Abstract
Background and purpose:
The intravesical administration of dimethyl sulphoxide (DMSO) is used to alleviate the symptoms of interstitial cystitis. We investigated the relaxant effect of DMSO and its underlying mechanism in the detrusor muscle.
Experimental approach:
The effects of DMSO on contraction, on Ca2+ sensitivity of myofilaments, and on myosin light chain (MLC) phosphorylation were investigated in both intact and α-toxin-permeabilized strips of rabbit detrusor muscle.
Key results:
In fura-PE3-loaded strips, DMSO (>1%) induced a significant relaxation during sustained contractions induced by 60 mM K+-depolarization or 10 μM carbachol, while having no effect on the [Ca2+]i level. DMSO decreased the level of MLC phosphorylation during the contractions induced by 60 mM K+ and 10 μM carbachol. DMSO also inhibited both the contraction and MLC phosphorylation induced by calyculin-A in intact strips. In the α-toxin-permeabilized preparations, DMSO relaxed the Ca2+-induced contraction and also inhibited the tension development induced by a stepwise increment of Ca2+ concentrations. Such a relaxant effect of DMSO was enhanced in the presence of phosphate.
Conclusions and implications:
DMSO relaxes rabbit detrusor muscle by decreasing the Ca2+ sensitivity of myofilaments. Inhibition of the kinase activities involved in myosin phosphorylation may play a major role in DMSO-induced Ca2+ desensitization. Inhibition of the cross-bridge cycling at the step of phosphate release may also contribute to the relaxant effect of DMSO. Such relaxant effects of DMSO could be linked to the therapeutic effect of DMSO in interstitial cystitis.
Keywords: bladder, smooth muscle, calcium sensitivity, dimethyl sulphoxide
Introduction
Interstitial cystitis is a disease of unknown aetiology, characterized by symptoms such as excessive urgency and frequency of urination, dyspareunia and chronic pelvic pain (Metts, 2001). Intravesical administration of dimethyl sulphoxide (DMSO) has been performed to alleviate the symptoms associated with interstitial cystitis (Parkin et al., 1997). A number of clinical investigations have proven a significant therapeutic effect of DMSO in the relief of symptoms (Rossberger et al., 2005). However, the mechanism for the therapeutic effect of DMSO in the interstitial cystitis remains unclear.
DMSO is frequently used as a solvent for drugs and reagents in biological studies and clinical practice, but it is also known to exert some cellular effects (Santos et al., 2003). Among these cellular effects, anti-inflammatory effects, stimulation of bladder afferent pathways and the resultant NO release (Birder et al., 1997) and a peripheral analgesic effect (Castroman and Ness, 2002) have all been proposed to contribute to the mechanism of the therapeutic effect of DMSO in interstitial cystitis.
The relaxation of the detrusor muscle is also considered to contribute to the therapeutic effect of DMSO (Jacob and Herschler, 1986). However, a limited number of studies have evaluated the effect of DMSO on the contractility of the detrusor muscle. The amplitude of contractile activity induced by the electrical field stimulation in rat urinary bladder smooth muscle strips has been shown to be depressed in the presence of DMSO (Melchior et al., 2003). However, the effects of DMSO under other contractions, especially those induced by physiologically relevant stimulations such as muscarinic agonists, and the precise mechanisms for the DMSO-induced urinary bladder relaxation still remain to be elucidated.
In this report, we attempted to highlight the key mechanisms involved in the relaxant effects of DMSO in rabbit urinary bladder, in terms of the Ca2+ signalling, by simultaneously measuring the changes in the cytosolic Ca2+ concentrations ([Ca2+]i) and tension in bladder strips loaded with fura-PE3. The effect of DMSO on the Ca2+ sensitivity of the contractile apparatus was also evaluated in strips permeabilized with α-toxin. The present study demonstrated, for the first time, that DMSO-induced relaxation in rabbit detrusor muscle without having any effects on [Ca2+]i. DMSO was also found to inhibit the phosphorylation of 20 kDa regulatory myosin light chain (MLC) and the cross-bridge cycling at the step of the phosphate release, thus decreasing the Ca2+ sensitivity of myofilaments.
Materials and methods
Tissue preparation
The study protocol was approved by the Animal Care and Committee of Graduate School of Medical Sciences, Kyushu University. Male Japanese white rabbits (2–3 kg) were killed by an intravenous administration of a lethal dose of sodium pentobarbitone (120 mg kg−1 weight). The urinary bladder was immediately excised, while taking special care so as not to damage or overstretch the tissue. The mucosa was mechanically removed, and then, the bladder preparation was cut into strips measuring approximately 1.0 × 1.0 × 4.0 mm in a longitudinal direction, under a binocular microscope. The strips were then equilibrated in a physiological saline solution (PSS).
Fura-PE3 loading and measurement of [Ca2+]i in intact strips of rabbit bladder
The changes in [Ca2+]i were recorded in intact strips loaded with fura-PE3, as described previously (Seguchi et al., 1998; Kanaide, 2006). In brief, the bladder strips were loaded with the Ca2+ indicator fura-PE3, by incubating them in Dulbecco's modified Eagle's medium (DMEM) containing 50 μM fura-PE3, as the acetoxymethyl ester (fura-PE3/AM) and 5% fetal bovine serum for 7 h at 37°C under aeration with a mixture of 5% CO2 and 95% O2. The fura-PE3-loaded strips were washed with PSS to remove the dye in the extracellular space, and then were equilibrated in PSS at 37°C for at least 1 h before the initiation of measurements. The changes in the fluorescence ratio were monitored with a front-surface fluorimeter CAM-OF-1 (JASCO, Tokyo, Japan). The fluorescence (500 nm) intensities obtained with the alternating (400 Hz) 340 and 380 nm excitation and their ratio (F340/F380) were continuously measured. The data were stored in a Macintosh computer using a data acquisition system MacLab (Analog Digital Instruments, Australia). The fluorescence ratio, which indicates [Ca2+]i, was expressed as a percentage, thus assigning the resting level and the sustained level of precontraction induced by 60 mM K+ or 10 μM carbachol to be 0 and 100%, respectively, unless otherwise specified. All simultaneous measurements of [Ca2+]i were performed at 37°C.
Tension measurement in the intact strips of rabbit bladder
The changes in [Ca2+]i and tension were simultaneously monitored. The bladder strips were mounted vertically in a quartz organ bath filled with 6 ml PSS (maintained at 37°C and aerated with 5% CO2, 95% O2) and connected to a force transducer (TB-612 T, Nihon Koden, Japan). During the equilibration period, the strips were stimulated with 60 mM K+ every 15 min, while the resting load was increased in a stepwise manner. The extent of the developed tension induced by 60 mM K+ increased as the resting load was elevated. When the resting load was 200 mg, the maximal response to 60 mM K+ was obtained. When the difference in the amplitude of the developed tension obtained with consecutive stimulations with 60 mM K+ was within 10%, the experimental protocol was then started. The levels of tension obtained at rest (in PSS) and at 10 min after initiating the contraction by 60 mM K+ or 10 μM carbachol were assigned values of 0 and 100%, respectively, unless otherwise specified.
Tension measurement in α-toxin-permeabilized strips of rabbit bladder
The strips of the rabbit bladder was permeabilized with Staphylococcus aureus α-toxin as described previously (Nishimura et al., 1988) with minor modifications. In brief, small strips (about 0.5 mm in width and 1.2 mm in length) of the rabbit bladder were permeabilized with Staphylococcal α-toxin (15 000 units ml−1) for 1 h at room temperature (25°C) in a relaxing solution composed of (in mM): potassium methansulphonate 100, Na2-ATP 2.2, MgCl2 3.38, ethyleneglycol bis (β-aminoethylether)-N′,N′,N′,N′-tetraacetic acid (EGTA) 10, creatine phosphate 10, Tris–maleate 20 (pH 6.8). The strips were then mounted between two tungsten wires, one of which was fixed and the other was attached to a force transducer (UL2; Minebea Co., Japan). The cytosolic substitution solution (CSS) containing the indicated concentrations of free Ca2+ was made by adding an appropriate amount of CaCl2 to the relaxing solution, according to the Ca2+-EGTA binding constant of 106 M−1 (Saida and Nonomura, 1978). The tension measurements of the permeabilized tissues were performed at 25°C. The levels of tension obtained in the relaxing solution and at maximal tension development induced by 100 μM Ca2+ were assigned values of 0 and 100%, respectively.
Tension measurement in the intact strips of rat aorta
The intact strips (2 mm in width, 10 mm in length) of the aortas of Wister–Kyoto rats were prepared as described previously (Watanabe et al., 1992). The development of tension of the strips of rat aorta was monitored as described above.
Measurement of MLC phosphorylation
The extent of MLC phosphorylation was determined using the urea–glycerol gel electrophoresis technique (Persechini et al., 1986), followed by immunoblot detection of 20 kDa MLC (Zhou et al., 1999). The strips were pulled to 1.5-fold of the resting length and pinned onto a rubber block to keep the resting load similar to that given in the force measurement. At the indicated time after simulation, the bladder strips were transferred into a solution containing 90% acetone, 10% trichloroacetic acid and 10 mM dithiothreitol (DTT) prechilled by dry ice to stop the reaction. The tissue specimens were then extensively washed and stored in acetone containing 10 mM DTT at −80°C. After the tissue was air-dried to remove acetone, the cellular protein was extracted in the sample buffer (8 M urea, 20 mM Tris (hydroxymethyl) aminomethane, 23 mM glycine, 0.004% bromophenol blue and 10 mM DTT) at room temperature for 1 h. The supernatant was then subjected to electrophoresis on 10% polyacrylamide gel containing 40% glycerol, followed by transfer onto nitrocellulose membrane (BioRad, Hercules, CA, USA) in 10 mM Na2HPO4 (pH, 7.6). The amount of extracted protein was not determined due to the interference by the high concentration of urea in the extract, but the same volume of the extract was loaded onto the gel. The amount of loaded protein thus could vary to some extent due to the variation in the extraction efficiency. The 20 kDa MLC, both unphosphorylated and phosphorylated, was detected by anti-MLC antibody (200 × dilutions) and a horseradish peroxidase-conjugated secondary antibody (1000 × dilution). In a separate experiment with purified MLC, this antibody was confirmed to detect, equally, the unphosphorylated and phosphorylated forms of MLC (data not shown). The immune complex was detected using the enhanced chemiluminescence technique (ECL plus kit; Amersham, Buckinghamshire, UK). The light emission was detected and analysed with ChemiDoc XRS-J and the computer programme Quantity One (BioRad, Hercules, CA, USA). The percentage of the phosphorylated form in total MLC (sum of unphosphorylated and phosphorylated forms) was calculated to indicate the extent of MLC phosphorylation.
In vitro phosphorylation assay
Phosphorylation reactions were performed at 25°C in the mixture containing 0.3 mg ml−1 mixture of 17 and 20 kDa MLC (MLC17+20), 5 μg ml−1 MLC kinase (MLCK), 5 μg ml−1 calmodulin, 30 mM Tris–HCl, 85 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2 and 1 mM ATP, with and without 10% DMSO. When the assay was performed with the active MLCK, active MLCK was added to the final concentration of 2 μg ml−1, while calmodulin and CaCl2 were omitted from the reaction mixture. The phosphorylation reaction was started by adding ATP, and then terminated 30 s later by making the reaction mixture 6 M in urea. The phosphorylation of MLC20 was evaluated by subjecting the samples to urea–glycerol gel electrophoresis, followed by coomassie brilliant blue protein staining. Each lane contained approximately 4.5 μg MLC17+20. The identification of the bands corresponding to MLC20 on the protein staining was confirmed by the western blot findings (data not shown).
Data analysis and statistical procedures
All data were expressed as the mean±s.e.m. of the number of experiments. One strip obtained from one animal was used for each experiment, and therefore the number of experiments (n-value) indicates the number of animals. Paired Student's t-test or analysis of variance was used to determine any statistical differences. P<0.05 was considered to be statistically significant. The four parameter logistic model was used to fit the concentration-response curves to the sigmoidal curve, and to estimate the concentrations of reagents to induce a half of the maximal effect (De Lean et al., 1978).
Drugs, chemicals reagents and other materials
The composition of PSS was as follows (in mM): NaCl 123, KCl 4.7, CaCl2 1.25, MgCl2 1.2, KH2PO4 1.2, NaHCO3 15.5 and D-glucose 11.5. High K+ PSS was prepared by replacing NaCl with equimolar KCl. PSS was bubbled with a mixture of 95% O2 and 5% CO2, with the resulting pH being 7.4.
Fura-PE3/AM was purchased from Teflab (Austin, TX, USA); dimethyl-sulphoxide, calyculin A, S. aureus α-toxin and a monoclonal anti-MLC antibody (clone MY-21) were purchased from Sigma (St Louis, CA, USA); wortmannin was obtained from Calbiochem (San Diego, CA, USA). Turkey gizzard MLCK, turkey gizzard MLC17+20 and bovine testis calmodulin were kindly donated by Dr Dmitry N Derkach and Dr DJ Hartshorne (University of Arizona, Tucson, Arizona, USA). An active form of the recombinant human MLCK (catalog no. 14–638) was purchased from Upstate (Charlottesville, VA, USA).
Results
Relaxant effect of DMSO in the intact strips of rabbit detrusor muscle
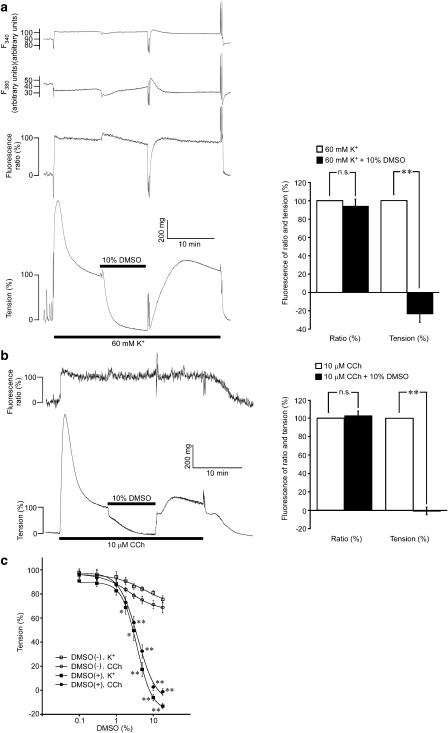
Both 60 mM K+-depolarization and 10 μM carbachol induced an initial phasic contraction, followed by a sustained contraction in the intact strips of rabbit detrusor muscle (Figure 1a and b). In both cases, the tension rapidly reached a peak within 1–2 min, and then declined to the sustained level within 10 min. Thereafter, the tension gradually and slightly declined (data not shown). The levels of tension obtained 20 min after initiating the contraction by 60 mM K+ and 10 μM carbachol were 94.9±4.7% (n=5) and 93.4±2.2% (n=5) of those seen at 10 min, respectively. Both 60 mM K+ and carbachol-induced sustained elevations of [Ca2+]i, while the phasic response of the [Ca2+]i elevation was less obvious than that seen with the tension development (Figures 1a and b). The levels of [Ca2+]i, seen 20 min after initiating the contraction by 60 mM K+ and 10 μM carbachol were 98.5±7.8% (n=5) and 98.7±1.3% (n=5) of those seen at 10 min, respectively.
Figure 1.
The effect of dimethyl sulphoxide (DMSO) on [Ca2+]i and tension during the contractions induced by 60 mM K+ or 10 μM carbachol in intact strips of rabbit detrusor muscle. Representative traces and summaries showing the effect of DMSO on [Ca2+]i and tension during the sustained contractions induced by 60 mM K+ (a) and 10 μM carbachol (b). DMSO was added 10 min after the initiation of the precontraction. After the 10-min exposure, DMSO was then washed out. (c) The concentration–response curves for the DMSO-induced relaxation during the precontractions induced by 60 mM K+ (K) or 10 μM carbachol (CCh). During the sustained phase of the precontraction, the fractional amount of DMSO (DMSO (+)) or distilled water (DMSO (−)) was increased in a stepwise manner at 5-min intervals. The levels of [Ca2+]i and tension obtained at rest and those obtained just before the applications of DMSO or distilled water were assigned values of 0 and 100%, respectively. The data represent the mean±s.e.m. (n=5). **P<0.01; *P<0.05; n.s., not significantly different, in comparison to the values obtained just before the application of DMSO (a, b) and those obtained with the corresponding fractional amount of distilled water (c).
The addition of 10% DMSO 10 min after initiating the contraction by 60 mM K+ or 10 μM carbachol decreased the level of tension to the resting level within 10 min, while it had no significant effect on the sustained level of [Ca2+]i (Figures 1a and b). When 10% DMSO was applied to the organ bath containing 6 ml buffer, 600 μl of the bathing buffer was removed, and then 600 μl of 100% DMSO was applied. The same volume of distilled water was thus applied in place of DMSO for the control. However, the levels of [Ca2+]i and tension obtained 10 min after adding 10% distilled water did not significantly differ from those obtained 20 min after initiating the contraction without the addition of distilled water (data not shown). In both 60 mM K+ and 10 μM carbachol-induced contractions, the removal of DMSO rapidly reversed the level of tension (Figures 1a and b). On removal of DMSO, the tension initially was higher than that seen before the application of DMSO and then it gradually returned to the level seen before the application of DMSO (Figures 1a and b).
Concentration–response curves for the relaxant effects of DMSO were obtained by increasing the concentrations of DMSO in a stepwise manner at a 5-min interval. The addition of DMSO was started 10 min after initiating the contraction, and then it took 40 min to reach the final concentration of 18% (Figure 1c). When distilled water was added to the control, the level of tension slightly decreased (Figure 1c, DMSO(−)). When the fraction of the added distilled water reached 18%, the levels of tension induced by 60 mM K+ and 10 μM carbachol decreased to 75.5±3.1% (n=5) and 68.6±4.4% (n=5), respectively. However, these levels of tension did not significantly differ from those obtained 50 min after initiating the contraction and without adding distilled water (77.3±4.9% (n=5) for 60 mM K+; 72.5±3.1% (n=5) for 10 μM carbachol). These observations thus suggested distilled water to have no significant effect on the contractions induced by 60 mM K+ and 10 μM carbachol, up to a proportion of 18%. On the other hand, the increase in the concentrations of DMSO-induced significantly greater decreases in the level of tension than that seen with distilled water (Figure 1c). A significant relaxant effect of DMSO was observed at concentrations higher than 1%, while the maximal effect was obtained at around 10% (Figure 1c). The concentrations of DMSO required to induce half the maximal relaxant effect for the 60 mM K+ and 10 μM carbachol-induced contraction were 3.7±0.4% (n=5) and 3.9±0.5% (n=5), respectively.
We also examined the effect of DMSO on the contraction in vascular smooth muscle, using the intact trips of the rat aorta. High K+ (118 mM K+) induced a sustained elevation of [Ca2+]i and tension in rat aorta. The addition of 10% DMSO during the sustained phase of the contraction decreased the level of tension to the resting level, while having no effect on the level of [Ca2+]i, as observed with the strips of rabbit detrusor muscle (data not shown).
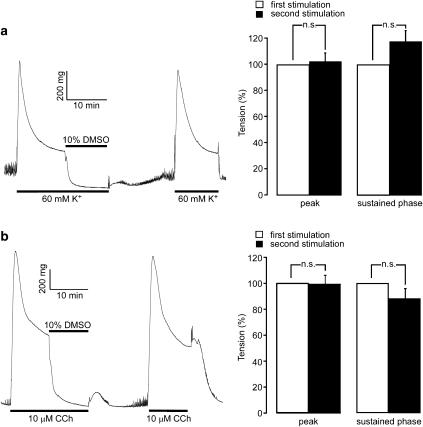
Reversibility of the relaxant effect of DMSO in the intact strips of rabbit detrusor muscle
The reversibility of the relaxant effect of DMSO was evaluated as shown in Figure 2. The contractions were first induced by 60 mM K+ or 10 μM carbachol. DMSO (10%) was then added 10 min after the initiation of the contractions. After the 10-min treatment with DMSO, the strips were washed and equilibrated in PSS for 15 min, and then the strips were challenged to the second stimulation with 60 mM K+ or 10 μM carbachol. The levels of tension obtained both at the peak and during the sustained phase (10 min after initiating the contractions) of the second contractions did not significantly differ from those obtained with the first contractions (Figure 2).
Figure 2.
The reversibility of the relaxant effect of dimethyl sulfoxide (DMSO) in intact strips of rabbit detrusor muscle. Representative traces and summaries showing the effect of the preceding treatment with DMSO on the subsequent contractile responses to 60 mM K+ (a) or 10 μM carbachol (b). The strips were first contracted with 60 mM K+ or 10 μM carbachol. DMSO (10%) was applied 10 min later. After 10 min treatment with DMSO, the strips were then incubated in PSS for 15 min and then they were stimulated with the second applications of 60 mM K+ or 10 μM carbachol. The contractile response was evaluated both at the peak of the contraction (peak) and 10 min after initiating the contraction (sustained phase), while the values obtained with the first contraction were considered to be 100%. Data represent the mean±s.e.m. (n=5). n.s., not significantly different.
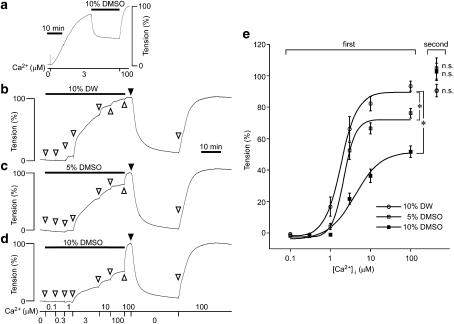
The effect of DMSO on the contractions induced by Ca2+ in the α-toxin-permeabilized strips of rabbit detrusor muscle
The observation in the intact strips that DMSO-induced relaxation with no significant decrease in [Ca2+]i in both 60 mM K+ and 10 μM carbachol-induced contractions suggested that DMSO decreased the Ca2+ sensitivity of the contractile apparatus. We thus examined the effect of DMSO on the Ca2+ sensitivity using α-toxin-permeabilized preparations. Elevation of the Ca2+ concentration to 3 μM induced a significant contraction in the α-toxin-permeabilized strips, while the addition of 10% DMSO induced a significant relaxation despite the maintained concentration of Ca2+ (Figure 3a). Higher concentrations of DMSO, however, did not induce any further decrease in the level of tension (data not shown), thus indicating that the relaxant effect of DMSO in the permeabilized strips reached a maximum at 10% as observed in the intact strips. The effect of DMSO on the Ca2+ sensitivity was then quantitatively examined (Figures 3b–e). A stepwise increase in the concentrations of Ca2+ induced a stepwise increase in tension in the presence of 10% distilled water, with the maximal contraction being obtained with 100 μM Ca2+ (Figure 3b). The subsequent removal of 10% distilled water from the buffer containing 100 μM Ca2+ induced little further increase in tension, thus suggesting that 10% distilled water had no significant effect on the contraction induced by 100 μM Ca2+. Therefore, this level of contraction was assigned a value of 100%. In the presence of 5 and 10% DMSO, the development of tension induced by stepwise increment of the Ca2+ concentrations was significantly inhibited (Figures 3c and d). The concentration of Ca2+ required to induce half a maximal contraction obtained with 10% DMSO (4.62±1.12 μM, n=5) was significantly (P<0.05) higher than that obtained in the presence of 10% distilled water (1.01±0.04 μM, n=5) (Figure 3e). Furthermore, the maximal contraction induced by 100 μM Ca2+ in the presence of 10% DMSO (51.8±3.6%, n=5) was significantly (P<0.001) smaller than that obtained in the presence of 10% distilled water (93.4±3.2%, n=5) (Figure 3e). However, the level of contraction induced by the second stimulation with 100 μM Ca2+ after washing out 5 or 10% DMSO, or distilled water, did not significantly differ from the 100% level (Figure 3e), also implying the reversibility of the effect of DMSO in the permeabilized strips.
Figure 3.
The effects of dimethyl sulphoxide (DMSO) on the Ca2+-induced contractions in α-toxin-permeabilized strips of rabbit detrusor muscle. (a) Representative traces of tension showing the effect of 10% DMSO on the contraction induced by 3 μM Ca2+. (b–e) Representative traces and summary of the contractile responses to a stepwise increase in the concentrations of Ca2+ in the presence of 10% distilled water (DW) (b), or 5% (c) and 10% (d) DMSO. The concentrations of Ca2+ were increased from 0 to 100 μM (first). After recording the contraction induced by 100 μM Ca2+ in the presence of distilled water or DMSO, the strips were then stimulated with 100 μM Ca2+ in their absence to record 100% level, as indicated by closed triangles. The strips were then completely relaxed in the Ca2+-free CSS for 30 min, and they were again stimulated with 100 μM Ca2+ (second). Data represent the mean±s.e.m. (n=5). *P<0.05 vs the values obtained with the corresponding concentrations of Ca2+ in the presence of 10% distilled water. n.s., not significantly different vs 100% level.
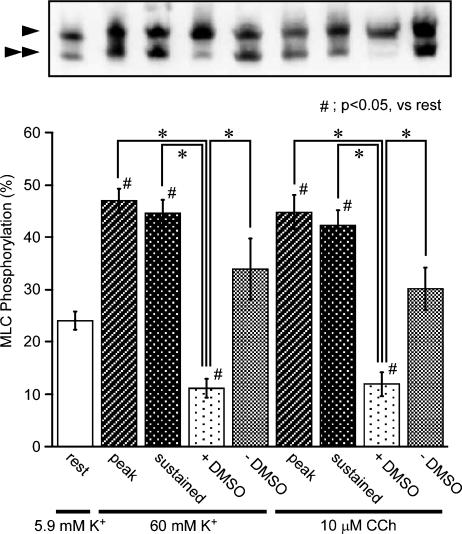
Effect of DMSO on the phosphorylation of MLC in the intact strips of rabbit detrusor muscle
An alteration of the level of MLC phosphorylation serves as a major mechanism for the changes in Ca2+ sensitivity (Hirano et al., 2003). We thus examined the effect of DMSO on the level of MLC phosphorylation during the contraction induced by 60 mM K+ and 10 μM carbachol (Figure 4). Under the resting state, 24.0±1.7% (n=7) of MLC was phosphorylated, which is consistent with the observations reported in previous studies (Hypolite et al., 2001; Rembold et al., 2004; Chang et al., 2006). Stimulation with 60 mM K+ or 10 μM carbachol increased the level of the MLC phosphorylation to almost twice resting values, at the peak of contraction (Figure 4, peak). In both cases, the level of the MLC phosphorylation seen just prior to the application of DMSO did not significantly differ from that seen at the peak (Figure 4, sustained). However, the application of 10% DMSO markedly decreased the levels of the MLC phosphorylation (Figure 4, +DMSO). The addition of 10% distilled water, on the other hand, had no significant effect on the level of the MLC phosphorylation (Figure 4, −DMSO)
Figure 4.
The effects of dimethyl sulphoxide (DMSO) on the phosphorylation of MLC in intact strips of rabbit detrusor muscle. Representative photograph of an immunoblot analysis and a summary of the phosphorylation of MLC obtained in 5.9 mM K+-PSS before the contractile stimulation with 60 mM K+ or 10 μM carbachol (rest), 1–2 min after the stimulation (peak), and 10 min after the stimulation and just before the application of DMSO (sustained) and at 10 min after adding 10% DMSO (DMSO +) or 10% distilled water (DMSO −) during the contractile stimulation. The arrowheads and double arrowheads indicate the unphosphorylated and mono-phosphorylated form of MLC, respectively. Data represent the mean±s.e.m. (n=7). *P<0.05. #P<0.05 vs rest.
The effect of DMSO on the contraction and MLC phosphorylation induced by calyculin-A in the intact strips of rabbit detrusor muscle
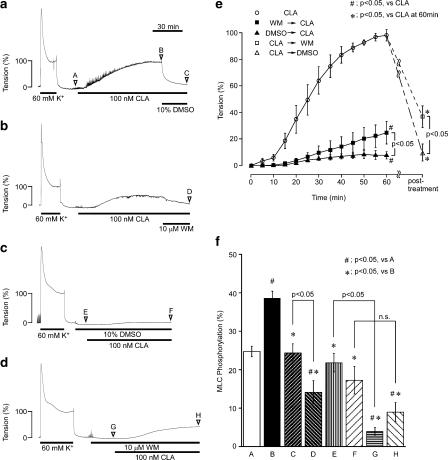
Either inhibition of kinase activity or activation of phosphatase activity could decrease the level of MLC phosphorylation. To investigate the possible effect of DMSO on the kinase activity, we examined the effect of DMSO on the contraction induced by a phosphatase inhibitor (Figure 5). The inhibition of the phosphatase activity by 100 nM calyculin-A induced a gradual increase in tension, which reached a maximal level (98.3±4.1%, n=4) at 60 min (Figures 5a, b, and e). This contraction was almost completely inhibited in the presence of 10% DMSO (Figures 5c and e). Pretreatment with 10 μM wortmannin, a MLC kinase inhibitor, also markedly inhibited the development of tension induced by calyculin-A (Figures 5d and e). The addition of 10% DMSO or 10 μM wortmannin during the calyculin-A-induced contraction decreased tension close to the resting level by 30 min (Figures 5a, b and e). The contraction induced by 100 nM calyculin-A was associated with a significant increase in MLC phosphorylation (Figures 5f-B). This increase in MLC phosphorylation was significantly inhibited by adding 10% DMSO and 10 μM wortmannin (Figures 5f-C, D). DMSO (10%) had no significant effect on the resting level of MLC phosphorylation (Figures 5f-E); however, it almost completely inhibited the increase in MLC phosphorylation induced by the subsequent application of calyculin-A (Figures 5f-F). Pretreatment with 10 μM wortmannin significantly decreased the resting level of MLC phosphorylation, thus inhibiting the increase in MLC phosphorylation induced by calyculin-A (Figures 5f-G, H). The inhibitory effect of DMSO on the calyculin-A-induced MLC phosphorylation was significantly (P<0.05) smaller than that seen with wortmannin (Figure 5f), while the inhibitory effect on the contraction was significantly (P<0.05) greater than that seen with wortmannin (Figure 5e).
Figure 5.
The effects of dimethyl sulphoxide (DMSO) and wortmannin (WM) on the contraction and the phosphorylation of MLC induced by calyculin-A (CLA) in the intact strips of rabbit detrusor muscle. (a-e) Representative traces and a summary of the contraction induced by 100 nM CLA, in the absence ((a), (b); CLA in (e)) and presence of 10% DMSO ((c); DMSO → CLA in (e)) or 10 μM WM ((d); WM → CLA in (e)). In (a) and (b), 10% DMSO and 10 μM WM were applied 60 min after initiating the contraction with calyculin-A. In (c) and (d), DMSO and WM were applied 10 min before initiating the contraction. The effects of the post-treatment of DMSO (CLA → DMSO in (e)) and WM (CLA → WM in (e)) on the CLA-induced contraction were evaluated 30 min after the application. The reference response to 60 mM K+ was recorded before starting the experimental protocols. The levels of tension at rest and those obtained 10 min after the stimulation with 60 mM K+ were assigned values of 0 and 100%, respectively. (f) A summary of the levels of the MLC phosphorylation obtained at the time points as indicated on the representative traces in (a-d) (marked with A-H in traces). Data represent the mean±s.e.m. (n=4 for (e); n=7 for (f)).
The synergistic effect of DMSO and phosphate on the Ca2+-induced contraction in α-toxin-permeabilized strips of rabbit detrusor muscle
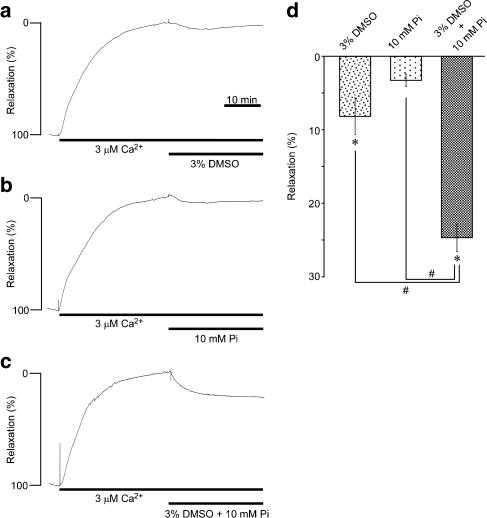
DMSO has been suggested to interfere with the cross-bridge cycling at the step of phosphate release in skeletal muscle (Mariano et al., 2001). We thus examined the possible synergistic effect of DMSO and phosphate on the contraction in the α-toxin-permeabilized strips (Figure 6). The addition of 3% DMSO during the contraction induced by 3 μM Ca2+ slightly but significantly decreased the level of tension in the α-toxin-permeabilized strips (Figures 6a and d). The addition of 10 mM phosphate, on the other hand, had no significant effect on the 3 μM Ca2+-induced contraction (Figures 6b and d). However, the combination of 3% DMSO and 10 mM phosphate decreased the tension to a significantly greater extent than that obtained with DMSO or phosphate alone (Figures 6c and d).
Figure 6.
The synergistic effects of dimethyl sulphoxide (DMSO) and phosphate on the contractions in the α-toxin-permeabilized strips of rabbit detrusor muscle. Representative traces (a–c) and a summary (d) of the effects of 3% DMSO, 10 mM phosphate and their combination on the 3 μM Ca2+-induced contraction in the α-toxin-permeabilized strips. DMSO and phosphate were applied 30 min after initiating the contraction. The level of tension obtained just before the application of DMSO or phosphate and that obtained in the relaxing solution were assigned values of 0 and 100% relaxation, respectively. Data represent the mean±s.e.m. (n=4). *P<0.05 vs the precontraction level (0% relaxation). #P<0.05.
The effect of DMSO on in vitro phosphorylation of MLC
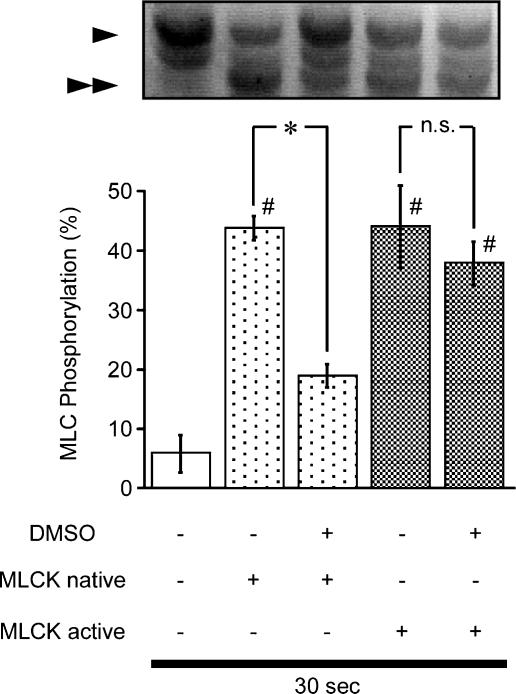
We directly examined the effect of DMSO on MLC phosphorylation using in vitro phosphorylation assays with purified MLC and MLCK. The native MLCK in the presence of Ca2+ and calmodulin and the active MLCK in the absence of Ca2+ and calmodulin-induced comparable levels of MLC phosphorylation, at 30 s in the absence of DMSO (Figure 7), which was similar to the level observed during the sustained contraction induced by either 60 mM K+ or 10 μM carbachol (Figure 4). Under this condition, DMSO significantly and markedly inhibited the MLC phosphorylation induced by the native MLCK. However, it had no significant effect on the phosphorylation induced by the active MLCK. A longer incubation (>3 min) induced almost 100% phosphorylation with the native MLCK and DMSO had no significant effect on this phosphorylation (data not shown).
Figure 7.
The effect of dimethyl sulphoxide (DMSO) on in vitro MLC phosphorylation. Representative photograph of Coomassie brilliant blue staining and a summary of in vitro MLC phosphorylation by native MLCK or active MLCK in the presence and absence of 10% DMSO. The reaction was terminated at 30 s. The arrowheads and double arrowheads indicate the unphosphorylated and monophosphorylated form of MLC, respectively. Data represent the mean±s.e.m. (n=7). *P<0.05. #P<0.05 vs DMSO(−) and MLCK native (−).
Discussion
The present study provides, for the first time, direct evidence that DMSO causes smooth muscle relaxation by decreasing the Ca2+ sensitivity of the contractile apparatus in rabbit detrusor muscle. The relaxant effect of DMSO was observed in the contractions induced by both high K+-depolarization and carbachol, with similar EC50 values. The latter is the physiologically most important contractile stimulation in the detrusor muscle. Furthermore, the relaxant effect of DMSO was not limited to the detrusor muscle, but it was also observed in the rat aorta. These observations thus suggest the site of action of DMSO to be on common contractile mechanisms. In this regard, the most important observation is that the DMSO-induced relaxation was not accompanied by a decrease in [Ca2+]i, thus indicating that DMSO decreased the Ca2+ sensitivity of the contractile apparatus. Decrease in the myofilament Ca2+ sensitivity by DMSO was further supported by observations that DMSO relaxed the α-toxin-permeabilized smooth muscle at fixed concentrations of Ca2+.
A significant relaxant effect of DMSO was observed with 3–18% DMSO. When DMSO was added to the buffer, an equal volume of the buffer was replaced with 100% DMSO. It is thus possible that the dilution of the components of the buffer is related to the relaxant effect of DMSO. However, this was not the case, because replacement with distilled water had no significant effect on the contractility. Furthermore, the relaxant effect of DMSO was readily reversible in both the intact and α-toxin-permeabilized strips, thus ruling out the possibility that the relaxant effect of DMSO was due to irreversible damage to the contractile apparatus. The reversibility of the effect of DMSO was consistent with the observations in the previous reports (Mariano et al., 2001; Melchior et al., 2003). Mariano et al. (2001) demonstrated the relaxant effect of DMSO in rabbit psoas skinned fibers to be reversible up to 30%. However, the inhibitory effect of 40% DMSO on the contraction in rabbit detrusor muscle was reported to be irreversible (Melchior et al., 2003). In the present study, since DMSO exerted its maximal relaxant effect at around 10% in both intact and α-toxin-permeabilized strips, the reversibility of the effect of higher concentrations of DMSO was not examined.
The mechanism regarding how DMSO decreased the myofilament Ca2+ sensitivity is the next important question. DMSO-induced relaxation during the contractions induced by K+-depolarization and carbachol was found to be associated with decreases in the level of MLC phosphorylation. The alteration to MLC phosphorylation is thus considered to play an important role in the decrease of the Ca2+ sensitivity. The level of MLC phosphorylation is determined by the balance between the activities of protein kinases and phosphatases (Hirano et al., 2003). Either inhibition of kinase or the activation of phosphatase can cause a decrease in the myofilament Ca2+ sensitivity. The inhibition of the phosphatase activity by calyculin-A shifted the balance between kinases and phosphatases toward the kinase-dominant state, thereby inducing an increase in MLC phosphorylation and contraction (Ishihara et al., 1989). The contraction induced by calyculin-A is thus considered to reflect the process of phosphorylation of MLC. The inhibitory effect of DMSO on the calyculin-A-induced MLC phosphorylation and contraction suggested that DMSO inhibited the phosphorylation reaction, thereby decreasing the MLC phosphorylation and the myofilament Ca2+ sensitivity. This conclusion is consistent with our observations of in vitro experiments. DMSO has been reported to inhibit the activity of apoptosis signal-regulating kinase (Gilot et al., 2002) and Src tyrosine kinase (Wang et al., 2003). However, our observations did not suggest DMSO could directly inhibit the catalytic activity of MLCK, but they did imply that DMSO inhibited the calmodulin-mediated activation of MLCK activity. This possibility, however, still remains to be established.
The observation that application of DMSO during the calyculin A-induced contraction induced a relaxation suggested that inhibition of the phosphatase activity by calyculin A was not complete. However, the relaxant effect of DMSO was markedly delayed in the presence of calyculin A. Furthermore, the levels of tension and MLC phosphorylation seen at the maximal relaxation induced by DMSO during the calyculin A-induced contraction was higher than those seen during the contraction induced by either 60 mM K+ or carbachol. These observations suggested that the phosphatase activity was substantially, if not completely, inhibited by calyculin A. The inhibition of the phosphorylation reaction by DMSO is thus considered to have caused the relaxation according to the residual amount of the phosphatase activity seen in the presence of calyculin A. In fact, the relaxant effect of DMSO was markedly delayed in the presence of calyculin A, in comparison to that seen during the contraction induced by either 60 mM K+ or 10 μM carbachol.
It should be noted that DMSO decreased the level of MLC phosphorylation to the level lower than the resting level during the sustained contractions induced by 60 mM K+ and 10 μM carbachol, while it had no significant direct effect on the resting level of MLC phosphorylation. In contrast, wortmannin significantly decreased the phosphorylation level during both resting and contracted states, suggesting effects on Ca2+-calmodulin-dependent MLCK. Our observations of the in vitro phosphorylation assay suggested DMSO was inhibiting MLC phosphorylation by interfering with the calmodulin-mediated activation of MLCK and not by directly inhibiting the catalytic activity. Such a mechanism of the inhibition of MLC phosphorylation may be consistent with the relative resistance of the resting level of MLC phosphorylation to DMSO. The MLC phosphorylation during the contraction would thus depend on the DMSO-sensitive mechanism to a greater extent than that seen during the resting state.
The inhibitory effect of DMSO on the calyculin-A-induced MLC phosphorylation was smaller than that seen with wortmannin, while the inhibitory effect on the contraction was greater. This finding suggested that some mechanism independent of MLC phosphorylation was also involved in the relaxant effect of DMSO. DMSO has been reported to enhance the apparent affinity of myosin toward phosphate and stabilize the ADP and phosphate-bound form of myosin in the rabbit psoas muscle (Mariano et al., 2001). DMSO may interfere with the cross-bridge cycling at the step of phosphate release (Mariano et al., 2001). In the present study, DMSO and phosphate exhibited a synergistic effect in relaxing the α-toxin-permeabilized strips of rabbit detrusor muscle. This observation suggested that an inhibitory effect on cross-bridge cycling was also involved in the relaxant effect of DMSO in smooth muscle.
In clinical practice, the urinary bladder is filled with 50% DMSO for the treatment of interstitial cystitis. The contractility of the bladder has been shown to be attenuated after the intravesical treatment with DMSO. Such a relaxant effect of DMSO is said to be related to the therapeutic effect of DMSO in relieving the symptoms associated with interstitial cystitis (Shirley et al., 1978). The concentrations of DMSO in the smooth muscle layers obtained by the intravesical administration of 50% DMSO remains unknown. However, DMSO is cell-permeable and also water-miscible. In the present study, DMSO induced a relaxant effect with its EC50 being about 3%, which is a much lower concentration than that applied to the bladder. The Ca2+-desensitizing and relaxant effect of DMSO observed in the present study is thus considered to be functionally relevant and it may therefore contribute to the therapeutic effect of DMSO in the treatment of interstitial cystitis.
In conclusion, the present study demonstrated for the first time that DMSO induced a relaxation in rabbit detrusor muscle by decreasing the Ca2+ sensitivity of the contractile apparatus, while having no effect on the level of [Ca2+]i, during the contractions induced by both membrane depolarization and muscarinic stimulation. The Ca2+-desensitizing effect of DMSO was suggested to be mainly due to the inhibition of MLC phosphorylation. Inhibition of the cross-bridge cycling at the step of phosphate release may also be involved in DMSO-induced relaxations of the detrusor muscle. Our findings thus help us to understand the molecular mechanism for the therapeutic effect of DMSO on interstitial cystitis. In addition, the present study suggests that a degree of caution is required when using DMSO in basic research. In the case of using DMSO as a solvent, care should be taken to keep the final concentrations below 1%, otherwise the cellular effects of DMSO, such as the relaxant effect as observed in the present study, may complicate the interpretation of the experimental results.
Acknowledgments
We thank Dr Dmitry N Derkach and Dr David J Hartshorne (University of Arizona, Tucson, AZ, USA) for their help with the in vitro phosphorylation assay and Mr Brian Quinn for linguistic comments and help with this manuscript. This study was supported in part by the grant from the Twenty-first Century COE Programme and a Grant-in-Aids for Scientific Research (No. 17590744) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- CSS
cytosolic substitution solution
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulphoxide
- DTT
dithiothreitol
- fura-PE3/AM
fura-PE3 in the form of acetoxymethyl ester
- MLC
myosin light chain
- MLC17+20
a mixture of 17- and 20-kDa MLC
- MLCK
myosin light chain kinase
- PSS
physiological saline solution
Conflict of interest
The authors state no conflict of interest.
References
- Birder LA, Kanai AJ, de Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol. 1997;158:1989–1995. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- Castroman PJ, Ness TJ. Spinal neurophysiologic correlates of the analgesic actions of intravesical dimethyl sulfoxide and capsaicin in the rat. J Pain. 2002;3:394–400. doi: 10.1054/jpai.2002.126789. [DOI] [PubMed] [Google Scholar]
- Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase beta and CPI-17. Am J Physiol Renal Physiol. 2006;290:F650–F656. doi: 10.1152/ajprenal.00235.2005. [DOI] [PubMed] [Google Scholar]
- De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Gilot D, Loyer P, Corlu A, Glaise D, Lagadic-Gossmann D, Atfi A, et al. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J Biol Chem. 2002;277:49220–49229. doi: 10.1074/jbc.M207325200. [DOI] [PubMed] [Google Scholar]
- Hirano K, Derkach DN, Hirano M, Nishimura J, Kanaide H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem. 2003;248:105–114. doi: 10.1023/a:1024180101032. [DOI] [PubMed] [Google Scholar]
- Hypolite JA, DiSanto ME, Zheng Y, Chang S, Wein AJ, Chacko S. Regional variation in myosin isoforms and phosphorylation at the resting tone in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C254–C264. doi: 10.1152/ajpcell.2001.280.2.C254. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Ozaki H, Sato K, Hori M, Karaki H, Watabe S, et al. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989;250:388–396. [PubMed] [Google Scholar]
- Jacob SW, Herschler R. Pharmacology of DMSO. Cryobiology. 1986;23:14–27. doi: 10.1016/0011-2240(86)90014-3. [DOI] [PubMed] [Google Scholar]
- Kanaide H. Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry. Methods Mol Biol. 2006;312:251–259. [PubMed] [Google Scholar]
- Mariano AC, Alexandre GM, Silva LC, Romeiro A, Cameron LC, Chen Y, et al. Dimethyl sulphoxide enhances the effects of Pi in myofibrils and inhibits the activity of rabbit skeletal muscle contractile proteins. Biochem J. 2001;358:627–636. doi: 10.1042/0264-6021:3580627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D, Packer CS, Johnson TC, Kaefer M. Dimethyl sulfoxide: does it change the functional properties of the bladder wall. J Urol. 2003;170:253–258. doi: 10.1097/01.ju.0000071520.73686.3d. [DOI] [PubMed] [Google Scholar]
- Metts JF. Interstitial cystitis: urgency and frequency syndrome. Am Fam Physician. 2001;64:1199–1206. [PubMed] [Google Scholar]
- Nishimura J, Kolber M, Van Breemen C. Norepinephrine and GTP-γ-S increase myofilament Ca2+ sensitivity in α-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis – a practical approach. Urology. 1997;49:105–107. doi: 10.1016/s0090-4295(97)00181-7. [DOI] [PubMed] [Google Scholar]
- Persechini A, Kamm KE, Stull JT. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. J Biol Chem. 1986;261:6293–6299. [PubMed] [Google Scholar]
- Rembold CM, Wardle RL, Wingard CJ, Batts TW, Etter EF, Murphy RA. Cooperative attachment of cross bridges predicts regulation of smooth muscle force by myosin phosphorylation. Am J Physiol Cell Physiol. 2004;287:C594–C602. doi: 10.1152/ajpcell.00082.2004. [DOI] [PubMed] [Google Scholar]
- Rossberger J, Fall M, Peeker R. Critical appraisal of dimethyl sulfoxide treatment for interstitial cystitis: discomfort, side-effects and treatment outcome. Scand J Urol Nephrol. 2005;39:73–77. doi: 10.1080/00365590410018738. [DOI] [PubMed] [Google Scholar]
- Saida K, Nonomura Y. Characteristics of Ca2+ - and Mg2+ -induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978;72:1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Seguchi H, Nishimura J, Zhou Y, Niiro N, Kumazawa J, Kanaide H. Expression of β3-adrenoceptors in rat detrusor smooth muscle. J Urol. 1998;159:2197–2201. doi: 10.1016/S0022-5347(01)63305-6. [DOI] [PubMed] [Google Scholar]
- Shirley SW, Stewart BH, Mirelman S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology. 1978;11:215–220. doi: 10.1016/0090-4295(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yamamoto H, Hirano K, Kobayashi S, Kanaide H. Mechanisms of caffeine-induced contraction and relaxation of rat aortic smooth muscle. J Physiol. 1992;456:193–213. doi: 10.1113/jphysiol.1992.sp019333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hirano K, Sakihara C, Nishimura J, Kanaide H. NH2-terminal fragments of the 130 kDa subunit of myosin phosphatase increase the Ca2+ sensitivity of porcine renal artery. J Physiol. 1999;516:55–65. doi: 10.1111/j.1469-7793.1999.055aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]