Abstract
Background and purpose:
Evidence indicates that the endocannabinoid, 2-arachidonoylglycerol (2-AG), increases food intake when injected into the nucleus accumbens shell (NAcS), thereby potentially activating hypothalamic nuclei involved in food intake regulation. We aimed to evaluate potential orexigenic effects of the endocannabinoid anandamide and of AA5HT, a fatty acid amide hydrolase (FAAH) inhibitor, and OMDM-1, an inhibitor of anandamide uptake, injected in the NAcS, as well as the effect of these treatments on activation of hypothalamic nuclei.
Experimental approach:
Drugs were given into the NAcS of rats and food intake quantified during the next 4 h. In other groups, after the same treatments the brains were processed for c-Fos immunohistochemistry with focus on hypothalamic nuclei. Additional groups were used to quantify endocannabinoid levels in the nucleus accumbens and the hypothalamus after AA5HT and OMDM-1 intra-NAcS injections.
Key results.
Our results indicate that the above treatments stimulate food intake during 4 h post-injection. They also increase c-Fos immunoreactivity in hypothalamic nuclei. The CB1 antagonist, AM251, blocked these effects. Finally, we found elevated levels of 2-AG, but not anandamide, after intra-NAcS injections of AA5HT.
Conclusions and implications:
These data support the involvement of the endocannabinoid system in feeding behavior at the level of the NAcS and hypothalamus. In addition, this is the first experimental demonstration that the pharmacological inhibition of endocannabinoid inactivation in the NAcS stimulates food intake, suggesting that the endocannabinoid degrading proteins can be a target for treating eating disorders.
Keywords: hyperphagia, anandamide, cannabinoid receptor 1, FAAH, hypothalamus, anandamide transporter, nucleus accumbens shell
Introduction
Mounting evidence supports the involvement of the endocannabinoid system in the control of feeding behaviour, mainly by regulating the activity of the brainstem, the hypothalamus and the nucleus accumbens (NAc) (Cota et al., 2003; Cooper, 2004; Di Marzo and Matias, 2005; Fride et al., 2005). For example, it has been demonstrated in several animal models that the endocannabinoids, anandamide (ANA), 2-arachidonoylglycerol (2-AG) and oleamide increase food intake, and that such effects are mediated by the CB1 receptor (Williams and Kirkham, 1999, 2002; Jamshidi and Taylor, 2001; Kirkham et al., 2002; Martínez-González et al., 2004). In this context, the CB1 antagonist SR141716A (rimonabant) has been used in humans (Van Gaal et al., 2005) and AM251, another CB1 antagonist, in animals, to reduce food intake and induce weight loss (Hildebrandt et al., 2003; Chambers et al., 2004). Moreover, mice that are CB1 null (CB1(−/−)) are lean and hypophagic (Cota et al., 2003; Osei-Hyiaman et al., 2005b; Wiley et al., 2005).
The sites of the orexigenic action of endocannabinoids have been studied. For example, both ANA and 2-AG have been detected in high concentrations in the hypothalamus, where food restriction increases 2-AG, but not ANA levels (Kirkham et al., 2002). However, leptin reduces the concentration of both endocannabinoids (Di Marzo et al., 2001). It has also been shown that mice lacking fatty acid amide hydrolase (FAAH) (FAAH(−/−)), the enzyme that metabolizes ANA, oleamide and 2-AG, exhibit reduced levels of the cocaine- and amphetamine-response transcript, an anorexigenic peptide, in the arcuate nucleus (ARC), in the dorsomedial hypothalamus (DMH) and in the NAc (Osei-Hyiaman et al., 2005a). In addition, ANA increases the release of neuropeptide Y, an orexigenic mediator, in the hypothalamus in vitro (Gamber et al., 2005), suggesting that high hypothalamic levels of endocannabinoids following food deprivation downregulate anorectic and upregulate orexigenic neuropeptides.
Administration of ANA into the ventromedial hypothalamus, a nucleus with CB1-expressing neurons, increases food intake in rats (Jamshidi and Taylor, 2001). On the other hand, infusion of GABAergic (Stratford and Kelley, 1997; Söderpalm and Berridge, 2000; Zhang et al., 2003) and opioidergic (MacDonald et al., 2003; Will et al., 2003) agonists, or glutamatergic antagonists (Kelley and Swanson, 1997; Stratford et al., 1998) into the NAc stimulates food intake. These treatments also produce activation of hypothalamic nuclei related to food intake (Maldonado-Irizarry et al., 1995; Stratford and Kelley, 1999). Furthermore, simultaneous inactivation of these hypothalamic nuclei (that is, lateral hypothalamus, LH) blocks the increase in food intake induced by these drugs (Maldonado-Irizarry et al., 1995; Will et al., 2003), thus indicating that stimulation of orexigenic mechanisms in the NAc is, at least in part, due to activation of hypothalamic nuclei. Infusion of 2-AG into the nucleus accumbens shell (NAcS), a subdivision of the NAc, produces a hyperphagic effect that is blocked by the systemic administration of rimonabant (Kirkham et al., 2002). However, whether or not also ANA enhances food intake when administered into the NAcS has not yet been determined. In the present study, we aimed at (a) demonstrating that ANA stimulates food intake when administered into the NAcS, (b) determining the ability of N-arachidonoyl-serotonin (AA5HT), an inhibitor of FAAH (Bisogno et al., 1998), and (R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine (OMDM-1: Ortar et al., 2003), a selective inhibitor of ANA cellular reuptake via a putative plasma membrane transporter, to enhance food intake when injected into the NAcS, (c) investigating whether these treatments in the NAcS activate the hypothalamus, and (d) determining endocannabinoid levels in the NAc and the hypothalamus after AA5HT or OMDM-1 administration.
Methods
Animals
Male Wistar rats (n=185) weighing between 250 and 280 g were used. After surgery, rats were housed individually in Plexiglas cages and maintained at constant temperature (23°C) in a reverse dark–light cycle (lights on from 2000 to 0800 hours) with food and water ad libitum. All the behavioural tests were carried out at the beginning of the dark period (0800 hours). None of the animals used in these experiments was food deprived at any time.
Rats were implanted bilaterally with 23-gauge stainless steel guide cannulae aimed at the NAcS (A=2.0; L=±1.0; V=−6.0; reference to Bregma, Paxinos and Watson, 1986). After fixation to the skull with dental cement, the cannulae were sealed with stylets to maintain their patency. Infusions were performed with the aid of 30-gauge injectors protruding 1.5 mm from the end of the guide cannula. The injections were performed while animals were temporarily restrained. After infusion, injectors were kept in place for 60 s to prevent outflow. All animals were treated in accordance with American Association for Accreditation of Laboratory Animal Care Policy.
Intra-NAcS administration of ANA, AA5HT and OMDM-1: food intake evaluation
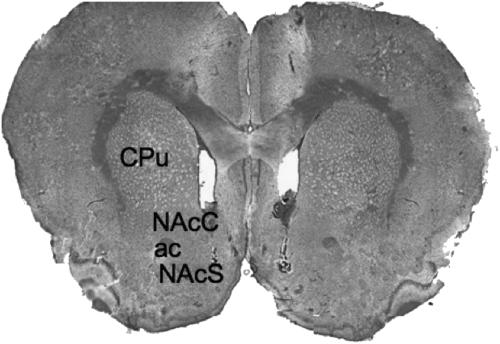
Rats (n=140) were gently handled for 2 h during 2 days before experimental manipulations to minimize stress. The number of experimental subjects for each group was 10. ANA was administered in three different doses: 0.1, 1.0 and 10.0 μg, in 0.5 μl per side (0.25 μl min−1). AA5HT and OMDM-1 doses were 1.0 μg per side. To determine if the behavioural effect produced by the most potent dose of ANA (1.0 μg) was exerted through the CB1 receptor, we prepared a cocktail with ANA and the CB1 receptor antagonist, AM251 (1.6 μg per side, equivalent in moles to ANA) (n=10). AA5HT and OMDM-1 were also mixed with AM251 (1.6 μg) to prevent the behavioural effect produced by these drugs. Since the OMDM-1 effects were not blocked by the AM251 at a dose of 1.6 μg, we proceeded to increase the dose until the effect was clearly blocked, that is, to 4.8 and 9.6 μg in 0.5 μl per side. In additional groups, AM251 was administered alone at the doses mentioned above. Rats under the effect of the different treatments received a pre-weighed amount of regular laboratory chow pellets, after intra-NAcS infusions. We estimated the amount of food ingested by the rats calculating the difference between the original food weight and the weight of the leftover food, corrected for by the spill-over, at 1 and 4 h after injection. Upon completion of food intake evaluation, rats were perfused transcardially with 200 ml phosphate-buffered saline (PBS) and 200 ml of 4% paraformaldehyde (4% PFH). The brains were prepared for histological analysis with cresyl violet staining to verify the correct placement of the injector tip (Figure 1). Animals with injector tips misplaced were discarded.
Figure 1.
Representative photomicrograph of injection sites. CPu, caudate putamen; ac, anterior commissure; NAcC, nucleus accumbens core; NAcS, nucleus accumbens shell.
Intra-NAcS administration of ANA, AA5HT and OMDM-1: hypothalamic c-Fos
In another group of rats (n=35), all drugs were administered as follows. For this experiment, we used the ANA dose (1.0 μg) that produced the largest effect in the first hour and the AM251 doses (1.6 and 9.6 μg) that prevented the hyperphagic effect induced by the various treatments. For the rest of treatments, the concentrations were the same. Five rats were used for each group.
Ninety minutes after administration of each compound or combination thereof, rats were anaesthetized with sodium pentobarbital and perfused transcardially with 200 ml PBS and 200 ml 4% PFH. Then, the brains were prepared for cryostat sectioning; 50-μm coronal slices were made at the hypothalamic level. For each slice taken for c-Fos immunohistochemistry, another immediately adjacent was selected for cresyl violet staining to determine accurately the limits of a given hypothalamic nucleus of our interest. Immunohistochemistry was developed with the peroxidase-diaminobenzidine reaction.
Sections were observed under a light microscope (Olympus BX41). Images were captured on a 12-bit digital camera (Evolution VF; MediaCybernetics) at a magnification of × 4 for panoramic resolution of the hypothalamic nuclei and at × 10 for cell-nuclei counting. The hypothalamic nuclei of interest were the following: paraventricular nucleus (PVN), ARC, DMH and LH. Images were displayed on a computer screen using the Image-Pro Plus software (MediaCybernetics) that allowed us to mark individual c-Fos immunoreactive (F-ir) cells. Counts of F-ir cells were obtained bilaterally from two brain sections for each region and averaged for subsequent analysis. Cells were counted in the defined nuclear areas as determined in the cresyl violet section for each region.
Intra-NAcS administration of AA5HT and OMDM-1: ANA and 2-AG quantification
The protocol of drug administration was the same as that in the experiments described above. The number of experimental subjects for each group (vehicle, AA5HT and OMDM-1) was four. Ninety minutes after infusions rats were killed. Rat brains were quickly removed, and the NAc and the hypothalamus were obtained by regional dissection on ice and immediately frozen in dry ice. Tissues were stored at −80°C until use. Tissues were then homogenized in 5 vol of chloroform/methanol/Tris-HCl 50 mM; (2:1:1) containing 100 pmol each of d8-anandamide and d5-2-AG as internal standards. Homogenates were centrifuged at 13 000 g for 16 min (4°C), the aqueous phase plus debris were collected and extracted again twice with 1 vol of chloroform. The organic phases from the three extractions were pooled and the organic solvents evaporated in a rotating evaporator. Lyophilized samples were then stored frozen at −80°C under nitrogen atmosphere until analysed. Lyophilized extracts were resuspended in chloroform/methanol (99:1; v/v). The solutions were then purified by open bed chromatography on silica. Fractions eluted with chloroform/methanol 9:1 by volume (containing ANA and 2-AG) were collected and the excess solvent evaporated with a rotating evaporator. The prepurified fractions were then analysed by isotope dilution liquid chromatography mass-spectrometry using chromatographic and mass spectrometric, as described previously (Di Marzo et al., 2001), but employing a single-quadrupole instrument from Shimadzu, Japan. Two liquid chromatography–mass spectrometry (LC–MS) peaks for both deuterated and undeuterated monoarachidonoylglycerol were found, corresponding to 2-AG and 1(3)-AG, in agreement with the previous observation that 2-AG undergoes isomerization during the purification procedure. Therefore, the amounts of 2-AG were calculated by adding the amounts of the two isomers. The amounts of endocannabinoids are expressed as pmol or nmol per gram of wet tissue extracted.
Data analysis
Food intake data obtained at the first or fourth hour were analysed, independently, using a one-way analysis of variance (ANOVA) test, followed by a post hoc Bonferroni test. Results are presented as mean±s.e.m. of the amount of food ingested.
Cell counts of F-ir cells were analysed by using a one-way ANOVA test with treatment and hypothalamic nucleus as factors, followed by a post hoc Bonferroni test. Results are presented as mean±s.e.m. Quantitative data of ANA and 2-AG content were analysed by a one-way ANOVA test followed by a post hoc Bonferroni test. Results are presented as mean±s.e.m. Significance was accepted at P<0.05 for all analyses.
Drugs
ANA was purchased from Sigma (St Louis, MO, USA), and AM251 from Cayman Chemical Company (Ann Arbor, MI, USA). AA5HT and OMDM-1 were synthesized as described previously (Bisogno et al., 1998; Ortar et al., 2003). The vehicle for ANA, AA5HT and OMDM-1 was 5% ethanol in saline solution. A mixture of dimethyl sulphoxide (25%), PBS (74%) and Tween 20 (1%) was used to dissolve AM251. The anti-c-Fos antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Results
Effects of ANA infusions into the NAcS on food intake
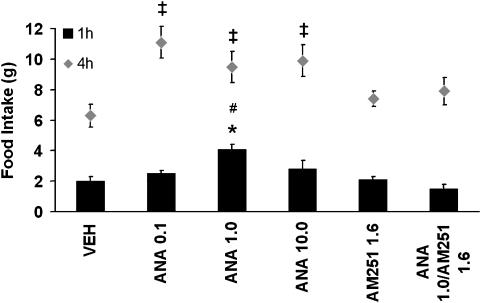
Results indicated that, in the first hour, ANA (1.0 μg) increased the amount of food ingested by about 100% compared to the vehicle (Figure 2). The CB1 antagonist, AM251, blocked this hyperphagic effect. Interestingly, AM251 alone did not produce any effect on food intake. The other two doses of ANA seemed to increase food intake but did not reach significance. Four hours after the administration, all doses of ANA increased food consumption, compared with the vehicle. Although AM251 given alone remained without effect, it prevented the orexigenic effects of ANA (Figure 2).
Figure 2.
Effects on food intake of infusions of three doses of ANA (0.1, 1.0 and 10.0 μg) and the CB1 antagonist, AM251, into the NAcS. Values are means±s.e.m. In the first hour, only 1.0 μg ANA increased food intake that was blocked by AM251. In the fourth hour, all doses of ANA increased food intake. * and ‡P<0.05 vs vehicle group. #P<0.05 vs AM251 and ANA1.0/AM251. ANA, anandamide; NAcS, nucleus accumbens shell.
Effects of AA5HT infusions into the NAcS on food intake
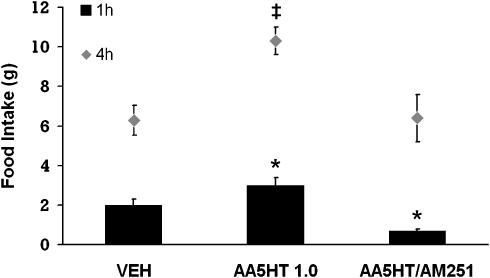
In this study, we tested whether the infusion of AA5HT, a FAAH inhibitor, into the NAcS affected food intake, and if such an effect was mediated through the CB1 receptor. We observed that AA5HT significantly stimulated food intake at the first and fourth hours post-injection, compared with the vehicle (Figure 3). This effect was prevented by AM251 at both times.
Figure 3.
Effects on food intake of infusions into the NAcS of the FAAH inhibitor, AA5HT (1.0 μg). Values are means±s.e.m. AA5HT increased food intake in the 1st and 4th hour. The effect was blocked by the coadministration of AM251. * and ‡P<0.05 vs vehicle and AA5HT/AM251. The combination of AA5HT and AM251 reduced food intake in the first hour. *P<0.05 vs vehicle and AA5HT.
Effects of OMDM-1 infusions into the NAcS on food intake
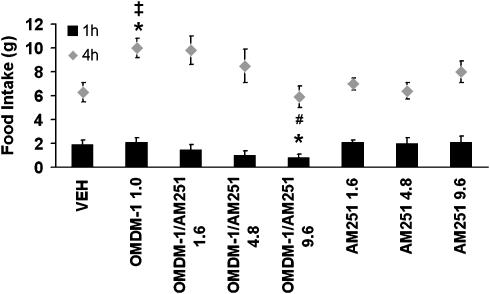
OMDM-1 significantly increases food intake only at the 4 h time point, post-injection compared with vehicle (Figure 4). However, this effect was not prevented by the dose of AM251 (1.6 μg) that blocked the effect of ANA and AA5HT or by a higher dose (4.8 μg). It was necessary to increase the dose to 9.6 μg for effective blockade. Interestingly, AM251 administered alone at the higher doses of 4.8 and 9.6 μg still did not affect ingestive behaviour (Figure 4).
Figure 4.
Effects on food intake of infusions into the NAcS of the ANA transporter inhibitor, OMDM-1 (1.0 μg). Values are means±s.e.m. OMDM-1 increased food intake only after 4 h. Such effects are reversed by AM251 at a dose of 9.6 μg. *P<0.05 vs vehicle and ‡P<0.05 vs OMDM-1/AM251 9.6, AM251 1.6, AM251 4.8 and AM251 9.6. In the first hour, OMDM-1 plus AM251 9.6 decreases food intake compared with vehicle and OMDM-1. *P<0.05, and #P<0.05 vs AM251 1.6, AM251 4.8 and AM251 9.6. ANA, anandamide; NacS, nucleus accumbens shell; OMDM-1, (R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine.
Effects of ANA, AA5HT and OMDM-1 infusions into the NAcS on hypothalamic c-Fos expression
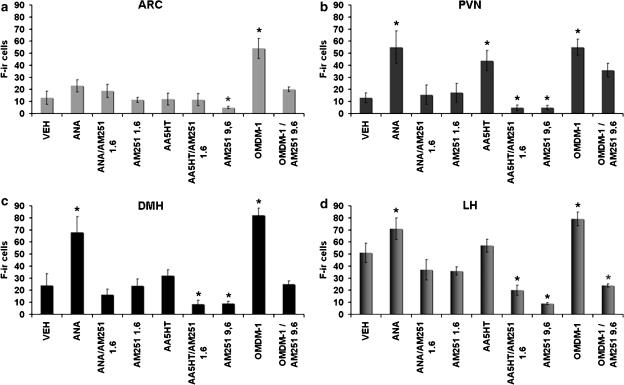
This experiment allowed us to determine whether endocannabinoid treatments into the NAcS affected hypothalamic nuclei related to feeding behaviour. Figure 5 shows representative microphotographs of F-ir cells in the four nuclei, ARC, PVN, DMH and LH, in which NAcS treatments induced significant changes. Mean values for F-ir cell counts after these treatments in the four hypothalamic nuclei are shown in Figure 6. No changes in ARC were documented except for those produced by OMDM-1 and such an effect was blocked by AM251. In turn, ANA, AA5HT and OMDM-1 increased the numbers of F-ir cells in the PVN, compared to the values after treatment with vehicle. These effects were prevented by AM251. Unlike AA5HT, ANA and OMDM-1 increased F-ir cell numbers in the DMH, compared to vehicle. This effect was also blocked by AM251. In the LH, only ANA and OMDM-1 increased c-Fos expression, compared to vehicle. AA5HT tended to increase F-ir cells but did not reach significance. Again, AM251 prevented both effects. Despite the fact that the administration of AM251 (1.6 μg) alone into the NAcS did not produce any change in the number of F-ir cells in any of the nuclei, the higher dose, 9.6 μg, significantly diminished Fos immunoreactivity in all four of the hypothalamic nuclei assayed.
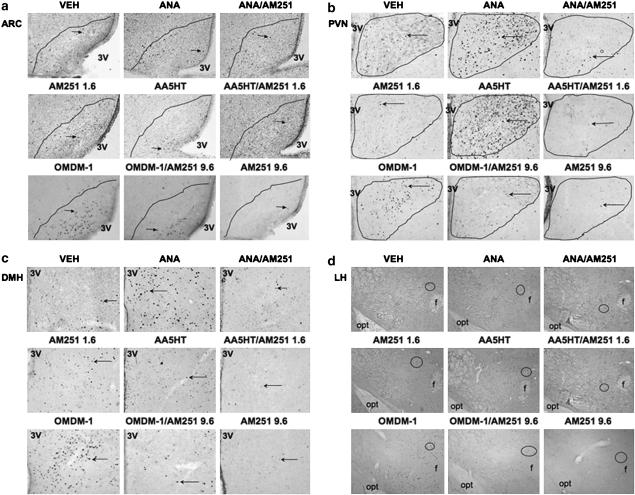
Figure 5.
Representative photomicrographs showing F-ir cells in the ARC (a), PVN (b), DMH (c) and LH (d) under NAcS treatments. Only OMDM-1 increased c-Fos expression in all nuclei. ANA and AA5HT increased F-ir cell numbers in the PVN, DMH and LH, compared to vehicle (VEH). This effect was blocked by AM251. Magnification: × 10. Scale bar=100 μm. The ARC and PVN areas are delineated by a continuous line, according to the cresyl violet slice for each section. 3V, third ventricle; opt, optic tract; f, fornix. Arrows indicate examples of F-ir cells in ARC, PVN and DMH. Red ellipses in LH indicate Fos-positive cells. ARC, arcuate nucleus; DMH, dorsomedial nucleus; F-ir, c-Fos immunoreactive; LH, lateral hypothalamus; NAcS, nucleus accumbens shell; OMDM-1, (R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine; PVN, paraventricular nucleus.
Figure 6.
Mean numbers of F-ir cells. In the ARC (a) only OMDM-1 increased significantly c-Fos expression. In the PVN (b) ANA and AA5HT increased significantly c-Fos expression. In the DMH (c) and LH (d) ANA and OMDM-1 effects reached significance compared with the vehicle. In all areas, the AM251 blocked the increase in Fos expression. AM251 (9.6 μg) reduced Fos immunoreactivity in all hypothalamic areas. Values are means±s.e.m. *P<0.05 compared with all treatments. ARC, arcuate nucleus; DMH, dorsomedial nucleus; F-ir, c-Fos immunoreactive; LH, lateral hypothalamus; OMDM-1, (R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine; PVN, paraventricular nucleus.
ANA and 2-AG levels after AA5HT or OMDM-1 treatments
The aim of this experiment was to determine if intra-NAcS administration of AA5HT and OMDM-1 increases endocannabinoid levels selectively in the NAcS and not in the hypothalamus. We found an increase in ANA and 2-AG levels in the NAc after AA5HT and OMDM-1 infusions, albeit only AA5HT increased 2-AG significantly compared to vehicle (Table 1). In the hypothalamus, a significant decrease of 2-AG levels was observed with both drugs, whereas no changes were detected for ANA.
Table 1.
Endocannabinoid levels in the nucleus accumbens and the hypothalamus after NAcS infusions of AA5HT and OMDM-1
|
Nucleus accumbens |
Hypothalamus |
|||
|---|---|---|---|---|
| ANA (pmol g−1) | 2-AG (nmol g−1) | ANA (pmol g−1) | 2-AG (nmol g−1) | |
| Vehicle | 64.7±5.5 | 1.6±0.2 | 45.7±4.7 | 5.2±0.3 |
| OMDM-1 | 67.1±3.1 | 2.1±0.6 | 55.4±2.5 | 3.3±0.8* (P=0.04) |
| AA5HT | 75.6±4.8 (P=0.08) | 2.6±0.4* (P=0.03) | 43.0±2.8 | 3.3±0.8* (P=0.04) |
Abbreviations: AA5HT, N-arachidonoyl-ethanolamine; 2-AG, 2-arachidonoylglycerol; ANA, anandamide; ANOVA, analysis of variance; NAcS, nucleus accumbens shell; OMDM-1, (R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine.
Values shown in the table are means±s.e.m. of four experiments. Differences between means were assessed by one-way ANOVA followed by Bonferroni's test.
P<0.05.
Discussion and conclusions
It has been previously demonstrated that injection in the NAcS of opioids and GABA agonists, glutamate antagonists and the endocannabinoid 2-AG enhances feeding behaviour (Kirkham et al., 2002; Hanlon et al., 2004). In this study, we show that administration into the NAcS of the other endocannabinoid, ANA, also increases food intake. We believe that ANA acts through the activation of the CB1 receptor, since its orexigenic effect was blocked here by coadministration with AM251, a selective CB1 antagonist. In general, all doses tested stimulated food intake but the effects of ANA can be classified, depending on the dose, either as an acute response produced by 1.0 μg of ANA at the first hour post-injection or as a slowly developing response after 4 h for the other two doses. Furthermore, we show, for the first time, that pharmacological inhibition by AA5HT (Bisogno et al., 1998) of the main enzyme (FAAH) involved in degrading endocannabinoids, as well as potential inhibition of the cellular uptake of ANA by OMDM-1 (Ortar et al., 2003), also stimulate food intake. These effects were also prevented by AM251, thus suggesting that these compounds facilitate food intake by activating the CB1 receptor indirectly, that is, by prolonging pharmacologically the lifetime of the endocannabinoids in the NAcS.
AM251 alone did not modify food intake at the doses used. This might seem in contrast with the extensive literature indicating that the CB1 antagonist, rimonabant, induces hyporexia. However, some differences were to be expected, since we applied here a single AM251 injection directly into the NAcS, whereas rimonabant has been given systemically. Also in a previous study, rimonabant given intra-NAcS was found to be inactive on food intake (Kirkham et al., 2002). Another possibility is that AM251 does not suppress food intake via actions on the NAc. Curiously, the combination of AA5HT and AM251 reduced food intake in the first hour, compared with vehicle. The same effect was observed with the OMDM-1/AM251 combination in a dose-responsive manner. This observation might suggest the existence of other endogenous compounds whose inactivation is still inhibited by AA5HT and OMDM-1 and whose ‘anorexic' activity is facilitated by CB1 receptor blockade. One such compound might be oleoylethanolamide, which given systemically together synergizes with rimonabant at reducing food intake (Serrano et al., 2007).
That the NAc is necessary for normal expression of feeding behaviour has been extensively documented (Kelley, 2004). It has also been suggested that the opioidergic system in the NAc is a crucial mediator of the hedonic sensation triggered by food (Peciña and Berridge, 2005). In addition, a functional interaction between opioidergic and cannabinergic systems has been described extensively (Vigano et al., 2005; Christie, 2006). Hence, it is plausible to think that endocannabinoids, either directly or by interacting with opioids in the NAcS, enhance the rewarding value of food (Cota et al., 2006). However, given the inhibitory nature of endocannabinoids and their localization, we believe that the direct infusion of these molecules into the NAcS would rather inhibit the activity of this nucleus (Hoffman and Lupica, 2000; Manzoni and Bockaert, 2001; Pistis et al., 2002; Robbe et al., 2002; Lupica et al., 2004). Actually, Kelley and Swanson (1997) and Stratford and Kelley (1999) demonstrated that intra-NAcS infusions of GABA agonists and glutamate antagonists stimulate food intake with a concurrent activation of food-related hypothalamic areas, mainly the LH. This effect might be due to the fact that infusions GABA agonists in this nucleus produce an inhibition of GABAergic medium spiny neurons (MSNs) and, as a consequence, disinhibition of the LH. Endocannabinoids, either exogenously injected or locally produced and degraded in a way inhibited by AA5HT and OMDM-1, might act at CB1 receptors to inhibit GABA release from MSNs, thus also resulting in the disinhibition of the LH. On the other hand, endocannabinoids could suppress the inhibitory tone on LH neurons acting directly in this nucleus (Jo et al., 2005). Our data reveal that the infusion of ANA, AA5HT and OMDM-1 into the NAcS readily activates not only the LH but also other hypothalamic nuclei, that is, the ARC, DMH and PVN. The role of these nuclei in the regulation of energy balance has been demonstrated by others (Bellinger and Bernardis, 2002; Bali and Kovács, 2003; Berthoud, 2004; Wynne et al., 2005; Gooley et al., 2006; Morton et al., 2006). At present, we do not know which neurotransmitters are released by these F-ir neurons. However, future experiments will shed a light into this question.
In support of the hypothesis that AA5HT injected into the NAcS acts selectively in this nucleus, and that, hence, the other drugs administered here in this manner, which have similar lipophilicity to AA5HT, also do not diffuse into other nuclei and do not cause their effects in the hypothalamus by acting directly in this brain area, we found that AA5HT caused a significant elevation of 2-AG levels in the NAc, whereas reducing it in the hypothalamus. There was also a trend to increase ANA levels in the NAc (P=0.08), whereas no changes were observed in the hypothalamus. OMDM-1 also tended to increase ANA and 2-AG levels in the NAc, whereas it significantly reduced 2-AG levels in the hypothalamus. The increase of endocannabinoids in the NAc is very likely a result of the pharmacological activity of the drugs, which, by inhibiting the degradation of endocannabinoids, should elevate their tissue levels. Conversely, it is possible that the reduction of 2-AG levels observed in the hypothalamus is a result of food intake. In fact, it was previously shown (Kirkham et al., 2002) that food intake is accompanied by a decrease in hypothalamic 2-AG, but not ANA, levels in rats. The lower efficacy of OMDM-1 at elevating endocannabinoid levels, compared to AA5HT, again supports our hypothesis that the two compounds are producing their pharmacological effects by inhibiting endocannabinoid degradation in the NAcS. In fact, endocannabinoid levels were measured in tissues dissected after 90 min from administration of the two drugs and OMDM-1 only produced significant effects on food intake after 4 h from its administration, whereas the effects of AA5HT were observed already after 1 h. The different time of onset of the effects of the two drugs might be due to pharmacokinetic factors and to their different mechanism of action.
In summary, our study indicates that infusion of ANA or of endocannabinoid level enhancers into the NAcS increases food intake very likely through the activation of CB1 receptors, as this effect was blocked with a CB1 antagonist. Cannabinoids administered in this way activate the hypothalamic nuclei involved in food intake regulation, thus supporting the notion that there is a functional relationship between the NAcS and the hypothalamus. This is the first study showing that indirect activators of CB1 receptors such as AA5HT and OMDM-1 can enhance food intake. This observation might have important clinical implications for the potential use of AA5HT to control eating disorders, like anorexia. Furthermore, it might suggest that conditions causing impairment of endocannabinoid inactivation with subsequent elevation of endocannabinoid levels in the NAcS might contribute to the development of hyperphagia and, eventually, obesity. Our study supports an important role of the endocannabinoids in the NAcS in the activation of brain regions such as the hypothalamus (Jamshidi and Taylor, 2001; Tucci et al., 2004) or the brainstem (Miller et al., 2004), that is, in other nuclei involved in feeding. Our work suggests the existence of an endocannabinoid/CB1-mediated functional relationship between the NAcS and the hypothalamus.
Acknowledgments
This study was supported by Grant IN224306 from DGAPA-UNAM to OPG and a fellowship from CONACyT to ESG. We thank Khalil Guzmán for help in animal handling. This work is part of ESG's Doctoral Dissertation in the Programa de Investigación Biomédica of UNAM.
Abbreviations
- AA5HT
N-arachidonoyl-serotonin
- 2-AG
2-arachidonoylglycerol
- ARC
arcuate nucleus
- DMH
dorsomedial hypothalamus
- FAAH
fatty acid amide hydrolase
- F-ir
c-Fos immunoreactive
- LH
lateral hypothalamus
- MSNs
medium spiny neurons
- NAc
nucleus accumbens
- NAcS
nucleus accumbens shell
- OMDM-1
(R)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine
- PBS
phosphate-buffered saline
- PFH
paraformaldehyde
- PVN
paraventricular nucleus
Conflict of interest
The authors state no conflict of interest.
References
- Bali B, Kovács KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci. 2003;18:1518–1526. doi: 10.1046/j.1460-9568.2003.02877.x. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, Bobrov MY, Gretskaya NM, Bezuglov VV, et al. Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun. 1998;248:515–522. doi: 10.1006/bbrc.1998.8874. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Christie MJ. Opioid and cannabinoid receptors: friends with benefits or just close friends. Br J Pharmacol. 2006;148:385–386. doi: 10.1038/sj.bjp.0706756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparison with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschp̂ M, Gŗbler Y, Flachskman C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids, and eating behavior: the molecular face of hedonism. Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkal S, JAral Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosc. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Fride E, Bregman T, Kirkham TC. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp Biol Med. 2005;230:225–234. doi: 10.1177/153537020523000401. [DOI] [PubMed] [Google Scholar]
- Gamber KM, Macarthur H, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increase in food intake or food seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger. Psychopharmacology. 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysio. 2000;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua C, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainite receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:999–1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–R5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez D, Bonilla-Jaime H, Morales-Otal A, Henriksen SJ, Velazquez-Moctezuma J, Prospero-Garcia O. Oleamide and anandamide effects on food intake and sexual behavior of rats. Neurosci Lett. 2004;364:1–6. doi: 10.1016/j.neulet.2004.03.080. [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Depetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ, et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of the endogenous anandamide. Neuroendocrinology. 2005a;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005b;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Raven Press: New York, NY; 1986. [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids caused increased hedonic impact of sweetness. J Neusci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur J Neurosci. 2002;15:1795–1802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, del Arco I, Pavón FJ, Macías M, Perez-Valero V, Rodríguez de Fonseca F.The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long term treatment with oleoylethanolamide in Zucker rats Neuropharmacology 2007(in press, available online 24 March 2007) [DOI] [PubMed]
- Söderpalm AH, Berridge KC. Food intake after diazepam, morphine or muscimol: microinjections in the nucleus accumbens shell. Pharmacol Biochem Behav. 2000;66:429–434. doi: 10.1016/s0091-3057(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley AE. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716A blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- Zhang H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]