuvastatin.
Results are mean±s.e.m (n=6 for each group).
The retinal plasmalogen composition (DMA 16:0/C16:0 and DMA 18:0/C18:0) of treated and non-treated WKY and SHR is presented in Table 3. No differences were observed between non-treated WKY and SHR animals for DMA 16:0/C16:0 and DMA 18:0/C18:0 ratios. However, rosuvastatin treatment increased DMA C16:0/C16:0 by 10% and DMA C18:0/C18:0 by 5% in SHR as compared to SHR control (P<0.05), due to increased DMA C16:0 and DMA C18:0 concentrations (P<0.05).
Table 3.
Retinal composition in plasmalogen composition in SHR and WKY treated or not with rosuvastatin for 3 weeks (% of total fatty acids)
| Rats Treatments |
WKY |
SHR |
||
|---|---|---|---|---|
| Control | ROSU | Control | ROSU | |
| Plasmalogens | ||||
| DMA 160 | 0.66±0.03 | 0.65±0.01 | 0.71±0.02 | 0.79±0.01* |
| C160 | 16.12±0.65 | 15.48±0.33 | 15.72±0.07 | 15.87±0.17 |
| DMA 160/C160 | 0.041±0.002 | 0.042±0.001 | 0.045±0.001 | 0.050±0.001* |
| DMA 180 | 2.43±0.04 | 2.48±0.02 | 2.36±0.02 | 2.47±0.04* |
| C180 | 21.91±0.20 | 22.02±0.06 | 21.51±0.13 | 21.57±0.08 |
| DMA 180/C180 | 0.111±0.002 | 0.113±0.001 | 0.110±0.001 | 0.114±0.001 |
Abbreviations: SHR, spontaneously hypertensive rats; WKY, Wistar Kyoto; DAM, dimethyacetals; ROSU, rosuvastatin.
Results are mean±s.e.m.
SHR control vs SHR ROSU, P<0.05 (n=6 for each group).
Discussion
Our study demonstrated that (1) the retinal functionality was lower in SHR than in WKY controls, (2) SHR displayed an increase in superoxide anion production in retinal ganglion cells, an increase in NAD(P)H oxidase activity and (3) that rosuvastatin was able to decrease these markers of retinal oxidative stress in SHR but has no effect on the retinal functionality.
SHR is a genetically engineered/selected model of hypertension and appears to have some visual dysfunctions (Rogers et al., 1993) caused by retinal capillary remodelling (Bhutto and Amemiya, 1997; Dosso et al., 1999) and probably related to increased blood pressure. Bellini et al. (1995) demonstrated that the electrical activity of the retina is altered early in the course of hypertension in humans. Population based-studies show that hypertensive retinopathy signs are strongly associated with blood pressure, but inconsistently associated with cholesterol and other risk factors of atherosclerosis (Yu et al., 1998; Wong and McIntosh, 2005). Hypertension leads to dysfunction of many organs including the eye, which is affected by retinal artery occlusion (Klein et al., 1997).
Electroretinography is an accurate method to record retinal electrical activity and gives more relevant data on retinal cells dysfunction than other techniques such as light discrimination and visual acuity measurement, used to characterize the visual function of SHR (Rogers et al., 1993). Rogers et al. (1993) suggested that the reason for retinal dysfunction in SHR was a reduced number of photoreceptors. Loss of photoreceptors is linked to a reduction in ERG a-wave amplitude (Machida et al., 2000). As we did not observe any differences in the ERG a-wave amplitude between SHR and WKY, our results do not support this hypothesis. However, we clearly observed a retinal functional defect in SHR that may be linked to dysfunction of the inner retinal cell layers, mainly amacrine and bipolar cells, since the b-wave is generated by the response of these cells (Bui and Fortune, 2004). Nevertheless the mechanisms for this finding remain partly unresolved. Studies suggested that the amplitude of the b-wave can be in some situations an indicator of retinal oxygenation (Block and Schwarz, 1998; Luhmann et al., 2005). It is now well known that OPs are one of the most sensitive parameters to be affected in retinal ischemia (Wachtmeister, 1998; Tzekov and Arden, 1999). However, in our studies, we were not able to show any modification in OPs of SHR when compared to WKY.
Two experimental studies have suggested that retinal damage induced by ischemia/reperfusion in SHR was closely related to oxidative stress and was reduced by antioxidant treatment such as superoxide dismutase (Szabo et al., 1992; Hirose et al., 2004). While it is well known that SHR exhibit increased levels of oxidative stress in several organs including the kidney, vessels (Rodriguez-Iturbe et al., 2003; Touyz, 2004) and the brain (Ikeda et al., 2003), no studies have evaluated the basal oxidative stress level in SHR retinas. Our study showed, for the first time, that superoxide anion production was higher in SHR ganglion cell layers than in WKY rats and that this was associated with an increase in NAD(P)H oxidase activity. NAD(P)H oxidase activity is known to be upregulated in SHR organs (vessels, heart, kidneys) (Griendling et al., 2000; Rodriguez-Iturbe et al., 2003), and could play an important role in the development of hypertension. Only a few results are available on the role of NAD(P)H oxidase in the retina. Al-Shabrawey et al. (2005) demonstrated that NAD(P)H oxidase activity was required for any hypoxia-stimulated increase in vascular endothelial growth factor expression and retinal neovascularization. The oxidative damage resulted in cell death, in part by apoptosis, causing morphologic changes in the retina and a decline in retina function (Cingolani et al., 2006).
Lipid composition in the retina, in particularly the level of DHA, and the balance between n−6 and n−3 fatty acids are very important for visual functioning (SanGiovanni and Chew, 2005). Chronic dietary DHA deficiency alters the recovery of rod photoreceptor response in rodents and in nonhuman primates (Jeffrey et al., 2002; Niu et al., 2004). The ERG a-wave and b-wave could also be defective in rats consuming a balanced diet containing small amounts of n−3 polyunsaturated fatty acids with isomerized double bonds (Acar et al., 2002), thus reinforcing the crucial role of DHA in retinal functionality. In our models, there was no difference between SHR and WKY retinas with regard to lipid content including DHA, plasmalogen and the n−6/n−3 ratio.
In our experimental conditions, the animals were given 10 mg kg−1 day−1 of rosuvastatin and this treatment did not lower plasma LDL, HDL, total cholesterol or triglyceride levels. These results confirm other studies showing that chronic rosuvastatin treatment (3 months) with the same concentration did not modify plasma cholesterol levels (Susic et al., 2003). Furthermore, Davignon (2004) showed that the anti-inflammatory, antiaggregant and antioxidant effects of statin therapy were independent of the plasma cholesterol lowering effects.
In contrast, we observed an antihypertensive effect of rosuvastatin in the SHR model from the first week of treatment as has already been described by our group and others (Susic et al., 2003; Sicard et al., 2005). Statin therapy decreases inducible NO synthase and increases endothelial NO synthase expression in the vascular wall, which contributes to an enhancement of NO availability and can therefore restore endothelial function in hypertensive rats (Bayorh et al., 2005). A small, but possibly clinically relevant, reduction in blood pressure associated with statin therapy has been reported in a number of studies; this effect has been observed in hypertensive patients (Glorioso et al., 1999; Borghi et al., 2000; Ferrier et al., 2002).
To our knowledge, there are no studies available on the effect of rosuvastatin treatment in retinal function and oxidative stress levels. In SHR, rosuvastatin was able to decrease the oxidative stress, demonstrated by the reduction in NAD(P)H oxidase activity in the ganglion cells. This effect was not associated with a decrease in retinal cholesterol levels. These results are in accordance with those of Erdos et al. (2006), who demonstrated that rosuvastatin inhibited specific NAD(P)H oxidase activity. However, rosuvastatin did increase phospholipid plasmalogens by 10% for DMA C16:0/C16:0 and by 5% for DMA C18:0/C18:0. Previous studies have suggested that plasmalogens may be protective by serving as antioxidants during lipoprotein oxidation (Zoeller et al., 1988; Engelmann, 2004). Lovastatin increased the plasmalogen portion in erythrocyte membrane phospholipids (Brosche et al., 1996). The increase in plasmalogen concentration in the retina of SHR treated with rosuvastatin could then be taken as a reinforcement of antioxidant defences.
Ko et al. (2005) suggested that an elevation of antioxidant concentrations might diminish the increase in ROS, but could not prevent subsequent damage. A number of studies concerning the role of oxidative stress in ocular degenerative pathways (Du et al., 2003; Moreno et al., 2004) concluded that the use of antioxidants could be a good therapeutic tool to prevent retinal degeneration. In addition, the relationship between eye diseases such as glaucoma, AMD and the use of cholesterol-lowering drugs has been examined in several clinical studies, but the results have been contradictory (Sen et al., 2002; McGwin et al., 2004, 2005; Wilson et al., 2004). However, some of the results suggest that primary prevention with statins might reduce the risk of developing AMD or retinopathy (Sen et al., 2002; McGwin et al., 2005).
In conclusion, our study demonstrated, for the first time, that the increase in oxidative stress in retinal ganglion cells, if the SHR, was associated with increased NAD(P)H oxidase activity. In this genetically hypertensive model, rosuvastatin therapy could decrease production of retinal superoxide anion through the inhibition of NAD(P)H oxidase activity, independently of a reduction in plasma and tissue cholesterol levels, but might not be sufficient to restore retinal function. These findings point to a complex relationship between oxidative stress in the pathogenesis of retinal disease and hypertension. Additional investigations are warranted to investigate whether statins or other antioxidant agents may provide additional protection against retinal hypertensive diseases, particularly the role of isoprenoid metabolism, in the regulation of fundamental cellular processes in the retina.
Acknowledgments
We are grateful to M Philip Bastable for the English correction. This work was supported in part by grants from the Regional Council of Burgundy, the Faculty of Medicine of Dijon and the French Minister for Research.
Abbreviations
- AMD
age-related macular degeneration
- APO
apocyanin
- DAPI
4′,6-diamidino-2-phenylindole
- DHA
docosahexaenoic acid
- DHE
dihydroethidium
- DMA
dimethyacetals
- ERG
electroretinogram
- HDL
high-density lipoprotein
- HMGCoA
3-hydroxy-3-methylglutaryl-CoA
- HR
heart rate
- LDL
low-density lipoprotein
- OPs
oscillatory potentials
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- SBP
systolic blood pressure
- SOD
superoxide dismutase
- WKY
Wistar Kyoto
Conflict of Interest
The authors state no conflict of interest.
References
- Acar N, Chardigny JM, Bonhomme B, Almanza S, Doly M, Sebedio JL. Long-term intake of trans (n−3) polyunsaturated fatty acids reduces the b-wave amplitude of electroretinograms in rats. J Nutr. 2002;132:3151–3154. doi: 10.1093/jn/131.10.3151. [DOI] [PubMed] [Google Scholar]
- Ackman RG. Flame ionization detection applied to thin-layer chromatography on coated quartz rods. Methods Enzymol. 1981;72:205–252. doi: 10.1016/s0076-6879(81)72013-5. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Mamdani MM. Impact of the pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22/Reversal of Atherosclerosis with Aggressive Lipid Lowering trials on trends in intensive versus moderate statin therapy in Ontario, Canada. Circulation. 2005;112:1296–1300. doi: 10.1161/CIRCULATIONAHA.104.531582. [DOI] [PubMed] [Google Scholar]
- Bayorh MA, Ganafa AA, Eatman D, Walton M, Feuerstein GZ. Simvastatin and losartan enhance nitric oxide and reduce oxidative stress in salt-induced hypertension. Am J Hypertens. 2005;18:1496–1502. doi: 10.1016/j.amjhyper.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bellini G, Bocin E, Cosenzi A, Sacerdote A, Molino R, Solimano N, et al. Oscillatory potentials of the electroretinogram in hypertensive patients. Hypertension. 1995;25:839–841. doi: 10.1161/01.hyp.25.4.839. [DOI] [PubMed] [Google Scholar]
- Bhutto IA, Amemiya T. Vascular changes in retinas of spontaneously hypertensive rats demonstrated by corrosion casts. Ophthalmic Res. 1997;29:12–23. doi: 10.1159/000267986. [DOI] [PubMed] [Google Scholar]
- Block F, Schwarz M. The b-wave of the electroretinogram as an index of retinal ischemia. Gen Pharmacol. 1998;30:281–287. doi: 10.1016/s0306-3623(97)00359-5. [DOI] [PubMed] [Google Scholar]
- Borghi C, Prandin MG, Costa FV, Bacchelli S, Degli Esposti D, Ambrosioni E. Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 2000;35:549–555. doi: 10.1097/00005344-200004000-00006. [DOI] [PubMed] [Google Scholar]
- Brosche T, Kral C, Summa JD, Platt D. Effective lovastatin therapy in elderly hypercholesterolemic patients – an antioxidative impact. Arch Gerontol Geriatr. 1996;22:207–221. doi: 10.1016/0167-4943(95)00694-x. [DOI] [PubMed] [Google Scholar]
- Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol. 2004;555:153–173. doi: 10.1113/jphysiol.2003.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemtob S, Hardy P, Abran D, Li DY, Peri K, Cuzzani O, et al. Peroxide–cyclooxygenase interactions in postasphyxial changes in retinal and choroidal hemodynamics. J Appl Physiol. 1995;78:2039–2046. doi: 10.1152/jappl.1995.78.6.2039. [DOI] [PubMed] [Google Scholar]
- Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA. Retinal degeneration from oxidative damage. Free Radic Biol Med. 2006;40:660–669. doi: 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- Doly M, Droy-Lefaix MT, Braquet P.Retinal electrophysiology and retinotoxicity Advances in Ginkgo biloba Extract Research: Ginkgo biloba Extract (EGb 761) as a Free-Radical Scavenger 1993Paris, France; 61–71.In: Ferradini C, MD-L, Christen Y (eds) [Google Scholar]
- Dosso AA, Leuenberger PM, Rungger-Brandle E. Remodeling of retinal capillaries in the diabetic hypertensive rat. Invest Ophthalmol Vis Sci. 1999;40:2405–2410. [PubMed] [Google Scholar]
- Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Engelmann B. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochem Soc Trans. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290:H1264–H1270. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Glorioso N, Troffa C, Filigheddu F, Dettori F, Soro A, Parpaglia PP, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–1286. doi: 10.1161/01.hyp.34.6.1281. [DOI] [PubMed] [Google Scholar]
- Grech E, Dodd N, Jackson M, Morrison W, Faragher E, Ramsdale D. Evidence for free radical generation after primary percutaneous transluminal coronary angioplasty recanalization in acute myocardial ischemia. Am J Cardiol. 1996;77:122–127. doi: 10.1016/s0002-9149(96)90580-9. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Zhao K, Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: a personal view of recent controversies. Free Radic Res. 1999;31:651–669. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]
- Hirose F, Kiryu J, Miyamoto K, Nishijima K, Miyahara S, Katsuta H, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004;43:1098–1102. doi: 10.1161/01.HYP.0000123069.02156.8a. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Negishi H, Yamori Y. Antioxidant nutrients and hypoxia/ischemia brain injury in rodents. Toxicology. 2003;189:55–61. doi: 10.1016/s0300-483x(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Jeffrey BG, Mitchell DC, Gibson RA, Neuringer M. n−3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2806–2814. [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE.The relation of systemic hypertension to changes in the retinal vasculature: the Beaver Dam Eye Study Trans Am Ophthalmol Soc 199795329–348.discussion 348–350 [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39:365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Kortuem K, Geiger LK, Levin LA. Differential susceptibility of retinal ganglion cells to reactive oxygen species. Invest Ophthalmol Vis Sci. 2000;41:3176–3182. [PubMed] [Google Scholar]
- Luhmann UF, Lin J, Acar N, Lammel S, Feil S, Grimm C, et al. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, et al. P23 H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41:3200–3209. [PubMed] [Google Scholar]
- McGwin G, Jr, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122:822–826. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]
- McGwin G, Jr, Xie A, Owsley C. The use of cholesterol-lowering medications and age-related macular degeneration. Ophthalmology. 2005;112:488–494. doi: 10.1016/j.ophtha.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes. 2005;54:2977–2982. doi: 10.2337/diabetes.54.10.2977. [DOI] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, Salem N, Jr, et al. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n−3 fatty acid deficiency. J Biol Chem. 2004;279:31098–31104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- Otto A, Fontaine D, Fontaine J, Berkenboom G. Rosuvastatin treatment protects against nitrate-induced oxidative stress. J Cardiovasc Pharmacol. 2005;46:177–184. doi: 10.1097/01.fjc.0000167010.98177.78. [DOI] [PubMed] [Google Scholar]
- Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Zhan CD, Quiroz Y, Sindhu RK, Vaziri ND. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension. 2003;41:341–346. doi: 10.1161/01.hyp.0000052833.20759.64. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Bolden SW, Patrech AS, Ehrlich D. Visual dysfunction in the spontaneously hypertensive rat. Physiol Behav. 1993;54:903–907. doi: 10.1016/0031-9384(93)90300-5. [DOI] [PubMed] [Google Scholar]
- Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK. Oxygen free radicals in essential hypertension. Mol Cell Biochem. 1992;111:103–108. doi: 10.1007/BF00229580. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56:1–11. doi: 10.1016/s0168-8227(01)00341-2. [DOI] [PubMed] [Google Scholar]
- Sicard P, Lauzier B, Oudot A, Busseuil D, Collin B, Duvillard L, et al. A treatment with rosuvastatin induced a reduction of arterial pressure and a decrease of oxidative stress in spontaneously hypertensive rats. Arch Mal Coeur Vaiss. 2005;98:804–808. [PubMed] [Google Scholar]
- Sicard P, Oudot A, Guilland JC, Moreau D, Vergely C, Rochette L. Dissociation between vascular oxidative stress and cardiovascular function in Wistar Kyoto and spontaneously hypertensive rats. Vascul Pharmacol. 2006;45:112–121. doi: 10.1016/j.vph.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Susic D, Varagic J, Ahn J, Slama M, Frohlich ED. Beneficial pleiotropic vascular effects of rosuvastatin in two hypertensive models. J Am Coll Cardiol. 2003;42:1091–1097. doi: 10.1016/s0735-1097(03)00926-4. [DOI] [PubMed] [Google Scholar]
- Szabo ME, Droy-Lefaix MT, Doly M. Modification of reperfusion-induced ionic imbalance by free radical scavengers in spontaneously hypertensive rat retina. Free Radic Biol Med. 1992;13:609–620. doi: 10.1016/0891-5849(92)90035-f. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance. Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Wilson HL, Schwartz DM, Bhatt HR, McCulloch CE, Duncan JL. Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol. 2004;137:615–624. doi: 10.1016/j.ajo.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull. 2005;73–74:57–70. doi: 10.1093/bmb/ldh050. [DOI] [PubMed] [Google Scholar]
- Yu T, Mitchell P, Berry G, Li W, Wang JJ. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116:83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]
- Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988;263:11590–11596. [PubMed] [Google Scholar]