Abstract
Background and purpose:
Studies were designed to examine the effects of dietary fats on metabolic effects of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy). These effects included hyperthermia, expression of uncoupling protein (UCP1 and 3) in brown adipose tissue or skeletal muscle and plasma free fatty acid (FFA) levels.
Experimental approach:
Male Sprague-Dawley rats were fed either a high-fat diet (HFD, 60% kcal) or a lower fat isocaloric controlled diet (LFD, 10% kcal) for 28 days before MDMA challenge.
Key results:
No significant differences were observed between LFD and HFD groups in terms of body weight, plasma thyroxine (T4) levels and expression of brown fat UCP1 or skeletal muscle UCP3 protein. HFD significantly raised levels of circulating FFA and potentiated the thermogenesis induced by MDMA (10 mg kg−1, s.c.), compared to the effects of the LFD. Moreover, 30 and 60 min after MDMA administration, plasma FFA levels decreased in HFD animals, but were markedly elevated in the LFD group.
Conclusions and implications:
These results indicate that high-fat feeding regulates MDMA-induced thermogenesis by augmenting the activation of UCP rather than its expression.
Keywords: MDMA, hyperthermia, free fatty acids, uncoupling proteins
Introduction
Increased production and accumulation of body heat in response to activation of the sympathetic nervous system (SNS) induced by sympathomimetic agents, involves a combination of vasoconstriction-induced loss of heat dissipation (Pedersen and Blessing, 2001) and the induction of inducible or ‘facultative' metabolic thermogenic mechanisms (Mills et al., 2004). Mitochondrial uncoupling proteins (UCPs) are the most prominent direct mediators of facultative thermogenesis. UCPs generate heat by regulating inducible mitochondrial proton leak, a highly exergonic process, which ‘uncouples' the free energy stored in the electrochemical proton gradient from ATP synthesis. First identified three decades ago, UCP1 in brown adipose tissue (BAT) is both necessary and sufficient for the thermogenic adaptation to cold (Nicholls, 1974). In this complex physiological response, cold-induced activation of the SNS leads to noradrenaline release and activation of β-adrenoceptors on brown adipocytes, which in turn initiate the 3′,5′-cyclic adenosine monophosphate (cAMP) and protein kinase A-dependent activation of intracellular lipases and the accumulation of cellular free-fatty acids (FFA). Although the direct mechanisms and the relevance to in vivo conditions remain controversial, abundant in vitro evidence from isolated mitochondria and recombinant liposomal reconstitution systems show that FFA are required for UCP-dependent proton transport and respiratory uncoupling.
UCP1 is expressed exclusively in BAT; like most other large animals, adult humans have little or no BAT (nor UCP1). Thus, UCP1 is thought to play an insignificant, negligible role in human metabolic physiology. However, the recently identified UCP1 homologue UCP3 is enriched in skeletal muscle and similarly regulates mitochondrial proton leak and respiratory efficiency. Moreover, UCP3, like UCP1, is strongly regulated by SNS-dependent noradrenaline release, β3-adrenoceptors, cAMP, and FFA (see Brand and Esteves, 2005). Consistent with its potential involvement in the regulation of human thermogenesis and metabolism, UCP3 has been implicated in clinical studies as a candidate susceptibility gene in hypometabolic diseases including obesity (Walder et al., 1998) and type II diabetes mellitus (Gable et al., 2006). Although its relevance to normal thermoregulatory physiology remains to be determined, strong support for the hypothesis that UCP3 is a candidate thermogenic mediator in intact animals stems from recent observations in mice indicating that the hypermetabolic hyperthermic responses to treatment with the amphetamines, 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and methamphetamine, are almost entirely lost in UCP3 knockout mice (Mills et al., 2003; Sprague et al., 2004). Clinical case reports have demonstrated that MDMA and methamphetamine often induce a catastrophic hypermetabolic hyperthermic response that leads to skeletal muscle breakdown and death (Fahal et al., 1992; Screaton et al., 1992; Mueller and Korey, 1998), suggesting that pathological mitochondrial uncoupling and heat production within muscle may contribute to human fatalities to these widely abused drugs.
A variety of different FFAS bind and activate proton transport in recombinant UCP3 liposomal preparations (Zackova et al., 2003) and in isolated muscle mitochondria (Echtay et al., 2002). These in vitro studies, showing that FFA are second messengers for UCP3 activation, are supported by recent findings in animal models that show that MDMA-induced peak thermogenesis (which is >80% dependent upon UCP3) is preceded by a significant elevation in circulating FFA (Sprague et al., 2007). Strong support for the notion that FFA act as mediators of thermogenic signalling comes from the very recent observation that cold-induced thermogenesis, a response thought to be exclusively mediated by brown fat UCP1, is lost in animals that lack the plasma membrane fatty acid transport protein 1 (Wu et al., 2006). High-fat feeding is known to increase significantly the levels of circulating and intramyocellar triglyceride FFA precursors (Hoeks et al., 2006). Therefore, to test the hypothesis that FFA may similarly act as UCP-activating thermogenic second messengers in vivo, we compared several physiological metabolic parameters, along with the acute thermogenic responses to MDMA administration in rats subjected to isocaloric high and control, low fat and dietary regimens.
Materials and methods
Animals
All studies were carried out in accordance with the protocols approved by the Virginia Tech Animal Use and Care Committees. Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN, USA) were obtained weighing between 175 and 200 g and were housed in groups of two and given access ad libitum to either the high-fat diet (HFD) or low-fat diet (LFD), depending on treatment group, and water. Housing conditions were maintained at a temperature of 22–24°C and a 12:12 light–dark cycle. Rats were randomly allocated to either HFD (n=12) or LFD (n=12) groups. All animals were allowed 1 week of acclimation before dietary treatment. Each treatment group was fed the appropriate diet 4 weeks before MDMA treatment and blood sampling. Weights were recorded once every week for 4 weeks following the start of dietary treatment.
High- and low-fat diets
Both HFD and LFD were purchased from Research Diets Inc. (New Brunswick, NJ, USA). The HFD was composed of 60% kcal fat, 20% kcal carbohydrate, and 20% kcal protein. The LFD was composed of 10% kcal fat, 70% kcal carbohydrate and 20% kcal protein.
MDMA treatment and blood sampling
Baseline, 30 and 60 min post-MDMA (10 mg kg−1, subcutaneous (s.c.)) blood samples were obtained by retro orbital sinus bleeds under isoflurane anaesthesia. Owing to possible interactions between isoflurane anaesthesia and MDMA, MDMA blood samples were taken 24 h after baseline samples and HFD rats and LFD rats were divided into two groups: 30 min blood draw (n=6) and 60 min blood draw (n=6). Each was divided so that core body temperatures (Tc) and FFA samples would not be affected by isoflurane anaesthesia.
Core temperature assessment
Tc values were measured in all animals before and after MDMA administration, using a Thermalert TH-8 (Physitemp Instruments, Clifton, NJ, USA) monitor with a (RET-2) rectal probe attached to the thermocouple and white petrolatum was applied to the probe before insertion. The probe was inserted 3 cm into the rectum while the rat was gently restrained. A steady readout was obtained within 30 s of probe insertion. Tc values were recorded at 0, 30 and 60 min, as 60 min has been shown as the peak thermogenic response associated with MDMA (Sprague et al., 2007).
Thyroxine analysis
Plasma thyroxine (T4) levels were determined by the DRI Thyroxine Assay method at the College of Veterinary Medicine (Blacksburg, VA, USA). The homogenous enzyme used in this immunoassay contains ready-to-use liquid reagents. This assay uses 8-anilino-1-naphthalene sulphonic acid to dissociate T4 from plasma-binding proteins. The dissociated T4 is then allowed to compete with glucose-6-phosphate dehydrogenase (G6PDH) labelled T4 within the sample for a fixed amount of anti-T4 specific antibody binding sites in the solution. A lack of T4 within the sample allows the enzymatic activity of T4-labelled G6DPH to be inhibited, creating a relationship between T4 concentration in the sample and enzymatic activity. G6PDH activity was determined spectrophotometrically at 340 nm by measuring its ability to convert nicotinamide adenine dinucleotide to NADH, which proportionally represents T4 levels present within the plasma sample.
Free-fatty acid determination
Plasma non-esterified fatty acid (NEFA) levels were determined by the acyl-CoA synthetase–acyl-CoA oxidase (ACS-ACOD) method at the Diagnostic Laboratory of the College of Veterinary Medicine at Cornell University (Ithaca, NY, USA). Briefly, acyl-coenzymeA (acyl-CoA) was combined with the plasma to create CoA thiol esters. Acyl-CoA oxidase was then added, generating hydrogen peroxide which, along with included peroxidase, oxidatively condensed 3-methyl-N-ethyl-N-(β-hydroxy-ethyl)-aniline and 4-aminoantipyrine. This created a purple adduct, which enabled measurement of NEFA by spectrophotometry at 550 nm.
Mitochondrial isolation and Western blotting
Skeletal muscle mitochondrial lysates were prepared as described (Sprague et al., 2007). Briefly, gastrocnemius and BAT biopsies were minced in isolation buffer (100 mM KCl, 50 mM Tris-HCl, 2 mM ethylene glycol bis(β-aminoethylether)-N,N,N',N',-tetraacetic acid, pH 7.4, 4°C), homogenized and centrifuged at 500 g (4°C). Mitochondria were pelleted at 10 500 g for 10 min and resuspended in 20 μl lysis buffer (0.1% Triton-100, phosphate-buffered saline pH 7.4) and incubated on ice for 60 min. BAT samples required additional centrifugation to clear adequately the lysates. A total of 40 μg of mitochondrial protein were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Filters were probed with rabbit polyclonal antibodies to UCP1 or UCP3 (Abcam ab10983 and ab3477, respectively). Levels of cytochrome c were evaluated with anti-cytochrome c (Santa Cruz catalogue no. sc-7159) as a control for total mitochondrial protein loading. Bands were visualized with secondary anti-rabbit antibodies linked to horseradish peroxidase using standard chemiluminescence (Pierce Biotechnology, Rockford, IL, USA).
Statistical analysis
Data within a treatment group were analysed by an analysis of variance (ANOVA) with a Dunnett's post hoc test. Data between groups were analysed by an ANOVA with a Student–Newman–Keuls post hoc test. When only two groups were compared, a Student's t-test was used. All statistical analysis was done using GraphPad Prism (Version 4.0). Significance was set a priori at P<0.05.
Drugs
MDMA was generously donated by Dr David E Nichols, Purdue University (West Lafayette, IN, USA).
Results
Characterization of the physiological effects of the HFD and LFD – thyroid hormone (T4) and body weight
No significant differences were observed between the LFD and HFD groups in either the initial or final body weights over the 4 weeks of dietary treatment. Similarly, levels of plasma T4 – the principal endocrine thermoregulatory hormone and transcriptional regulator of UCP3 – were identical in LFD and HFD groups at the conclusion of the study (Table 1).
Table 1.
Initial weight, final weight, baseline NEFA and baseline T4 levels
| Diet | Initial weight (g) | Final weight (g) | NEFA levels (mEq l−1) | T4 levels (μg l−1) |
|---|---|---|---|---|
| LFD (n=12) | 182.92±1.48 | 356.92±4.17 | 0.50±0.04 | 49.2±2.4 |
| HFD (n=12) | 180.08±1.83 | 361.58±6.87 | 0.71±0.06* | 44.2±3.0 |
Abbreviations: NEFA, non-esterified free fatty acids; T4, thyroxine.
The values in the table are means±s.e.m. from the number of animals shown in parentheses.
Significance between groups (P< 0.03).
Core body temperature assessment
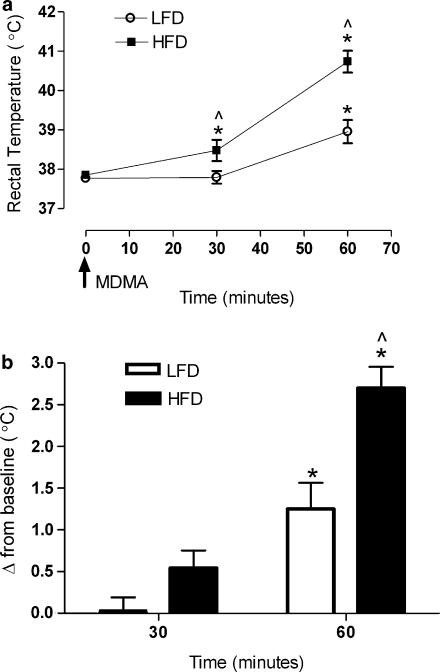
Basal Tc values did not significantly differ between the HFD and LFD group (Figure 1a). However, subsequent to stimulation of the SNS with MDMA, the rise in body temperature exhibited in the LFD group was significantly augmented in the HFD animals. At 30 min post-MDMA treatment, Tc values in the HFD were significantly higher than baseline temperatures (P<0.05) and temperatures of the LFD group (P<0.05). At 60 min, the HFD group differed significantly from both its own baseline (P<0.001) and from the LFD group at 60 min post treatment (P<0.001). At 60 min post treatment, both the HFD and LFD groups had significantly higher Tc values compared to baseline (P<0.01). Moreover, the HFD group maintained a significantly higher temperature 60 min post-MDMA compared to LFD (P<0.01). These data indicate that high fat feeding significantly potentiates the peak (60 min) acute thermogenic/hyperthermic response to MDMA both in terms of the change from baseline and the absolute temperature response comparisons between groups.
Figure 1.
(a) Effects of MDMA (10 mg kg−1, s.c.) on Tc values at an ambient temperature of 23°C in Sprague–Dawley rats consuming either HFD or LFD diets for 28 days. (b) Effects of MDMA (10 mg kg−1, s.c.) on Tc change from baseline at 23°C in Sprague–Dawley rats consuming either HFD or LFD diets for 28 days. Tc values were taken post-MDMA treatment in both LFD and HFD. *Significance from baseline (P<0.01) and ^ Significance between groups (P<0.02). Each value is the mean±s.e.m. (n=12 for 0 and 30 min; n=6 for 60 min). HFD, high-fat diet; LFD, low-fat diet; MDMA, 3,4-methylenedioxymethamphetamine.
Free-fatty acid levels
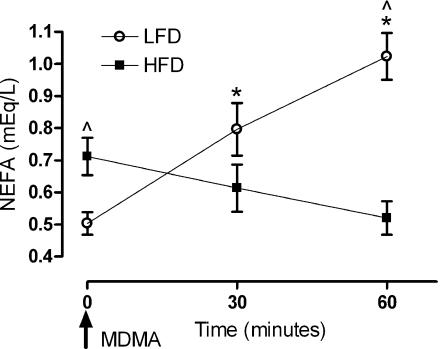
Baseline non-esterified FFA levels before MDMA challenge were significantly higher in HFD versus LFD animals levels 28 days after the start of feeding regimens (Figure 2; P<0.05). To determine if any differences in FFA utilization occurred in response to diet, animals were challenged with MDMA, and FFA levels were measured 30 and 60 min after treatment. At 30 min, no differences were apparent in each group when FFA levels were compared to baseline; however, LFD plasma FFA levels at this time point were significantly higher than those of the HFD group (P<0.01). At 60 min post-MDMA treatment, the LFD group demonstrated a significant elevation in FFA compared to baseline and relative to FFA levels in the HFD group (P<0.001; Figure 2). These data indicate that LFD and HFD fed animals mobilize and/or utilize FFA differently after acute stimulation of the SNS with MDMA.
Figure 2.
Effects of MDMA (10 mg kg−1, s.c.) on non-esterified FFA levels (NEFA) in Sprague–Dawley rats consuming either HFD or LFD diets for 28 days. NEFA levels were taken at baseline and post-MDMA treatment in both LFD and HFD. *Significance from baseline (P<0.01) and ^ Significance between groups (P<0.05). Each value is the mean±s.e.m. (n=12 for 0 min; n=6 for 30 min and n=5 for 60 min). HFD, high-fat diet; LFD, low-fat diet; MDMA, 3,4-methylenedioxymethamphetamine; NEFA, non-esterified fatty acid.
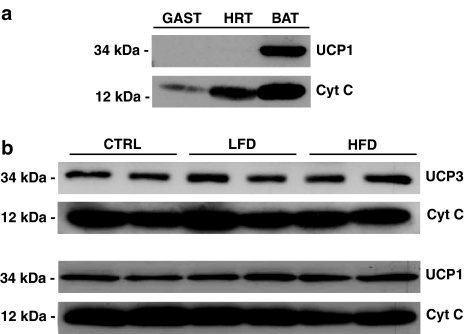
UCP1 and UCP3 levels in gastrocnemius and BAT samples
HFD or LFD feeding for 28 days did not alter the expression of UCP3 protein in mitochondria isolated from skeletal muscle nor did it change levels of UCP1 in brown fat mitochondria. We confirmed the specificity of the Abcam UCP1 antibody (ab10983) by comparing UCP1 immunoreactivity in mitochondria isolated from gastrocnemius (GAST), heart (HRT), and BAT (Figure 3a). This antibody detected a ∼34 kDa immunoreactive band only in BAT. We have previously used skeletal muscle mitochondria from UCP3 knockout mice to confirm the specificity of the anti-UCP3 antibody (Abcam no. 3477) used in these studies (Sprague et al., 2007). No differences were observed in levels of BAT UCP1 (lower) and skeletal muscle UCP3 (upper) protein in response to chronic changes in dietary fat content (Figure 3b). These data indicate that this particular HFD dietary regimen has no effect on the expression of UCP1 and UCP3 at the protein level in mitochondria.
Figure 3.
Effects of chronic manipulations in dietary fat on UCP expression. (a) Specificity of the Abcam UCP1 antibody (ab10983) comparing UCP1 immunoreactivity in gastrocnemius (GAST), heart (HRT), and BAT. The antibody detected a ∼34 kDa immunoreactive band only in BAT. (b) No differences were observed in levels of BAT UCP1 (lower) and skeletal muscle UCP3 (upper) protein in response to chronic changes in dietary fat content. BAT, brown adipose tissue; UCP, uncoupling protein.
Discussion
Recent evidence indicates that the acute thermogenic responses to stimulation of the SNS with MDMA and methamphetamine are largely UCP3-dependent (Mills et al., 2003; Sprague et al., 2004). The peak rise in body temperature induced by these agents ranges from 1 to 2 h in rodents and is preceded by a significant increase in plasma levels of noradrenaline and FFA (Sprague et al., 2005, 2007). Noradrenaline stimulates thermogenesis in target tissues by binding most avidly to α1- and β3-adrenoreceptors (Kuusela et al., 1997; Zhao et al., 1997). β3-Adrenoreceptors activate the cAMP-dependent, hormone-sensitive lipase mediated conversion of stored intracellular triacylglycerols into FFA in brown adipocytes and skeletal muscle. Noradrenaline also stimulates the release of FFA into the general circulation from triglyceride depots in white adipose tissue (Garofalo et al., 1996). On the basis of the abundant in vitro data, FFAs activate UCP1-3-dependent respiratory uncoupling through thermogenic proton leak, but the mechanisms by which fatty acids and UCPs interact to mediate proton leak are highly controversial (Garlid et al., 1996). Dietary manipulations have been shown to modify UCP1-dependent thermoregulatory responses to cold. However, these data may have little or no relevance to thermoregulation in adult humans thought not to express metabolically relevant amounts of UCP1 and brown fat. Moreover, there is a lack of studies to support a role for FFA in the regulation of an UCP3-mediated thermogenic function in animals. Here, we demonstrate for the first time that thermogenesis induced by MDMA administration is significantly enhanced by high-fat feeding. We also ruled out the possibilities that the enhanced thermogenic responses exhibited in HFD fed animals were due to changes in body weight, plasma thyroid hormone levels (T4), or changes in the protein levels of UCPs 1 and 3 in brown fat and skeletal muscle, respectively. Following MDMA administration, the HFD animals displayed a reduction in FFA levels and a significant elevation in body temperature. Conversely, animals maintained on a LFD demonstrated a rise in FFA levels, which was also correlated with a rise in core temperature; however, the LFD group never reached the same magnitude of elevation in core temperature as the HFD group.
FFAs are the required ligand activators of UCPs. FFA-induced uncoupling of oxidative phosphorylation within skeletal muscle mitochondria has been shown to play an important role in regulating body temperature in animals exposed to cold environmental conditions (Brustovetsky et al., 1992). FFAs also activate UCP3 and accumulate in skeletal muscle during contraction or exercise (Tsutsui et al., 2001; Watt et al., 2003; McArdle et al., 2004). Curtin et al. (2002) found that skeletal muscle UCP3 overexpression enhances the thermogenic response to contraction ex vivo, but no differences in muscle temperatures were apparent at rest. Moreover, nonhuman primate studies have demonstrated that increases in muscle contractions (namely locomotor activity) are not required for MDMA to induce hyperthermia (Crean et al., 2006). Skeletal muscle mitochondria from hyperthyroid rats exhibit increased proton conductance (Brand et al., 1992) along with increased levels of FFAs (Lombardi et al., 2002); moreover, increased mitochondrial proton leak in hyperthyroid skeletal muscle is abolished when FFAs are removed (Silvestri et al., 2005). We have previously shown that hyperthyroid animals are more sensitive to the thermogenic effects of MDMA and have elevated levels of skeletal muscle UCP3 (Sprague et al., 2007). Together, these observations strongly suggest that FFAs are likely to be required for uncoupling-mediated thermogenesis.
Previous studies have shown that a HFD can increase plasma FFA levels (Chou et al., 2001) along with skeletal muscle triacylglycerides, and that these differences in skeletal muscle FFA accumulation are greatly enhanced in the absence of UCP3 (Hoeks et al., 2006). In obese and lean rats, fasting has been demonstrated to increase FFA levels (Cha et al., 2005) and in these rats, central administration of C75, an inhibitor of fatty acid synthase, and peripheral administration of phentolamine, a nonselective α-adrenoreceptor blocker, antagonized fatty acid oxidation in the periphery significantly more than when C75 was used alone (Cha et al., 2005). These latter findings confirm that the SNS and α-adrenoreceptors are directly involved in fatty acid breakdown.
Cha et al. (2005) further demonstrated that the combination of C75 and phentolamine decreased mRNA levels of UCP3 present in skeletal muscle. Studies on the expression of UCP3 protein in muscle in response to dietary fat are equivocal. HFD feeding has been shown to apparently increase UCP3 protein expression in skeletal muscle (Chou et al., 2001), but others have observed no change in mRNA expression (Kusunoki et al., 2005). In the present study, we have demonstrated that a HFD versus an isocaloric control LFD affected neither UCP3 nor UCP1 expression levels in skeletal muscle and brown fat, respectively. However, we confirmed that HFD increased plasma FFA compared to LFD, and significantly augmented hyperthermia induced by MDMA. Furthermore, UCP1 and UCP3 expression in both BAT and skeletal muscle are highly regulated by thyroid hormone (Sprague et al., 2007). Consistent with a lack of effect of HFD on UCP levels in mitochondria from BAT and skeletal muscle, we found that high-fat feeding had no effect on levels of circulating T4. We have recently verified two antibodies/detection methodologies that are appropriate for UCP3 detection in mouse and rat tissues by western blotting (Sprague et al., 2007). In the same article, we report that another purchased UCP3 antibody detects a strong 34 kDa non-specific immunoreactive band in UCP3 knockout mitochondria and wild type equally well. We anticipate that many of the discrepancies reported in the literature are due to the use of methodologies and reagents not verified for specificity, using knockout systems. Furthermore, the lack of change in UCP1 and UCP3 proteins we observed between LFD and HFD animals may also reflect the duration (28 days) and/or the quality of nutritional components and fats in various dietary regimens.
Together, these data demonstrate that dietary fat is a significant and positive regulator of inducible thermogenesis and suggest that the thermoregulatory effects of high-fat feeding involve changes in UCP activity, most likely as the result of elevated FFAs.
Abbreviations
- BAT
brown adipose tissue
- G6PDH
glucose-6-phosphate dehydrogenase
- HFD
high-fat diet
- LFD
low-fat diet
- MDMA
3,4-methylenedioxymethamphetamine
- SNS
sympathetic nervous system
- T4
thyroxine
- Tcs
core body temperatures
- UCP
uncoupling protein
Conflict of interest
The authors state no conflict of interest.
References
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brand MD, Steverding D, Kadenbach B, Stevenson PM, Hafner RP. The mechanism of the increase in mitochondrial proton permeability induced by thyroid hormones. Eur J Biochem. 1992;206:775–781. doi: 10.1111/j.1432-1033.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky NN, Egorova MV, Gnutov DYU, Gogvadze VG, Mokhova EN, Skulachev VP. Thermoregulatory, carboxyatractylate-sensitive uncoupling in heart and skeletal muscle mitochondria of the ground squirrel correlates with the level of free fatty acids. FEBS Lett. 1992;305:15–17. doi: 10.1016/0014-5793(92)80645-w. [DOI] [PubMed] [Google Scholar]
- Cha S, Hu Z, Chohnan S, Lane M. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, Cha M, Jung D, Boozer C, Hashim S, Pi-Sunyer X. High-fat diet feeding elevates skeletal muscle uncoupling protein 3 levels but not its activity in rats. Obes Res. 2001;9:313–319. doi: 10.1038/oby.2001.39. [DOI] [PubMed] [Google Scholar]
- Crean RS, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (±)3, 4-methylenedioxymethamphetamine, (±)3, 4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NA, Clapham JC, Barclay CJ.Excess recovery heat production by isolated muscles from mice overexpressing uncoupling protein-3 J Physiol 2002542231–235.Part 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Fahal IH, SallomiD F, Yoqoob M, Bell GM. Acute renal failure after ecstasy. BMJ. 1992;305:29. doi: 10.1136/bmj.305.6844.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable DR, Stephens JW, Cooper JA, Miller GJ, Humphries SE. Variation in the UCP2-UCP3 gene cluster predicts the development of type 2 diabetes in healthy middle-aged men. Diabetes. 2006;55:1504–1511. doi: 10.2337/db05-1645. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Orosz DE, Modriansky M, Vassanelli S, Jezek P. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J Biol Chem. 1996;217:2615–2620. doi: 10.1074/jbc.271.5.2615. [DOI] [PubMed] [Google Scholar]
- Garofalo MAR, Kettelhut IC, Roselino JES, Migliorini RH. Effects of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J Auton Nerv Sys. 1996;60:206–208. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- Hoeks J, Hesselink MK, Sluiter W, Schaart G, Willems J, Morrison A, et al. The effect of high-fat feeding on intramuscular lipid and lipid peroxidation levels in UCP3-ablated mice. FEBS Lett. 2006;580:1371–1375. doi: 10.1016/j.febslet.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Tsutsumi K, Iwata K, Yin W, Nakamura T, Ogawa H, et al. NO-1886 (ibrolipim), a lipoprotein lipase activator, increases the expression of uncoupling protein 3 in skeletal muscle and suppresses fat accumulation in high-fat diet-induced obesity in rats. Metabolism. 2005;54:1587–1592. doi: 10.1016/j.metabol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kuusela P, Rehnmark S, Jacobsson A, Cannon B, Nedergaard J. Adrenergic stimulation of lipoprotein lipase gene expression in rat brown adipocytes differentiated in culture: mediation via beta3- and alpha1-adrenergic receptors. Biochem J. 1997;321:759–767. doi: 10.1042/bj3210759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A, Silvestri E, Moreno M, De Lange P, Farina P, Goglia F, et al. Skeletal muscle mitochondrial free-fatty-acid content and membrane potential sensitivity in different thyroid states: involvement of uncoupling protein-3 and adenine nucleotide translocase. FEBS Lett. 2002;532:12–16. doi: 10.1016/s0014-5793(02)03690-6. [DOI] [PubMed] [Google Scholar]
- McArdle A, van der Meulen J, Close GL, Pattwell D, Van Remmen H, Huang TT, et al. Role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space. Am J Physiol Cell Physiol. 2004;286:C1152–C1158. doi: 10.1152/ajpcell.00322.2003. [DOI] [PubMed] [Google Scholar]
- Mills E, Rusyniak D, Sprague J. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxy-methamphetamine. J Mol Med. 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- Mills EM, Banks ML, Sprague JE, Finkel T. Uncoupling the agony from ecstasy. Nature. 2003;426:403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- Mueller PD, Korey WS. Death by ‘ecstasy': the serotonin syndrome. Ann Emer Med. 1998;32 (3 Part 1):377–380. doi: 10.1016/s0196-0644(98)70018-6. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Hamster brown-adipose-tissue mitochondria. The control of respiration and the proton electrochemical potential gradient by possible physiological effectors of the proton conductance of the inner membrane. Eur J Biochem. 1974;49:573–583. doi: 10.1111/j.1432-1033.1974.tb03861.x. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3, 4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton GR, Singer M, Cairns HS, Thrasher A, Sarner M, Cohen SL. Hyperpyrexia and rhabdomyolysis after MDMA (‘ecstasy') abuse. Lancet. 1992;339:677–678. doi: 10.1016/0140-6736(92)90834-p. [DOI] [PubMed] [Google Scholar]
- Silvestri E, Moreno M, Lombardi A, Ragni M, de Lange P, Alexson SE, et al. Thyroid-hormone effects on putative biochemical pathways involved in UCP3 activation in rat skeletal muscle mitochondria. FEBS Lett. 2005;579:1639–1645. doi: 10.1016/j.febslet.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Mallet NM, Rusyniak DE, Mills E. UCP3 and thyroid hormone involvement in methamphetamine-induced hyperthermia. Biochem Pharmacol. 2004;68:1339–1343. doi: 10.1016/j.bcp.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Moze P, Caden D, Rusyniak DE, Holmes C, Goldstein DS, et al. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) in an animal model. Crit Care Med. 2005;33:1311–1316. doi: 10.1097/01.ccm.0000165969.29002.70. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Yang X, Sommers J, Gilman TL, Mills EM. Roles of norepinephrine, free fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J Pharmacol Exp Ther. 2007;320:274–280. doi: 10.1124/jpet.106.107755. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, et al. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation. 2001;104:134–136. doi: 10.1161/01.cir.104.2.134. [DOI] [PubMed] [Google Scholar]
- Walder K, Norman RA, Hanson RL, Schrauwen P, Neverova M, Jenkinson CP, et al. Association between uncoupling protein polymorphisms (UCP2-UCP3) and energy metabolism/obesity in Pima Indians. Human Mol Gen. 1998;7:1431–1435. doi: 10.1093/hmg/7.9.1431. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Heigenhauser GJ, Spriet LL, Dyck DJ. Hormone-sensitive lipase activity and triacylglycerol hydrolysis are decreased in rat soleus muscle by cyclopiazonic acid. Am J Physiol Endocrinol Metab. 2003;285:E412–E419. doi: 10.1152/ajpendo.00023.2003. [DOI] [PubMed] [Google Scholar]
- Wu Q, Kazantzig M, Doege H, Ortegon AM, Tsang B, Falcon A, et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes. 2006;55:3229–3237. doi: 10.2337/db06-0749. [DOI] [PubMed] [Google Scholar]
- Zackova M, Skobisova E, Urbankova E, Jezek P. Activating omega-6 polyunsaturated fatty acids and inhibitory purine nucleotides are high affinity ligands for novel mitochondrial uncoupling proteins UCP2 and UCP3. J Biol Chem. 2003;278:20761–20769. doi: 10.1074/jbc.M212850200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Cannon B, Nedergaard J. alpha1-Adrenergic stimulation potentiates the thermogenic action of beta3-adrenoreceptor-generated cAMP in brown fat cells. J Biol Chem. 1997;272:32847–32856. doi: 10.1074/jbc.272.52.32847. [DOI] [PubMed] [Google Scholar]