Abstract
Background and purpose:
Assessing the proarrhythmic potential of compounds during drug development is essential. However, reliable prediction of drug-induced torsades de pointes arrhythmia (TdP) remains elusive. Along with QT interval prolongation, assessment of the short-term variability of the QT interval (STV(QT)) may be a good predictor of TdP. We investigated the relative importance of IKs and IKr block in development of TdP together with correlations between QTc interval, QT interval variability and incidence of TdP.
Experimental approach:
ECGs were recorded from conscious dogs and from anaesthetized rabbits given the IKr blocker dofetilide (DOF), the IKs blocker HMR-1556 (HMR) and their combination, intravenously. PQ, RR and QT intervals were measured and QTc and short-term variability of RR and QT intervals calculated.
Key results:
DOF increased QTc interval by 20% in dogs and 8% in rabbits. HMR increased QTc in dogs by 12 and 1.9% in rabbits. Combination of DOF+HMR prolonged QTc by 33% in dogs, by 16% in rabbits. DOF or HMR given alone in dogs or HMR given alone in rabbits induced no TdP. Incidence of TdP increased after DOF+HMR combinations in dogs (63%) and following HMR+DOF (82%) and DOF+HMR combinations (71%) in rabbits. STV(QT) markedly increased only after administration of DOF+HMR combinations in both dogs and rabbits.
Conclusion and implications:
STV(QT) was markedly increased by combined pharmacological block of IKr and IKs and may be a better predictor of subsequent TdP development than the measurement of QTc interval prolongation.
Keywords: torsades de pointes, beat-to-beat short-term variability, QT interval, dofetilide, HMR 1556, repolarization reserve
Introduction
Numerous cardiovascular and non-cardiovascular drugs can cause torsades de pointes (TdP) polymorphic ventricular tachycardia that may degenerate into ventricular fibrillation causing sudden death (for a recent review, see Fenichel et al., 2004). TdP arrhythmia is generally considered to be a consequence of abnormal cardiac repolarization, that is impairment of the repolarization process often but not necessarily associated with, or preceded by, a prolonged QT interval. Predicting TdP in clinical settings is an extremely difficult task since the incidence of drug-induced TdP can be very low (1:100 000), and such events are not closely related to obvious changes in the QT interval. Recent evidence suggests that a prolonged QT interval cannot reliably predict the development of TdP since the repolarization reserve may be reduced without noticeable changes in the duration of cardiac repolarization. The concept of repolarization reserve, suggested by Roden (1998), refers to the multiple mechanisms responsible for cardiac repolarization. Loss or impaired function of one of these mechanisms does not necessarily lead to clinically manifest repolarization abnormalities but makes the heart more vulnerable to arrhythmia development (Roden, 1998; Roden and Yang, 2005; Varró and Papp, 2006). Accumulating evidence strongly suggests that the circumstances favouring TdP development may need to be re-defined from ‘prolonged repolarization' to the more appropriate ‘compromised repolarization reserve'.
One of the most elegant experimental models for drug-induced TdP is chronic atrioventricular block in dogs (Chezalviel-Guilbert et al., 1995; Vos et al., 1995). In this setting, the downregulation of potassium currents, particularly the slow delayed rectifier potassium current (IKs) has been demonstrated and suggested to play a vital role in arrhythmia development (Volders et al., 1999). Decrease of IKs itself without sympathetic stimulation does not seem to affect repolarization in dog, rabbit and human-isolated ventricular muscle but it has been assumed that as an important part of the repolarization reserve it can significantly contribute to the safety margin of repolarization (Varró et al., 2000; Lengyel et al., 2001; Volders et al., 2003; Jost et al., 2005; Abi-Gerges et al., 2006).
In the canine chronic AV block model, amiodarone, which markedly prolonged QTc interval, did not cause substantial increase in the incidence of TdP, and these results were similar to observations made in humans (van Opstal et al., 2001). In the same model, it was found that the incidence or lack of arrhythmias correlated well with the degree of the short-term variability (STV) of repolarization (Thomsen et al., 2004). After interventions that led to increased STV of repolarization, the incidence of TdP was also increased and following interventions that resulted in no change in STV of repolarization, the likelihood of TdP development was not altered in spite of significant QTc prolongation (Thomsen et al., 2004). Therefore, STV or short-term QT variability was suggested as a better predictor for TdP than the traditional assessment of QTc interval.
As in the dog model of chronic AV block and TdP, IKs was markedly downregulated and the additional IKr block resulted in a high incidence of TdP, we have set out to investigate the effect of combined pharmacological block of the slow (IKs) and rapid delayed rectifier potassium currents (IKr) on the development of arrhythmias, TdP in particular, on repolarization and STV of repolarization in conscious dogs and in anaesthetized rabbits.
Part of this work has been recently presented in abstract form (Lengyel et al., 2006).
Methods
The study was conducted in accordance with the standards of the European Community Guidelines on the Care and Use of Laboratory Animals and the protocol had been approved by the Ethical Committee for the Protection of Animals in Research of the Albert Szent-Györgyi Medical Centre, University of Szeged, Szeged, Hungary.
Conscious dogs
Beagle dogs of both sexes (10–15 kg) were used for the experiments. The animals were allowed to accommodate to experimental personnel and equipment, including a loosely fitting jacket containing electrocardiogram (ECG) electrodes, every day for a week before the commencement of the actual studies. After a 20-min equilibration period, baseline recordings were obtained. The animals were then randomly assigned to the following two groups: (i) eight dogs received 25 μg kg−1 dofetilide first, followed by 1 mg kg−1 HMR 1556 after a 20-min equilibration period and (ii) six dogs were given 1 mg kg−1 HMR 1556 first, followed by 25 μg kg−1 dofetilide intravenously after a 20-min equilibration period. The drugs were administered as a 5 min continuous intravenous infusion (Terufusion TE-3, Terumo Europe, Leuven, Belgium). The ECG was obtained using precordial leads and was digitized and stored on a computer for later analysis using National Instruments data acquisition hardware (National Instruments, Austin, TX, USA) and SPEL Advanced Haemosys software (v2.7, Experimetria Ltd., Budapest, Hungary). The PQ, RR and QT intervals were measured as the average of 30 consecutive beats (the minimum number of beats required for the calculation of STV of an interval, see below), and the frequency-corrected QT interval (QTc) was calculated using a formula recommended for Beagle dogs: QTc=QT–(0.087*(RR−1000)) (Van de Water et al., 1989; Tattersall et al., 2006). The intervals were measured at the following time points during the experiments: (i) 2 min before the start of drug infusion (baseline) and (ii) 15 min after the end of drug infusions.
Anaesthetized rabbits
Male New Zealand white rabbits (2–3 kg) were used for the experiments. The animals were anaesthetized with thiopentone (50 mg kg−1 intravenously) given into the marginal vein of the right ear. A catheter filled with isotonic saline containing 500 IU ml−1 heparin was inserted into the left carotid artery for the measurement of arterial blood pressure. The right jugular vein was cannulated for subsequent intravenous drug administration. The animals were allowed to stabilize for 20 min and baseline measurements were taken. The first group of rabbits received 25 μg kg−1 dofetilide in a volume of 2 ml kg−1 during a 5-min infusion followed by 0.1 mg kg−1 HMR 1556 after a 20-min equilibration period. The second group was administered 0.1 mg kg−1 HMR 1556 followed by 25 μg kg−1 dofetilide 20 min after HMR administration. The third group received 1 mg kg−1 HMR 1556 followed by 25 μg kg−1 dofetilide 20 min after HMR administration.
The blood pressure and the ECG (leads I–III) were continuously recorded (at 200 Hz), digitized and stored on a computer for analysis using National Instruments data acquisition hardware (National Instruments) and SPEL Advanced Haemosys software (v2.7, Experimetria Ltd.). The PQ, RR and QT intervals were measured as the average of 30 beats (the minimum number of beats required for the calculation of STV of an interval, see below). During the measurement of the QT interval in anaesthetized rabbits, the guidelines described by Farkas et al. (2004) were followed. In animals, such as rabbits, with a significantly faster heart rate than that of humans, QTc calculated with Bazett's formula does not accurately reflect the heart rate-dependent changes in QT interval. Therefore, QTc was calculated by a formula specifically suggested for anaesthetized rabbits by Batey and Coker (2002) as follows: QTc=QT–(0.704*(RR−250)).
For the determination of plasma concentrations of K+, Na+ and Cl−, blood samples were collected from anaesthetized rabbits just before drug administration.
Short-term beat-to-beat variability of the RR and QT intervals
To characterize the instability of beat-to-beat heart rate and repolarization, Poincaré plots of the RR and QT intervals were constructed, where each RR and QT value is plotted against its former value in dogs and rabbits (Figures 2a and b, 4a and b, 5a and b and 6a and b). The plots are the result of 30 consecutive interval measurements in sinus rhythm at a given time point during the experiments. In the case of combined IKs and IKr block where TdP commonly occurred, the measurements were taken before the development of TdP. The beat-to-beat STV of RR or QT intervals was calculated using the following formula: STV= ∑∣Dn+1−Dn∣(30 × √2)−1, where D is the duration of the QT or RR interval. The STV represents the mean orthogonal distance to the line-of-identity on the Poincaré plot, and this estimation of RR and QT interval instability is based on previous analysis of the value of qualitative and quantitative Poincaré plot examination (Brennan et al., 2001).
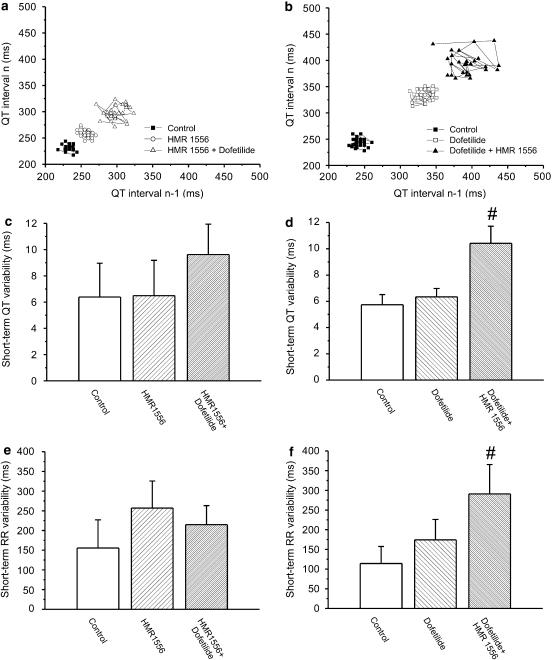
Figure 2.
(a and b) Representative Poincaré plots illustrate the increase in short-term variability of the QT interval (STV(QT)) following combined IKr and IKs inhibition in conscious dogs. Right and upward shift on this figure represents QT interval prolongation while the scatter of data points represents QT interval variability. (c and d) Grouped data show that STV(QT) was not increased by HMR 1556 (1 mg kg−1 intravenously) or dofetilide (25 μg kg−1 intravenously) alone, only by their combination given in either order. (e and f) STV(R) increased only when pharmacological inhibition of IKr preceded IKs block. #P<0.05 vs control in the same group of conscious dogs, n=6–8 animals in each group. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current; STV(QT), short-term variability of the QT interval; STV(R), short-term variability of the RR interval.
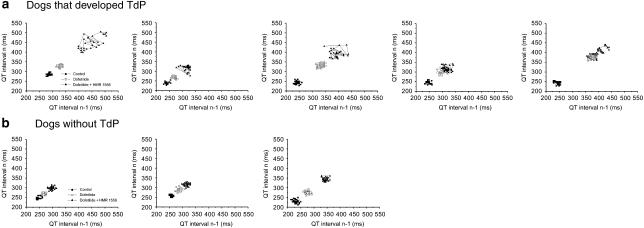
Figure 4.
(a) Individual Poincaré plots showing increased STV(QT) after combined IKs+IKr block in dogs that later developed TdP but (b) no change in STV(QT) when TdP did not occur. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current; STV(QT), short-term variability of the QT interval.
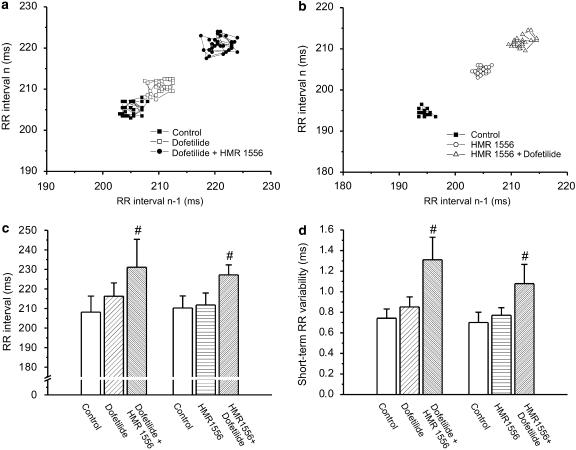
Figure 5.
(a) Representative example of a Poincaré plot of the RR interval from an anaesthetized rabbit treated with the IKr blocker dofetilide (25 μg kg−1 intravenously) followed by HMR 1556 (0.1 mg kg−1 intravenously) and (b) from another treated with the IKs blocker HMR 1556 (0.1 mg kg−1 intravenously) followed by dofetilide (25 μg kg−1 intravenously). (c) Combined IKr and IKs inhibition significantly increased RR interval and (d) the RR variability in anaesthetized rabbits. #P<0.05 vs control in the same group, n=7–11 animals in each group. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current.
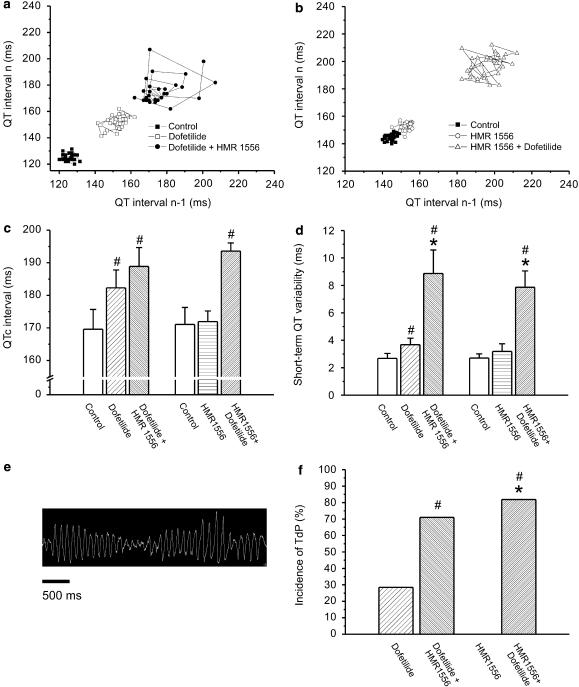
Figure 6.
(a) Representative example of a Poincaré plot of the QT interval from an anaesthetized rabbit after the administration of the IKr blocker dofetilide (25 μg kg−1 intravenously) followed by HMR 1556 (0.1 mg kg−1 intravenously) and (b) from another after the combined administration of the IKs blocker HMR 1556 (0.1 mg kg−1 intravenously) and dofetilide (25 μg kg−1 intravenously). (c) The QTc interval was significantly increased by dofetilide and the combination of dofetilide+HMR 1556 given in any order, but HMR 1556 alone did not lengthen QTc. (d) STV(QT) was increased after dofetilide administration. (e and f) Combined IKr and IKs block markedly increased STV(QT) and provoked TdP in anaesthetized rabbits. #P<0.05 vs control in the same group, *P<0.05 vs dofetilide treatment, n=7–11 animals in each group. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current; STV(QT), short-term variability of the QT interval; STV(R), short-term variability of the RR interval.
Statistical analysis
The incidence of arrhythmias (%) was compared by using the χ2-test. All other data are expressed as means±s.e.m. After one-way analysis of variance, the groups were compared in pairs by means of Student's t-test. A level of P<0.05 was considered to be statistically significant.
Drugs
HMR 1556 (Aventis Pharma, Frankfurt am Main, Germany) was dissolved in dimethylsulphoxide (0.1%) as a stock solution of 10 μM. Dofetilide (Gedeon Richter Ltd., Budapest, Hungary) was dissolved in saline as a stock solution of 5 μM. Each stock solution was diluted immediately before use.
Results
Effect of intravenous infusions on heart rate, QT, QTc intervals and the STV(RR) and STV(QT) in conscious dogs
To test whether intravenous infusions influenced the heart rate, QT, QTc intervals, STV(QT) and STV(RR) in conscious dogs, a small group of dogs (n=4) received vehicle intravenously during a 5-min infusion while their ECG was continuously recorded. Intravenous vehicle administration did not increase the RR (720.7±41.4 vs 715.5±32.3 ms at baseline, P>0.05), QT (265.7±12.3 vs 258.3±14.5 ms at baseline, P>0.05) and QTc intervals (290±11.9 vs 283.1±12.8 ms at baseline, P>0.05) significantly in conscious dogs.
Vehicle administration resulted in a small but non-significant increase in STV(QT) in conscious dogs (7.4±2.16 ms after vehicle administration vs 6.5±1.19 ms at baseline, P>0.05). The increase in STV(R) following intravenous vehicle infusion proved to be non-significant (106.2±20.3 ms after vehicle vs 87.6±12.5 ms at baseline, P>0.05).
Effect of combined IKr and IKs block on heart rate, QT and QTc intervals in conscious dogs
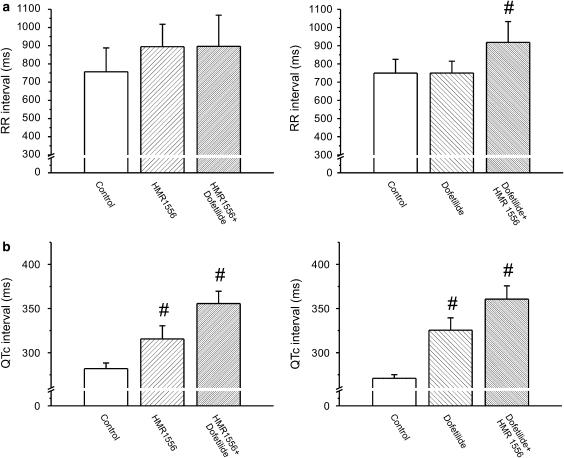
The heart rate of the animals in the two groups was not different at baseline (84±6.4 and 88±11.5 beats per min, P>0.05). Administration of the IKr blocker dofetilide (25 μg kg−1) alone did not alter the RR interval (Figure 1a). The IKs blocker HMR 1556 (1 mg kg−1) alone had a tendency to increase the RR interval but this increase did not prove to be statistically significant (Figure 1a). Dofetilide did not increase the RR interval when administered after HMR 1556 injection, but HMR 1556 significantly increased the RR interval following dofetilide infusion (Figure 1a). Figure 1b shows that pharmacological block of IKs or IKr alone resulted in a statistically significant QTc prolongation, calculated with the formula suggested by Van de Water et al. (1989) in conscious dogs, and the combination of dofetilide and HMR 1556 administered in either order further increased the QTc interval. The corresponding QT intervals yielded similar results: 306.4±24.3 ms after 1 mg kg−1 HMR 1556 and 346.7±24.4 ms after 1 mg kg−1 HMR 1556+25 μg kg−1 dofetilide vs 260.7±15.0 ms at baseline; 303.8±14.2 ms after 25 μg kg−1 dofetilide and 354.0±20.3 ms after 25 μg kg−1 dofetilide+1 mg kg−1 HMR 1556 vs 249.2±6.3 ms at baseline, all P<0.05).
Figure 1.
Effect of inhibiting IKr and IKs, separately and in combination, on the RR and QTc intervals in conscious dogs. (a) IKr block by dofetilide (25 μg kg−1) did not increase the RR interval, but the IKs blocker HMR 1556 (1 mg kg−1) tended to increase the RR interval alone and in combination with dofetilide (25 μg kg−1). (b) Inhibition of IKs with HMR 1556 caused a moderate, whereas IKr block by dofetilide and combined IKs and IKr block caused a marked QTc prolongation in conscious dogs. #P<0.05 vs control in the same group, n=6–8 animals in each group. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current.
Effect of combined IKr and IKs block on the STV of QT and RR intervals in conscious dogs
Figure 2a shows Poincaré plots constructed from the QT intervals of an individual dog that received HMR 1556 (1 mg kg−1) first, followed by dofetilide (25 μg kg−1). Figure 2b illustrates the marked increase in the STV of the QT interval (STV(QT)) in a dog receiving dofetilide (25 μg kg−1) first, followed by the IKs blocker HMR 1556 (1 mg kg−1). As the individual examples (Figure 2a and b) and grouped average data (Figure 2c and d) show, pharmacological block of IKr or IKs alone did not result in increased STV(QT). However, combined IKr and IKs block elevated the STV(QT) independent of the order of the administration of dofetilide and HMR 1556 (Figure 2c and d). In the group where HMR 1556 was administered first, the increase of the STV(QT) did not reach significance, most probably due to (i) the large standard deviation of STV(QT) in this group at baseline that remained large during subsequent drug administration (Figure 2c) and due to (ii) the non-uniform increase in STV(QT) in the group (the variability of the QT interval increased significantly only in the animals later developing TdP, Figure 3b and c). The STV of the RR interval (STV(R)) did not increase significantly either when HMR 1556 or dofetilide was applied alone (Figure 2e and f). Interestingly, the variability of the RR interval increased after the combination of the two drugs, only when dofetilide was applied first (Figure 2f).
Figure 3.
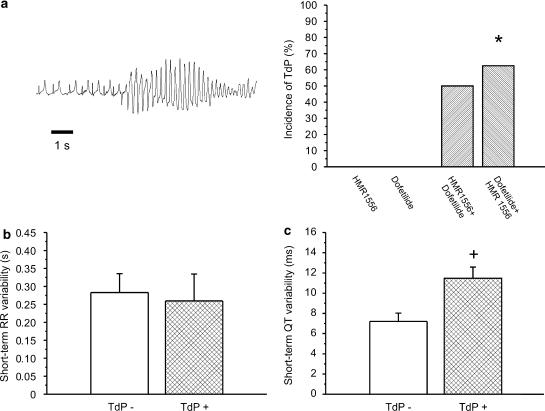
(a) Representative example of TdP in a conscious dog following the combined administration of dofetilide (25 μg kg−1) and HMR 1556 (1 mg kg−1). Grouped data showing that only combined IKr and IKs block led to the development of TdP in conscious dogs. (b) The STV(R) interval was not different in dogs developing TdP from those not developing TdP (TdP−) following combined IKs and IKr block. (c) Detailed analysis revealed that STV(QT) was increased only in dogs that later developed TdP (TdP+) following the combined administration of HMR 1556 and dofetilide, irrespective of the order of their administration. *P<0.05 vs dofetilide group. +P<0.05 vs TdP− group, n=6–8 animals in each group. IKs, the slow component of the delayed rectifier potassium current; IKr, the rapid component of the delayed rectifier potassium current; STV(QT), short-term variability of the QT interval; STV(R), short-term variability of the RR interval.
Effect of combined IKr and IKs block on the incidence of TdP in conscious dogs
The first panel of Figure 3a illustrates TdP ventricular tachycardia induced by the combined administration of 1 mg kg−1 dofetilide+1 mg kg−1 HMR 1556 in a conscious dog. No TdP was observed after administration of either dofetilide or HMR 1556 alone (Figure 3a). However, when dofetilide was administered, followed by HMR 1556, five of eight dogs exhibited TdP, when HMR 1556 was first administered, followed by dofetilide TdP developed in three of six animals (Figure 3a). Although the STV of the RR interval significantly increased following dofetilide+HMR 1556 administration (Figure 2c), the STV(R) was not different between animals that developed TdP from those not exhibiting this type of arrhythmia irrespective of what order dofetilide and HMR 1556 were administered (Figure 3b). On the other hand, while only the combination of dofetilide+HMR 1556 increased the overall STV(QT) significantly, the animals with TdP had a significantly higher STV(QT) compared to those without TdP, irrespective of the order of dofetilide and HMR 1556 administration (Figure 3c).
Figure 4 illustrates the increased STV(QT) in dogs with TdP in contrast to the unchanged STV(QT) in those without TdP after administration of dofetilide followed by HMR 1556 (1 mg kg−1).
Rationale for the use of anaesthetized rabbits
Since the introduction of Carlsson's rabbit TdP model (Carlsson et al., 1990), the anaesthetized rabbit has been extensively used in different in vivo proarrhythmia studies for the assessment of the risk for arrhythmia development associated with a compound of interest (for a recent review, see Lawrence et al., 2005). Therefore, three sets of experiments investigating the effect of pharmacological block of IKs, IKr and their combination on instability of repolarization and the development of TdP were also performed in anaesthetized rabbits.
Blood pressure and plasma [K+] in anaesthetized rabbits
There were no differences in blood pressure among the three groups at baseline (Table 1) The mean arterial blood pressure (MAP) was not altered by dofetilide (25 μg kg−1) or HMR 1556 (0.1 and 1 mg kg−1) alone, but their combination significantly decreased MAP in anaesthetized rabbits (Table 1). Similar changes of the systolic and diastolic blood pressure were observed in these animals (Table 1).
Table 1.
Effect of dofetilide (25 μg kg−1), HMR 1556 (0.1 and 1 mg kg−1) and their combination on arterial blood pressure in anaesthetized rabbits
| Group | Dose (mg kg−1) | N |
Arterial blood pressure (mm Hg) |
||
|---|---|---|---|---|---|
| Mean | Systolic | Diastolic | |||
| Control | 7 | 107±5.2 | 119±4.5 | 91±5.1 | |
| Dofetilide | 0.025 | 7 | 102±6.9 | 108±4.5 | 82±5.9 |
| Dofetilide+HMR 1556 | 0.025+0.1 | 7 | 87±3.9# | 94±4.8# | 76±5.4# |
| Control | 11 | 102±2.4 | 114±2.8 | 91±2.5 | |
| HMR 1556 | 0.1 | 11 | 104±1.8 | 117±2.4 | 93±2.3 |
| HMR 1556+dofetilide | 0.1+0.025 | 11 | 91±3.7# | 102±5.1# | 81±3.7# |
| Control | 6 | 102±4.6 | 116±3.8 | 87±3.8 | |
| HMR 1556 | 1 | 6 | 103±5.6 | 114±6.1 | 85±7.2 |
| HMR 1556+dofetilide | 1+0.025 | 6 | 92±5.7 | 107±5.3 | 73±5.4 |
Arterial blood pressure values were measured during sinus rhythm.
P<0.05 vs control values within the same group.
The plasma concentration of potassium can have marked effects on the development of TdP, therefore plasma [K+] along with [Na+] and [Cl−] were measured and found to be: 3.6±0.19 mM for [K+], 142.1±1.25 mM for [Na+] and 100.6±1.72 mM for [Cl−] (n=19) before drug administration in anaesthetized rabbits. There were no differences in plasma [K+], [Na+] and [Cl−] between any of the groups. These values are in good agreement with the results of Gil et al. (2004) showing moderate hypokalaemia in thiopentone-anaesthetized rabbits.
Effect of IKr and IKs block and their combination on heart rate and repolarization in anaesthetized rabbits
There were no differences in heart rate between the three groups at baseline (296±14.1, 279±8.1 and 274±4.6 beats per min, P>0.05). The QT intervals were similar in all groups at baseline (136.1±5.37, 144.4±4.75 and 143.9±6.2 ms, n=6–11, P>0.05) and the same applies to the QTc intervals.
When administered alone, neither the IKr blocker dofetilide (25 μg kg−1, intravenously) nor the IKs blocker HMR 1556 (0.1 mg kg−1) increased the RR interval (Figure 5c). Dofetilide significantly prolonged the QTc interval while HMR 1556 had no effect on QTc (Figure 6c). In contrast, the combination of dofetilide and HMR 1556 given in any order significantly increased the RR interval and lengthened QTc (Figures 5c and 6c). When administered in the higher dose (1 mg kg−1), HMR 1556 did not increase QTc in anaesthetized rabbits (165.7±3.86 vs 162.5±5.44 ms at baseline, P>0.05). However, administration of 25 μg kg−1 dofetilide 20 min following 1 mg kg−1 HMR 1556 significantly increased the QTc interval (184.5±8.1 vs 162.5±5.44 ms at baseline, P<0.05).
Effect of IKr and IKs block and their combination on variability of heart rate and repolarization in anaesthetized rabbits
The first two panels of Figure 5 (a and b) show representative examples of Poincaré plots constructed from the RR intervals measured before and after the administration of dofetilide, HMR 1556 (0.1 mg kg−1) and their combination. There were no differences in the STV(R) or STV(QT) among the three groups at baseline. In anaesthetized rabbits, no significant increase in STV(R) was observed after IKr or IKs block alone, but given in combination in any order, dofetilide and HMR 1556 increased the variability of heart rate (Figure 5d). To assess the stability of repolarization Poincaré plots, QT intervals were also drawn for each animal. Representative plots and grouped average data shows that IKs block did not increase STV(QT), IKr block caused a relatively small but significant elevation of STV(QT) and IKs and IKr combination led to an approximately fourfold increase in STV(QT) (Figure 6).
Application of HMR 1556 in the higher dose (1 mg kg−1) alone and in combination with dofetilide yielded similar results in anaesthetized rabbits: neither the STV(R) nor the STV(QT) increased following its administration alone, but significantly increased STV(QT) and STV(R) when combined with dofetilide (STV(QT) was 2.64±0.39 and 7.56±0.99 ms vs 2.31±0.25 at baseline; STV(R) was 0.81±0.09 and 1.61±0.28 vs 0.76±0.09 ms at baseline after 1 mg kg−1 HMR 1556, and following the combination of 1 mg kg−1 HMR 1556+25 μg kg−1 dofetilide, respectively).
No TdP was observed after HMR 1556 administration (0.1 or 1 mg kg−1). Two of seven animals exhibited TdP following dofetilide injection, but 9 of 11 developed TdP after the combined administration of 0.1 mg kg−1 HMR 1556 and 25 μg kg−1 dofetilide (Figure 6); five of seven after the combination when dofetilide was given first; and five of six developed TdP after 1 mg kg−1 HMR 1556 and 25 μg kg−1 dofetilide (data not shown). Importantly, the increase in STV(QT) paralleled the increase in the incidence of TdP in anaesthetized rabbits (Figure 6d and f).
Discussion
The main findings of the present study are as follows: lengthening of repolarization by IKr block in dogs did not evoke TdP and did not markedly increase STV(QT). Decreasing the repolarization reserve alone by IKs block did not evoke TdP and did not change STV(QT) in either conscious dogs or in anaesthetized rabbits. However, IKr block in the presence of decreased repolarization reserve greatly augmented the incidence of TdP and also increased STV(QT) both in conscious dogs and anaesthetized rabbits. The elevation of STV(QT) and incidence of TdP were correlated with each other. Finally, the increase of STV(QT) may be partially related to the irregularity of the cycle length.
Pharmacological block of IKs: a tool for reducing repolarization reserve
The main goal of this study was to compromise repolarization reserve by pharmacological means that resembles its decrease in pathological settings. Therefore, we investigated the effect of IKs and IKr block alone and in combination on repolarization, its beat-to-beat stability and role in the development of TdP. For this reason, it was important to selectively inhibit IKs and IKr without significantly affecting other ionic currents responsible for cardiac repolarization. For this reason, dofetilide was used to block IKr (Rasmussen et al., 1992) and HMR 1556 was used for selective pharmacological block of IKs. HMR 1556 was applied since it has been shown to be superior to chromanol 293B both in its potency and specificity for blocking IKs (Gögelein et al., 2000; Thomas et al., 2003). HMR 1556 blocked IKs with an IC50 of 10–50 nM and inhibited IKr, Ito and ICa,L only at much higher concentrations (IC50 values between 10–30 μM) while having no effect on IK1 in concentrations up to 50 μM in canine ventricular myocytes (Thomas et al., 2003). The dose of HMR 1556 used in this study (0.1 and 1 mg kg−1) can be expected to correspond to a plasma concentration of the compound in the submicromolar range similarly to that achieved by Volders et al. (2003) in conscious dogs.
Our results are in good agreement with previous studies suggesting that IKr block but not IKs block lengthens ventricular repolarization markedly. IKs block did not prolong QTc (Lengyel et al., 2001), the action potential duration (Lengyel et al., 2001), or monophasic action potential duration (MAPD) in rabbits (So et al., 2006). In the present study, neither 0.1 nor 1 mg kg−1 HMR 1556 caused a significant QTc prolongation in anaesthetized rabbits (∼1–2%). On the other hand, 1 mg kg−1 HMR 1556 caused a significant QTc prolongation in conscious dogs (∼12%). However, this amount of QTc prolongation is still relatively small compared to the QTc prolonging effect of HMR 1556 described by Volders et al. (2003), who found large (∼58%) and significant QTc lengthening after IKs block with HMR 1556 in conscious dogs. The discrepancy between the two studies is not fully understood. However, in the study of Volders et al. (2003), this large increase of QTc was observed after 3 h and with a 30 mg kg−1 oral dose, which resulted in a peak plasma concentration of 2.68 μM. This is much higher than the IC50 of HMR 1556 to block IKs (10–50 nM). Therefore, the possibility of HMR 1556 at such a high concentration affecting other potassium currents cannot be ruled out. The formation of metabolites, which may affect repolarization following oral administration, is also possible. In conscious dogs, we applied 1 mg kg−1 intravenously and the effect on QTc lengthening was smaller than that observed by Volders et al. (2003) with 3 mg kg−1 HMR 1556 after oral administration.
Our results suggest that the present in vivo model, where IKs is blocked pharmacologically and the repolarization reserve becomes severely compromised, can serve as a proper and simple tool for testing different compounds for their possible TdP-inducing side effects.
STV of the QT interval predicts TdP development
In recent years, different electrophysiological parameters have been suggested that could be used to predict drug-induced life-threatening arrhythmias, including TdP, both in patients and in experimental models. Such parameters include the prolongation of the QT and QTc intervals, spatial inhomogeneity of repolarization or QTc dispersion (Verduyn et al., 1997; Belardinelli et al., 2003; Kaab et al., 2003). Since the predictive power of these parameters is rather low, new and more sensitive methods are needed.
The results of the present study support the findings of some earlier investigators who proposed that susceptibility to proarrhythmia is related not only to spatial but also to temporal dispersion of repolarization (Berger et al., 1997; Hondeghem et al., 2001). Our results are in good agreement with those of Thomsen et al. (2004), who found that STV of repolarization, but not QTc changes, predicted the development of TdP in the dog. These observations also resemble the results of the MADIT II trial in which it was concluded that in post-infarction patients with severe left ventricular dysfunction, increased QT variability was associated with increased risk for ventricular tachycardia or fibrillation (Moss et al., 1999).
There was a difference in the STV(QT) response in conscious dogs following the combined administration of dofetilide and HMR 1556, that is the increase in STV(QT) was significant only when dofetilide was administered first. Detailed analysis of the STV(QT) data revealed that STV(QT) increased only in dogs, which later developed TdP and this was independent of the order of dofetilide and HMR 1556 administration.
In theory, the prolonged QT interval could inherently lead to increased STV(QT) in experimental conditions. A recent study by Thomsen et al. (2006) has found that, at longer pacing cycle lengths, both the MAPD and the STV of the MAPD were increased in anaesthetized dogs. However, when the pacing cycle length was increased from 700 to 1200 ms, the STV of the MAPD increased by approximately 0.5 ms. In our conscious dogs, we did not observe such large changes in heart rate and the calculated STV(QT) increased by more than 100% in the dogs exhibiting TdP. Therefore, we think that the slowing of heart rate in our study could only minimally influence the STV(QT) increase seen after combined dofetilide and HMR 1556 administration.
The cellular and ionic mechanisms responsible for the increased STV of repolarization are unknown but they seem to be related to the amount of available repolarization reserve. IKs, which has been shown to be a less important contributor to repolarization in normal settings is considered to be a key player in determining repolarization reserve. The pharmacological block of this current in our study and its downregulation in the study of Thomsen et al. (2004) strongly argues for the role of repolarization reserve and specifically IKs in the short-term repolarization variability. Also, short-term adaptation of repolarization to heart rate, which may depend on intracellular calcium levels, can be a factor since in our experiments regular rhythm was associated with less STV(QT) than irregular rhythm. However, the role of this latter relation is not clearly defined yet and has not been established in earlier observations, therefore it needs further investigation.
Limitations
Although the HMR 1556 plasma concentrations were not determined in the present study, in our conscious dog experiments, we observed a significant QTc prolonging effect of intravenous HMR 1556 that is in agreement with the results of Volders et al. (2003), where HMR 1556 was administered orally in conscious dogs.
It is not clear how beat-to-beat variability of heart rate as well as respiratory sinus arrhythmia influences STV(QT). A recent study by Thomsen et al. (2007) has demonstrated no correlation between STV(R) and the STV of repolarization in anaesthetized dogs with chronic AV block. Our results on the effect of combined IKs and IKr block on STV(QT) and incidence of TdP were similar in conscious dogs with intact adrenergic responses and marked respiratory sinus arrhythmia and in anaesthetized rabbits. Anaesthetized rabbits exhibited no respiratory sinus arrhythmia and smaller STV(R) compared to conscious dogs.
The plasma concentration of K+ can significantly influence the development of arrhythmias, including TdP. In our anaesthetized rabbits, the mean [K+] showed that the animals had borderline hypokalaemia (3.6±0.19 mM), a condition that favours TdP development. However, there were no differences among groups in plasma [K+] and previous studies showed similarly low or even lower plasma [K+] in rabbits anaesthetized with thiopentone or other compounds (Farkas and Coker, 2002; Gil et al., 2004).
Conclusions
In summary, this study further emphasizes that increased STV(QT) precedes TdP arrhythmias and as such may be used for the prediction of the pro-arrhythmic risk following drug administration in addition to using conventional parameters. Whether this method can also be applied to assessment of pro-arrhythmic risk in susceptible patients needs to be elucidated by further studies. However, on the basis of results of the present and recent studies, it is suggested that STV of repolarization has a better predictive value than QTc measurements in estimating the danger of developing drug-induced arrhythmias in patients at risk.
Acknowledgments
This study was supported by the Hungarian Academy of Sciences, by grants from the Hungarian National Research Foundation (OTKA T-048698, NI-61902 and F-61222), Hungarian Ministry of Health (458/2003; 360/2006), National Research and Development Programmes (NKFP 1A/0011/2002, NKFP 1A/046/2004 and Bio-37 KPI), by the János Bolyai Research Scholarship (Dr Lengyel) and József Öveges Programme by the National Office for Research and Technology (OMFB-01383/2006, Dr Baczkó). We thank Professor Gögelein at Aventis Pharma (Frankfurt am Main, Germany) for the generous gift of HMR 1556 and Gedeon Richter Ltd. (Budapest, Hungary) for the gift of dofetilide.
Abbreviations
- IKr
the rapid component of the delayed rectifier potassium current
- IKs
the slow component of the delayed rectifier potassium current
- STV(QT)
short-term variability of the QT interval
- STV(R)
short-term variability of the RR interval
- TdP
torsades de pointes
Conflict of interest
The authors state no conflict of interest.
References
- Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin JP, Pollard CE. Gender differences in the slow delayed (IKs) but not in inward (IK1) rectifier K+ currents of canine Purkinje fibre cardiac action potential: key roles for IKs, β-adrenoceptor stimulation, pacing rate and gender. Br J Pharmacol. 2006;147:653–660. doi: 10.1038/sj.bjp.0706491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey AJ, Coker SJ. Proarrhythmic potential of halofantrine, terfenadine and clofilium in a modified in vivo model of torsade de pointes. Br J Pharmacol. 2002;135:1003–1012. doi: 10.1038/sj.bjp.0704550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Antzelevitch C, Vos MA. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability. IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Almgren O, Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Chezalviel-Guilbert F, Davy JM, Poirier JM, Weissenburger J. Mexiletine antagonizes effects of sotalol on QT interval duration and its proarrhythmic effects in a canine model of torsade de pointes. J Am Coll Cardiol. 1995;26:787–792. doi: 10.1016/0735-1097(95)00234-U. [DOI] [PubMed] [Google Scholar]
- Farkas A, Batey AJ, Coker SJ. How to measure electrocardiographic QT interval in the anaesthetized rabbit. J Pharmacol Toxicol Methods. 2004;50:175–185. doi: 10.1016/j.vascn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Farkas A, Coker SJ. Limited induction of torsade de pointes by terikalant and erythromycin in an in vivo model. Eur J Pharmacol. 2002;449:143–153. doi: 10.1016/s0014-2999(02)01992-1. [DOI] [PubMed] [Google Scholar]
- Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Independent Academic Task Force et al. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–495. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil AG, Silvan G, Illera M, Illera JC. The effects of anesthesia on the clinical chemistry of New Zealand white rabbits. Contemp Top Lab Anim Sci. 2004;43:25–29. [PubMed] [Google Scholar]
- Gögelein H, Bruggemann A, Gerlach U, Brendel J, Busch AE. Inhibition of IKs channels by HMR 1556. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Dujardin K, De Clerck F. Phase 2 prolongation, in the absence of instability and triangulation, antagonizes class III proarrhythmia. Cardiovasc Res. 2001;50:345–353. doi: 10.1016/s0008-6363(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Jost N, Virág L, Bitay M, Takács J, Lengyel Cs, Biliczki P, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome – a case–control pilot study using i.v. sotalol. Eur Heart J. 2003;24:649–657. doi: 10.1016/s0195-668x(02)00806-0. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Pollard CE, Hammond TG, Valentin JP. Nonclinical proarrhythmia models: predicting torsades de pointes. J Pharmacol Toxicol Methods. 2005;52:46–59. doi: 10.1016/j.vascn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Lengyel Cs, Jost N, Virág L, Varró A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel CS, Udvary E, Papp JG, Varró A.Estimation of the proarrhythmic risk by short term variability of QT-interval and/or action potential duration in dog Heart Rhythm 20063(Suppl 1)S307P6–P18 (abstract) [Google Scholar]
- Moss AJ, Cannom DS, Daubert JP, Hall WJ, Higgins SL, Klein H, et al. Multicenter Automatic Defibrillator Implantation Trial II (MADIT II): design and clinical protocol. Ann Noninvasive Electrocardiol. 1999;4:83–91. doi: 10.1111/j.1542-474X.2005.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HS, Allen MJ, Blackburn KJ, Butrous GS, Dalrymple HW. Dofetilide, a novel class III antiarrhythmic agent. J Cardiovasc Pharmacol. 1992;20 (Suppl 2):S96–S105. [PubMed] [Google Scholar]
- Roden DM. Taking the idio out of idiosyncratic – predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. Circulation. 2005;112:1376–1378. doi: 10.1161/CIRCULATIONAHA.105.562777. [DOI] [PubMed] [Google Scholar]
- So PP, Hu XD, Backx PH, Puglisi JL, Dorian P. Blockade of IKs by HMR 1556 increases the reverse rate-dependence of refractoriness prolongation by dofetilide in isolated rabbit ventricles. Br J Pharmacol. 2006;148:255–263. doi: 10.1038/sj.bjp.0706721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall ML, Dymond M, Hammond T, Valentin JP. Correction of QT values to allow for increases in heart rate in conscious Beagle dogs in toxicology assessment. J Pharmacol Toxicol Methods. 2006;53:11–19. doi: 10.1016/j.vascn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Gerlach U, Antzelevitch C. HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol. 2003;41:140–147. doi: 10.1097/00005344-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Oros A, Schoenmakers M, van Opstal JM, Maas JN, Beekman JD, et al. Proarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarisation. Cardiovasc Res. 2007;73:521–530. doi: 10.1016/j.cardiores.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Volders PG, Beekman JDM, Vos MA. Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J Am Coll Cardiol. 2006;48:1268–1276. doi: 10.1016/j.jacc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Van de Water A, Verheyen J, Xhonneux R, Reneman RS. An improved method to correct the QT interval of the electrocardiogram for changes in heart rate. J Pharmacol Methods. 1989;22:207–217. doi: 10.1016/0160-5402(89)90015-6. [DOI] [PubMed] [Google Scholar]
- van Opstal JM, Schoenmakers M, Verduyn SC, de Groot SH, Leunissen JD, van Der Hulst FF, et al. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001;104:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- Varró A, Baláti B, Iost N, Takács J, Virág L, Lathrop DA, et al. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varró A, Papp JG. Low penetrance, subclinical congenital LQTS: concealed LQTS or silent LQTS. Cardiovasc Res. 2006;70:404–406. doi: 10.1016/j.cardiores.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Verduyn SC, Vos MA, van der Zande J, Kulcsar A, Wellens HJ. Further observations to elucidate the role of interventricular dispersion of repolarization and early after depolarizations in the genesis of acquired torsade de pointes arrhythmias: a comparison between almokalant and d-sotalol using the dog as its own control. J Am Coll Cardiol. 1997;30:1575–1584. doi: 10.1016/s0735-1097(97)00333-1. [DOI] [PubMed] [Google Scholar]
- Volders PG, Sipido KR, Vos MA, Spätjens RLHMG, Leunissen JDM, Carmeliet E, et al. Downregulation of delayed rectifier K+ currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100:2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Vos MA, Verduyn SC, Gorgels AP, Lipcsei GC, Wellens HJ. Reproducible induction of early after depolarizations and torsade de pointes arrhythmias by d-sotalol and pacing in dogs with chronic atrioventricular block. Circulation. 1995;91:864–872. doi: 10.1161/01.cir.91.3.864. [DOI] [PubMed] [Google Scholar]