Abstract
Background and purpose:
Sepsis is a systemic inflammatory response resulting from the inability of the host to restrict local infection. The failure of neutrophil migration to the infection site is one of the mechanisms involved in this process. Recently, it was demonstrated that this event is mediated by nitric oxide (NO). The present study addresses the possibility that peroxynitrite (ONOO-), a NO-derived powerful oxidizing and nitrating compound, could also be involved in neutrophil migration failure.
Experimental approach:
Male C57Bl/6 mice were subjected to moderate (MSI) or severe (SSI) septic injury, both induced by cecal ligation and puncture (CLP). The leukocyte rolling and adhesion in the mesentery was evaluated by intravital microscopy. Cytokines (TNF-α and MIP-1α) were measured by ELISA and 3-nitrotyrosine (3-NT) by immunofluorescence.
Key results:
Compared with saline pretreatment of SSI mice, pre-treatment with uric acid, a ONOO- scavenger, partially restored the failure of neutrophil rolling, adhesion and migration to the site of infection. These mice also presented low circulating bacterial counts and diminished systemic inflammatory response. Pretreatment with uric acid reduced 3-NT labelling in leukocytes in mesenteric tissues and in neutrophils obtained from peritoneal exudates. Finally, uric acid pretreatment enhanced significantly the survival rate in the SSI mice. Similarly, treatment with FeTPPs, a more specific ONOO- scavenger, re-established neutrophil migration and increased mice survival rate.
Conclusions and implications:
These results indicate that ONOO- contributed to the reduction of neutrophil/endothelium interaction and the consequent failure of neutrophil migration into infection foci and hence susceptibility to severe sepsis.
Keywords: Sepsis, peroxynitrite, neutrophil migration, nitric oxide, cecal ligation and puncture
Introduction
Sepsis is a systemic inflammatory response (SIR) that results from the inability of the immune system to control bacterial spread during an ongoing infection. During the infection, a complex cascade of events is initiated after the invasion of the host by pathogenic microorganisms (Medzhitov and Janeway, 2000). In this context, neutrophils play a crucial role in orchestrating host defence. They are the first cells that migrate to the infection site and are able to destroy microorganisms by producing bactericidal agents including reactive oxygen and nitrogen species (ROS and RNS) (Yamashiro et al., 2001).
Recently, our group showed that animals subjected to severe sepsis induced by cecal ligation and puncture (CLP) (Benjamim et al., 2000; Torres-Duenas et al., 2006) or Staphylococcus aureus inoculation (Crosara-Alberto et al., 2002) present failure of neutrophil rolling, adhesion and transmigration to the site of infection. This impairment of neutrophil migration was associated with high mortality and increased numbers of bacteria in peritoneal exudate and blood. On the other hand, in non-severe sepsis, the bacterial infection was restricted to the peritoneal cavity, neutrophil recruitment was not affected, and a higher survival rate was observed (Benjamim et al., 2000, 2002; Crosara-Alberto et al., 2002; Rios-Santos et al., 2003; Alves-Filho et al., 2006b). The factors contributing to this failure of neutrophil function include the excessive systemic release of pro-inflammatory cytokines/chemokines, which induce the production of nitric oxide (NO) by inducible nitric oxide synthase (iNOS) (Tavares-Murta et al., 1998, 2001; Benjamim et al., 2002; Crosara-Alberto et al., 2002; Rios-Santos et al., 2007).
There is much evidence to show that several deleterious effects ascribed to NO are, in fact, mediated by peroxynitrite (ONOO−). When produced together, superoxide anion (O2·−) and NO react at an almost diffusion-limited rate (6.7 × 109 M−1 s−1) to produce ONOO− (Huie and Padmaja, 1993). ONOO− is an oxidizing and nitrating agent that reacts with a variety of biomolecules, including lipids, proteins, carbohydrates and deoxyribonucleic acid (Moreno and Pryor, 1992; Beckmann et al., 1994; Pryor and Squadrito, 1995; Salgo et al., 1995). Potential patho-physiological effects of ONOO− include its action as a bactericidal agent (Evans et al., 1996), an initiator of lipid peroxidation (Rubbo et al., 1994), oxidation of sulphydryls (Radi et al., 1991), inactivation of sodium transport (Hu et al., 1994) and nitration of tyrosine residues in a variety of proteins, including inactivation of enzymes and/or receptors (Ischiropoulos et al., 1992). Recently, it was demonstrated that ONOO− inhibits neutrophil and monocyte migration in inflammation induced by the macrophage inflammatory protein-1α (MIP-1α) (Sato et al., 2000), as well as neutrophil-actin polymerization (Clements et al., 2003). It is important to add that the potential for ONOO− formation is high in severe sepsis because of the increased formation of O2·−. (Wang et al., 1994).
Therefore, the aim of this study was to assess the role of ONOO− in the failure of neutrophil migration to the site of infection and in the outcome of severe sepsis, induced by CLP. We demonstrated that animals pretreated with uric acid (UA), a naturally occuring scavenger of ONOO− (Whiteman and Halliwell, 1996), re-established leukocyte rolling, adhesion and neutrophil migration to the focus of infection (peritoneal cavity). Moreover, we observed effective bacterial clearance and a reduced SIR evaluated through both decreased neutrophil sequestration into lung tissue and reduced systemic levels of cytokines/chemokines. Consequently, the animals presented improved survival rates. The treatment of mice with FeTPPs (5,10,15,20-tetrakis(4-sulphonatophenyl) porphyrinato iron(III)chloride), another ONOO− scavenger with less apparent reactive oxygen intermediate scavenging activity, also improved the neutrophil migration, with a subsequently increased survival rate. Our data therefore provide evidence for the critical role of ONOO− in the failure of neutrophil to migrate to sites of infection in severe sepsis.
Methods
Animals
Male C57BL/6 mice obtained from the Animal Facility of the Faculty of Medicine of Ribeirão Preto, University of São Paulo, and weighing between 18 and 22 g, were used in this study. The animals were housed in cages in temperature-controlled rooms with a 12:12 h light–dark cycle and received water and food ad libitum. All experiments were conducted in accordance with the ethical guidelines of the School of Medicine of Ribeirão Preto (University of São Paulo, São Paulo, Brazil).
Sepsis model
Sepsis was induced through CLP as described elsewhere (Wichterman et al., 1980). Briefly, mice were anaesthetized with tribromoethanol (250 mg kg−1; intraperitoneally (i.p.)), and a 1 cm midline incision was made on the anterior abdomen. The cecum was exposed and ligated below the ileocecal junction without causing bowel obstruction. A single puncture was made through the cecum using a 30- or 18-gauge needle to induce moderated septic injury (MSI) or severe septic injury (SSI), respectively. Pressure was applied (the cecum was squeezed) to allow cecal contents to be expressed through the puncture. The cecum was placed back in the abdomen cavity, and the peritoneal wall and skin incision were closed. All animals received 1 ml of sterile isotonic saline subcutaneously (s.c.) immediately after the surgery. Sham-operated animals (controls) underwent identical laparotomy but without cecum ligation and puncture.
Neutrophil migration to the peritoneal cavity
Animals were killed in a CO2 chamber and the cells present in the peritoneal cavity were harvested by introducing 1.5 ml of phosphate-buffered saline (PBS) containing 1 mM ethylenediaminetetraacetic acid (EDTA). Total cell counts were performed using a Coulter Ac T series analyzer (Coulter Corp., Miami FL, USA). Differential cell counts were carried out on cytocentrifuge slides (Cytospin 3; Shandon Southern Products, Astmoore, UK) stained by the May-Grünwald-Giemsa (Rosenfeld) method (Laborclin, Pinhais, Pr, Br). The results are expressed as number of neutrophils per cavity.
Measurement of leukocyte rolling and adhesion to the mesenteric microcirculation by intravital microscopy
Leukocyte parameters were examined as described previously (Fortes et al., 1991). Briefly, mice were anaesthetized with tribromoethanol (250 mg kg−1 i.p.) and the mesenteric tissue was exposed for microscopic examination. The animals were maintained on a special board thermostatically controlled at 37°C. Images were registered on a video recorder with a long-distance objective lens (× 40) with a 0.65 numerical aperture. Vessels selected for study were third-order venules, defined according to their branch-order location within the microvascular network. These vessels corresponded to postcapillary venules, with a diameter of 12–18 μm. Rolling leukocytes were defined as those white blood cells that moved at a lower velocity than erythrocytes in the same stream and were determined at 10-min intervals. Adherent leukocytes were considered as those white blood cells that remained stationary on the venular endothelium at the end of observation. The venular area in which the adhesion process was determined varied from 350 to 450 μm2, and the results were expressed as the number of adherent leukocytes per 100 μm2 of venule (Alves-Filho et al., 2006a). Time points selected to determine rolling (2 h) and adhesion (4 h) were based on previous studies, where it was demonstrated that rolling and adhesion respectively peak at these times after injection of inflammatory stimuli (Secco et al., 2003; Freitas et al., 2006).
Bacterial counts in the blood
Animals were killed in a CO2 chamber and the blood was collected by cardiac puncture under sterile conditions. Bacterial count was assessed as described previously (Godshall et al., 2002). Briefly, 10 μl of blood from each animal was plated on a Muller–Hinton agar dishes and incubated at 37°C. Colony forming units (CFU) were analysed after 24 h and the results were expressed as median of log CFU per ml of blood.
Effects of UA on phagocytosis and bacterial killing by neutrophils
Mice were pretreated with sterile isotonic saline or UA and injected i.p. with 1 ml of thioglycollate (3%) to obtain peritoneal neutrophils. The peritoneal cells were harvested 6 h later by washing the cavities with RPMI-1640 medium. Cell viability (Trypan blue exclusion) was >98% and the population consisted of macrophages and neutrophils, with the latter representing >85% of total leukocytes. Cells were cultured for 1 h at 37°C in antibiotic-free RPMI-1640, and the non-adherent cells (>94% neutrophils) were collected.
Phagocytosis was measured by incubating these neutrophils (1 × 106 cells ml−1) with cecal bacteria (1 × 107 CFU) at 37°C with cecal shaking. After 90 min, the cells were washed with PBS at 4°C and centrifuged at 200 g (to remove extracellular bacteria). Following this, cells were deposited onto microscope slides, and cytocentrifuged at 200 g (Cytospin 3). Cells were then fixed with methanol and stained by the May-Grünwald-Giemsa (Rosenfeld) method (Laborclin, Pinhais, Pr, Brazil). The number of neutrophils that had ingested bacteria were counted under a phase contrast microscope. Results are expressed as the percentage of neutrophils that ingested bacteria.
Neutrophil killing was measured by incubating neutrophils (1 × 106 cells ml−1) with cecal bacteria (1 × 106 CFU) at 37°C for 3 h with mild shaking. At the end of this incubation, samples were collected by centrifugation (200 g) and lysed in 0.2% Triton X-100. Bacterial viability was assessed by serial log dilutions and plating on Mueller–Hinton agar dishes (Difco Laboratories, Detroit, USA). CFU were counted after 12 h and the results expressed as number of viable ingested bacteria (log CFU).
Determination of cytokine and chemokine levels
The animals were killed in a CO2 chamber; the cells present in the peritoneal cavity were harvested by introducing 1.5 ml of PBS containing 1 mM EDTA and the blood was collected by cardiac puncture. The concentrations of tumour necrosis factor-α (TNF-α) and MIP-1α were determined by using a double-ligand enzyme-linked immunosorbent assay (ELISA). For both peritoneal exudate and serum, the results are expressed as pg ml−1.
Lung tissue myeloperoxidase activity
The neutrophil sequestration in the lung was measured by measuring myeloperoxidase (MPO) activity as described previously (Souza et al., 2000). Briefly, the animals were killed in a CO2 chamber and the lungs perfused through the right ventricle with 10 ml of saline. Following this, lung tissue (50–100 mg) was harvested and homogenized in 2 volumes of ice-cold buffer (0.1 M NaCl, 20 mM Na-phosphate, 15 mM Na-EDTA, pH 4.7) and centrifuged at 800 g for 15 min. The pellet was then subjected to hypotonic lysis (0.2% NaCl solution followed 30 s later by the addition of an equal volume of a solution containing 1.6% NaCl). After further centrifugation, the pellet was re-suspended in 200 μl of sodium phosphate buffer (50 mM, pH 5.4) containing 0.5% H-TAB. MPO activity in the re-suspended pellet was assayed using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM) and by measuring the change in absorbance at 450 nm. Results are expressed as the mean of MPO activity (units per mg of tissue).
Immunofluorescence assay for 3-nitrotyrosine
Immunofluorescence staining for 3-nitrotyrosine (3-NT) was carried out in mesenteric tissue to count the numbers of 3-NT-positive leukocytes passing into the peritoneal exudate. The mesentery was removed and washed in PBS and frozen. Frozen serial sections were mounted on poly-L-lysine covered glass slides. For immunofluorescence staining, glass slides were defrosted for 30 min in a wet chamber at room temperature, then fixed for 10 min in cold acetone 100% (−20°C) and washed in PBS. In addition, leukocytes from animals of each group were harvested from the peritoneal cavity by introducing 1.5 ml of PBS containing 1 mM EDTA. Subsequently, cytocentrifuge slides were prepared (Cytospin 3), cells fixed with paraformaldehyde (4%) and frozen. For immunofluorescence glass slides containing the mesenteric tissues and leukocytes were defrosted for 30 min in a wet chamber at room temperature.
The glass slides were further incubated for 90 min in a wet chamber at room temperature with PBS containing 1% bovine serum albumin (blockade buffer), washed and then incubated overnight with anti-3-NT antibody (monoclonal antibody; Upstate, Charlottesville, VA, USA; 1:200 dilution). Subsequently, slides were washed 10 times and incubated at room temperature for 1 h with a secondary antibody conjugated with fluorescein isocyanate-FITC (diluted 1:400 in blockade buffer). Glass slides were then washed, mounted using DAPI (Vector Laboratories, Burlingame, CA, USA) and sealed with enamel.
Specificity of the antisera used in this study was examined in each immunohistochemical experiment to assist with the interpretation of the results. This was accomplished by omission of the primary antibody to determine the background generated during the detection assay. The results of quantitative analysis are expressed as number of stained cells present in the microscopic field (magnification × 40).
Experimental protocols
Protocol 1
The animals were divided into seven groups: sham, MSI, UA+MSI, SSI, UA+SSI, SSI+UA and SSI+FeTPPs. Mice subjected to sham, MSI and SSI procedures were pretreated (s.c.) with sterile isotonic saline 1 h before CLP. The UA+MSI group was pretreated (s.c.) with UA at doses of either 10 or 100 mg kg−1 1 h before sepsis induction. The UA+SSI group was pretreated with UA at doses of 1, 10, 50, 100 or 300 mg kg−1, 1 h before CLP. The SSI +FeTPPs group was treated i.p. with FeTPPs at doses of 1, 5 or 15 mg kg−1, 15 min after CLP. Another group of animals was treated with UA (100 mg kg−1) using a therapeutic protocol, that is 6 and 18 h after severe sepsis induction (SSI+UA). The following parameters were analysed: (a) neutrophil migration into the peritoneal cavity 6 h after septic injury and (b) survival rates of animals every 8 h up to 120 h after surgery.
Protocol 2
In an additional set of experiments, mice were pretreated s.c. with a single dose of UA (100 mg kg−1) and 1 h later the animals were subjected to the SSI procedure. The leukocyte rolling and adhesion in the mesenteric microcirculation venules were determined 2 and 4 h respectively after CLP. The number of bacteria in blood and cytokine levels in both the peritoneal exudate and in serum was evaluated 6 h after CLP. In addition, neutrophil sequestration to the lung tissues measured as the tissue MPO activity and immunofluorescence analysis of 3-NT in both the mesenteric tissues and neutrophils were also assessed 6 h after CLP.
Protocol 3
Mice were pretreated s.c. with sterile isotonic saline or with UA (100 mg kg−1). One hour later the animals were injected i.p. with 1 ml of thioglycollate (3%), a cell migration stimulus, and cells in the peritoneal cavity were harvested 6 h later. The neutrophils obtained were incubated in vitro with cecal bacteria for analysis of phagocytosis and killing activity.
Protocol 4
Mice were divided into four experimental groups, three groups were pretreated s.c. with sterile isotonic saline or UA (100 mg kg−1). Fifteen minutes later, these animals received an s.c. injection of either sterile isotonic saline, 3-morpholinosydnonimine (SIN-1; 3 mg kg−1) or S-nitroso-N-acetylpenicillamine (SNAP) (3 mg kg−1). After a further 15 min, these animals were injected i.p. with carrageenan (500 μg per cavity). The control (fourth) group was injected i.p. with sterile isotonic saline. The leukocyte rolling, adhesion and neutrophil migration were determined after 2, 4 and 6 h respectively.
Statistical analysis
The data (except for the survival curves and bacterial counts) are reported as the means±s.e.m. of values obtained from two different experiments. The means of different treatments were compared by multifactorial analysis of variance (ANOVA), followed by Tukey's test (EzANOVA: http://www.sph.sc.edu/comd/rorden/ezanova/home.html, Figure 1a) or one-way ANOVA. If significance was observed, individual comparisons were subsequently subjected to a Bonferroni's test for unpaired values (GraphPad Prism Software version 4, San Diego, CA, USA). Bacterial counts are reported as median of log CFU and were analysed by a Mann–Whitney U-test (GraphPad Prism Software version 4). The survival rate was expressed as the percentage of live animals, and a Mantel–Cox log-rank test was used to determine differences between survival curves (GraphPad Prism Software version 4). A P-value⩽0.05 was considered significant.
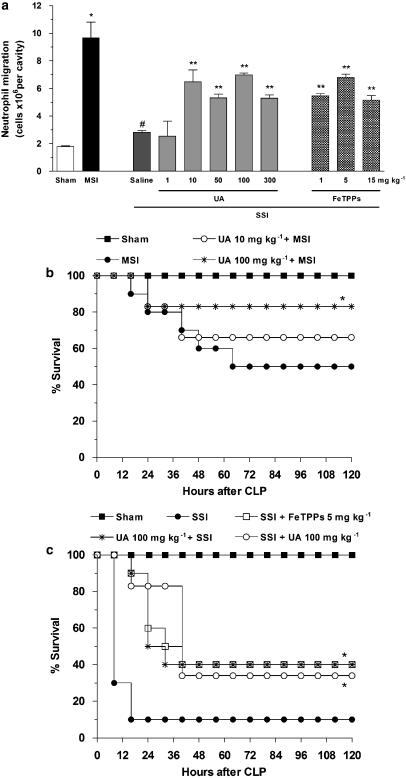
Figure 1.
ONOO− inhibition prevents the failure of neutrophil migration and improves survival rates in septic mice. Mice were subjected to sham, moderate (MSI) or severe (SSI) injury after CLP, as described in the Methods section. (a) Neutrophil migration into the peritoneal cavity was performed 6 h after CLP. Animals were pretreated s.c., with saline or with indicated doses of UA, 1 h before induction of SSI or with indicated doses of FeTPPS, 15 min after SSI. The results are expressed as mean±s.e.m. of 10 animals per group. *P<0.001 compared to sham group; #P<0.001 compared to MSI group; **P<0.05 compared to saline-pretreated mice with SSI (multifactorial ANOVA, followed by Tukey's test). (b) The mice were in sham or MSI groups. The MSI group were pretreated with saline or UA (10 or 100 mg kg−1) 1 h before the surgery. (c) The mice were in sham or SSI groups. The SSI group were pretreated with saline or UA (100 mg kg−1) 1 h before the surgery or treated with UA 6 and 18 h after SSI (100 mg kg−1). Another group of SSI was treated with FeTPPs, 15 min after surgery (5 mg kg−1). The survival rates of animals were determined every 8 h until 120 h after CLP. Results are expressed as % survival (n=6 animals per group). *P<0.01 compared to MSI or SSI group (Mantel–Cox log-rank test). ANOVA, analysis of variance; CLP, cecal ligation and puncture; FeTPPS, 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinato iron(III); MSI, moderate septic injury; ONOO−, peroxynitrite; s.c., subcutaneously; SSI, severe septic injury; UA, uric acid.
Drugs and reagents
Carrageenan, UA, SNAP and FeTPPs were purchased from Sigma-Aldrich (St Louis, MO, USA) and SIN-1 was purchased from Tocris Cookson (St Louis, MO, USA). All drugs used were dissolved in sterile isotonic saline.
Results
Role of ONOO− in neutrophil migration and survival rate in sepsis
Initial experiments were performed to determine the role of ONOO− in the failure of neutrophils to migrate to the site of infection. For this, the ONOO− scavengers, UA or FeTPPs, were given to mice at different doses. The results in Figure 1a show that animals with MSI showed a marked migration of neutrophils into the peritoneal cavity, compared with the sham group. In contrast, the saline-pretreated mice with SSI showed no accumulation of neutrophils in the peritoneal cavity and the pretreatment of these animals with UA restored in part, the neutrophil migration. A dose of 1 mg kg−1 was ineffective, whereas doses of 10, 50, 100 and 300 mg kg−1 were equally effective in restoring neutrophil migration. We also evaluated the effect of another ONOO− scavenger, FeTPPs, and found that this treatment also partially reversed the failure of neutrophil migration observed after SSI (Figure 1a).
We further investigated whether ONOO− scavengers could affect the survival rate of mice. Figures 1b and c show the survival curves of mice subjected to sham, MSI or SSI procedures and the effect of pretreatment with UA. The sham-operated group showed 100% survival throughout the experimental period (120 h). The MSI group showed 100% survival until 12 h after CLP, falling to 80% by 24 h and remaining at 50% for the duration of the experiment. The MSI animals pretreated with UA at doses of 10 or 100 mg kg−1 presented survival rates of approximately 70 and 80%, respectively (Figure 1b). The SSI group presented only 30% survival at 12 h after CLP and subsequently decreased to 10%. The mice pretreated with UA or treated with FeTPPs presented nearly 40% survival 120 h after SSI (Figure 1c). As preventive clinical intervention is not routinely performed, we also performed a set of experiments using a post-sepsis therapeutic treatment protocol. UA was administered 6 and 18 h after induction of SSI and this post-treatment increased survival as effectively as the pretreatment regimen, nearly 40% (Figure 1c, open circles). Because both the ONOO− scavengers used in this study (UA or FeTPPs) showed similar improvements in survival rate and neutrophil migration, and to avoid unnecessary use of animals, all subsequent experiments were conducted with only one scavenger, UA at a dose of 100 mg kg−1.
ONOO− inhibition improves endothelium–leukocyte interactions
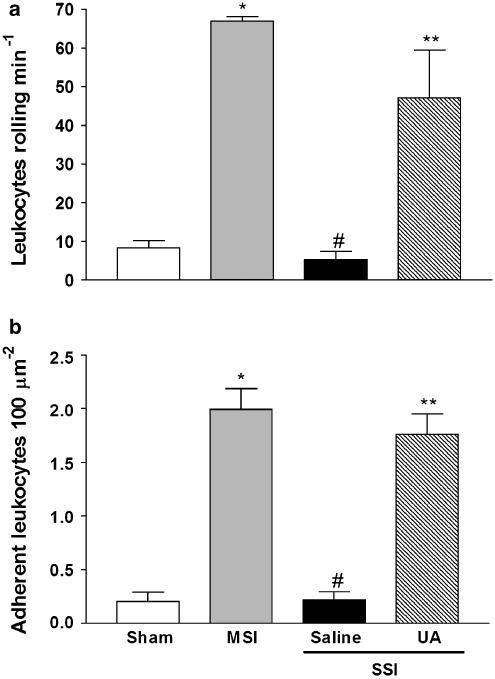
To clarify the mechanisms by which ONOO− scavengers preserve neutrophil migration, we investigated the effect of UA on endothelium–leukocyte interactions (rolling and adhesion) in mesenteric post-capillary venules. Pretreatment with UA significantly enhanced leukocyte rolling and adhesion in mice with SSI (UA+SSI), compared with mice pretreated with saline (Figures 2a and b, respectively). These results suggest that ONOO− inhibited the endothelium–leukocyte interactions and consequently could mediate the failure of neutrophil migration.
Figure 2.
ONOO− inhibition improves the endothelium–leukocyte interactions. Mice were in sham, MSI and SSI groups. The leukocyte rolling (a) and adhesion (b) were evaluated by intravital microscopy in the mesentery 2 and 4 h after CLP, respectively. Animals were pretreated, s.c., with saline or UA (100 mg kg−1) 1 h before SSI. The results are expressed as mean±s.e.m. of five animals per group. *P<0.001 compared to sham group; #P<0.001 compared to MSI group; **P<0.05 compared to saline-pretreated mice subjected to SSI (ANOVA, followed by Bonferroni's test). ANOVA, analysis of variance; CLP, cecal ligation and puncture; MSI, moderate septic injury; ONOO−, peroxynitrite; s.c., subcutaneously; SSI, severe septic injury; UA, uric acid.
Effect of ONOO− on bacterial spread in sepsis
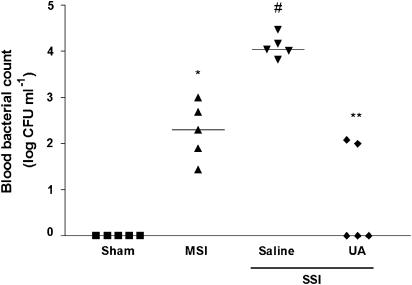
The bacterial load in blood samples obtained from saline-pretreated mice subjected to SSI was significantly higher than that observed in the MSI group (Figure 3). In contrast, the pretreatment of the SSI group with UA significantly reduced the bacterial count in blood compared with the count in blood from mice with SSI and pretreated with saline.
Figure 3.
ONOO− inhibition reduces bacterial count in blood from mice with sepsis. Mice were in sham, MSI and SSI groups. Bacterial count in blood was determined 6 h after CLP. Animals were pretreated, s.c., with saline or UA (100 mg kg−1) 1 h before SSI. Results are expressed as median of log CFU per ml of blood, (n=5 animals per group). *P<0.05 compared to Sham group; #P<0.05 compared to MSI group; **P<0.05 compared to saline-pretreated mice subjected to SSI (Mann–Whitney U-test). CFU, colony forming units; CLP, cecal ligation and puncture; MSI, moderate septic injury; ONOO−, peroxynitrite; s.c., subcutaneously; SSI, severe septic injury; UA, uric acid.
Effect of UA on phagocytosis and neutrophil killing
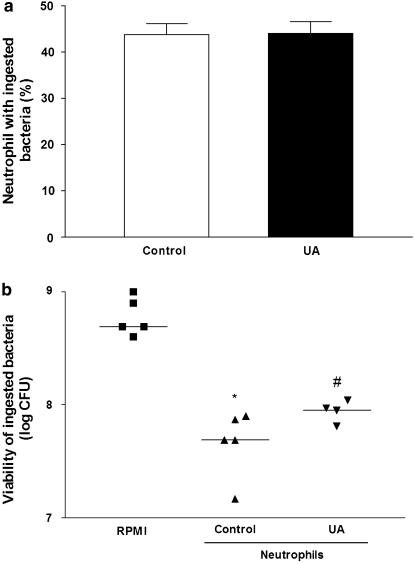
Experiments were carried out to evaluate the in vitro capacity of neutrophils, obtained from UA or saline-pretreated mice, to engulf cecal bacteria. Neutrophils from saline-pretreated (control) or UA-pretreated (100 mg kg−1) mice exhibited a similar phagocytic activity (Figure 4a). The subsequent killing of bacteria by neutrophils was then examined. Neutrophils from mice pretreated with UA displayed a significant decrease of killing activity when compared with control (Figure 4b). These results indicate that pretreatment with UA did not affect the phagocytic activity, but diminished the killing activity of neutrophils.
Figure 4.
UA does not affect phagocytosis but decreases the killing activity of the neutrophil. Neutrophils of mice pretreated s.c. with saline (control) or UA (100 mg kg−1) obtained from the peritoneal cavity after challenge with thioglycollate, were incubated in vitro with cecal bacteria for analysis of phagocytosis (90 min) and killing activity (3 h). (a) Phagocytosis is expressed as percentage of neutrophils that ingested bacteria (n=5 per group) and (b) killing activity, which was calculated as number of viable ingested bacteria (log CFU), n=5, as described in Methods section. *P<0.05 compared to RPMI; #P<0.05 compared to control (Mann–Whitney U test). CFU, colony forming units; ONOO−, peroxynitrite; s.c., subcutaneously; UA, uric acid.
Role of ONOO− on the systemic inflammatory response
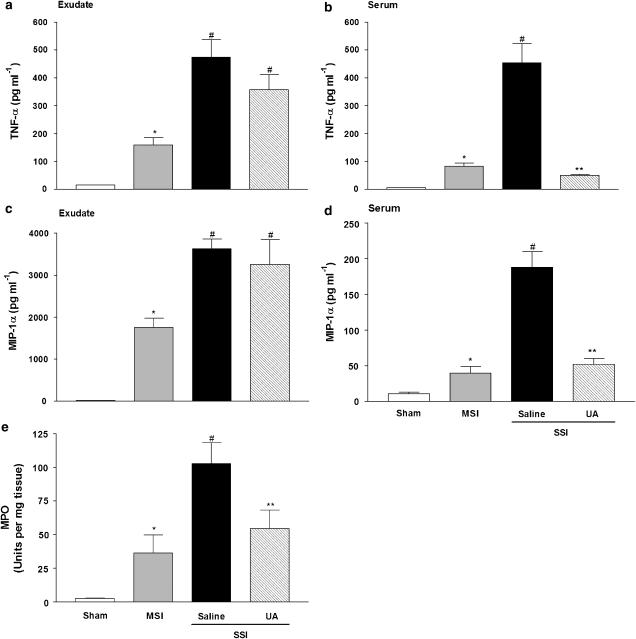
Figure 5 shows cytokine concentrations (TNF-α and MIP-1α) in peritoneal exudates (panels a and c) and serum (panels b and d) of mice after CLP-induced sepsis. The peritoneal exudate and serum concentrations of TNF-α and MIP-1α were significantly increased in saline-pretreated mice with SSI, when compared to the MSI group. Animals pretreated with UA showed diminished concentrations of cytokines in serum, but did not present significant changes in the cytokine levels in peritoneal exudates, demonstrating that efficient neutrophil recruitment and consequent control of infection reduces the development of a SIR.
Figure 5.
ONOO− inhibition reduces the SIR and diminishes the neutrophil accumulation in lung in severe sepsis. Mice were in sham, MSI and SSI groups after CLP. TNF-α and MIP-1α concentrations were quantified in the peritoneal exudate (a and c) and in serum (b and d) 6 h after CLP. Animals were pretreated, s.c., with saline or UA (100 mg kg−1) 1 h before SSI. Results are expressed as mean±s.e.m. of five animals per group. *P<0.05 compared to sham group; #P<0.05 compared to MSI group; **P<0.05 compared to saline-pretreated mice subjected to SSI (ANOVA, followed by Bonferroni's test). (Panel e), neutrophil sequestration in lung was assessed 6 h after CLP by the MPO assay in the lung homogenates. Results are expressed as means of units of MPO activity per mg of tissue±s.e.m. of five animals per group. *P<0.05 compared to sham group; #P<0.05 compared to MSI group; **P<0.05 compared to saline-pretreated mice subjected to SSI (ANOVA, followed by Bonferroni's test). ANOVA, analysis of variance; CLP, cecal ligation and puncture; MIP-1α; macrophage inflammatory protein-1α; MPO, myeloperoxidase; MSI, moderate septic injury; ONOO−, peroxynitrite; SIR, systemic inflammatory response; SSI, severe septic injury; TNF-α, tumour necrosis factor-α; UA, uric acid.
Neutrophils have been implicated as a source of mediators directed towards acute lung injury; therefore, lung neutrophil sequestration was assessed by the MPO assay. As shown in Figure 5e, the SSI group pretreated with saline presented an increase in MPO activity as compared to the MSI group. The pretreatment with UA significantly diminished MPO activity in the lung tissue in mice subjected to severe sepsis (UA+SSI) when compared to mice pretreated with saline and subjected to SSI.
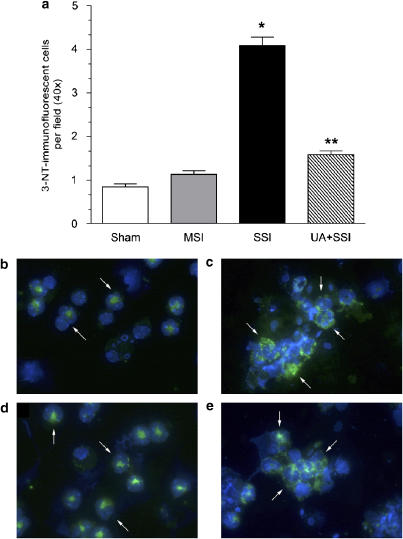
Role of ONOO− in 3-NT formation in mesentery (emigrating leukocytes) and in leukocytes in the peritoneal exudate of mice with sepsis
Mice subjected to SSI and saline pretreatment presented a significant enhancement of 3-NT-positive leukocyte numbers in mesenteric tissue compared with sham-operated animals (Figure 6a). In neutrophils from the peritoneal exudates more 3-NT fluorescence was observed in SSI mice compared with sham or MSI mice (Figures 6c vs b or d). UA pretreatment of mice subjected to SSI resulted in a significant decrease of 3-NT-positive cells counts in both mesentery (Figure 6a) and peritoneal leukocytes (Figure 6e), as compared to the saline-pretreated mice subjected to SSI.
Figure 6.
UA pretreatment reduces 3-NT in mesentery (emigrating leukocytes) and in peritoneal exudates leukocytes of septic mice. Mice were in sham MSI and SSI groups after CLP. (Panel a): The count of positive leukocyte number for 3-NT in histopathological sections from mesentery was evaluated 6 h after CLP. Animals were pretreated s.c., with saline or UA (100 mg kg−1) 1 h before SSI. Results are expressed as means of stained 3-NT-immunofluorescent cells per field±s.e.m. of 25 fields per section. *P<0.05 compared to MSI group; **P<0.05 compared to saline-pretreated mice subjected to SSI (ANOVA, followed by Bonferroni's test). In (b) sham) (c) SSI, (d) MSI and (e) UA+SSI the exudate cells obtained from peritoneal cavity 6 h after CLP were immunofluorescence stained for 3-NT (green) and DAPI (blue nuclear marker). The pre-treatments with saline or UA (100 mg kg−1, s.c.) were performed 1 h before SSI. The arrows point to 3-NT staining. ANOVA, analysis of variance; CLP, cecal ligation and puncture; MSI, moderate septic injury; 3-NT, 3-nitrityrosine; s.c., subcutaneously; SSI, severe septic injury; UA, uric acid.
Role of ONOO− in rolling, adhesion and migration
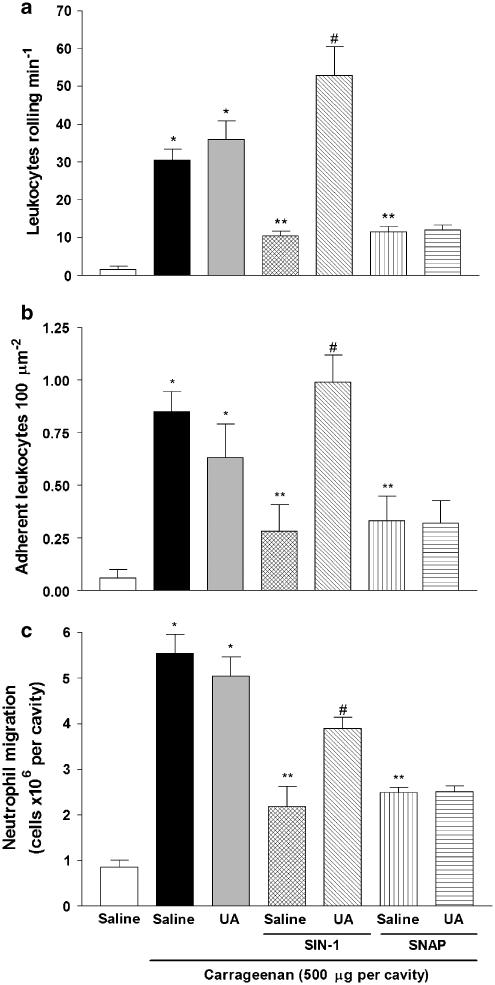
To demonstrate the selective scavenger effect of UA on ONNO− and exclude any possible effect by NO, we used a specific ONOO− donor (SIN-1) and a specific NO donor (SNAP). For this, we used a non-infectious model of inflammation induced by carrageenan. Animals pretreated with saline or UA and post-carrageenan challenge presented a significant leukocyte rolling, adhesion and neutrophil migration (Figures 7a–c). However, in mice post-treated with either SIN-1 or SNAP and challenged with carrageenan, there was a decrease in the neutrophil recruitment when compared to carrageenan-treated mice in the absence of NO or ONOO− donors (saline). Moreover, in mice treated with SIN-1 and carrageenan challenged but pretreated with UA, there was a reversal of the inhibitory effect of SIN-1 treatment. In contrast, UA pretreatment was unable to prevent the inhibitory effect of SNAP treatment on carrageenan-induced neutrophil migration.
Figure 7.
Uric acid pretreatment improves rolling, adhesion and migration by selective inhibition of ONOO−. Leukocyte rolling (a) and adhesion (b) were evaluated by intravital microscopy in the mesentery 2 and 4 h after carrageenan challenge, respectively. (c) Neutrophil migration into the peritoneal cavity was measured 6 h after carrageenan challenge. The mice were pretreated s.c. with saline or uric acid (UA; 100 mg kg−1). Fifteen minutes later these animals received an s.c. administration of saline, SIN-1 (3 mg kg−1) or SNAP (3 mg kg−1). After 15 min these animals were injected i.p. with carrageenan (500 μg per cavity). The control group was injected i.p. with saline. The results are expressed as mean±s.e.m. of ten animals per group. *P<0.05 compared to saline group; **P<0.05 compared with mice pretreated with saline and injected with carrageenan; #P<0.05 compared with mice pretreated with saline and SIN-1 and injected with carrageenan (ANOVA, followed by Bonferroni's test). ANOVA, analysis of variance; i.p., intraperitoneally; ONOO−, peroxynitrite; s.c., subcutaneously; SIN-1, 1,3-morphholinosydnonimine; SNAP, S-nitroso-N-acetylpenicillamine; UA, uric acid.
Discussion
The present study demonstrates for the first time that a reduction of neutrophil/endothelium cell interaction and consequently the failure of neutrophil migration observed in CLP-induced severe sepsis were partially mediated by ONOO−. Pretreatment of septic mice with UA, an ONOO− scavenger (Whiteman and Halliwell, 1996), improved neutrophil rolling, adhesion and migration and, as a consequence, decreased bacterial counts in the circulation. Moreover, a reduced SIR determined by circulating cytokine levels and neutrophil sequestration into lung tissue was observed, and consequently an enhanced survival rate. Similarly, FeTPPs, a more specific ONOO− scavenger with minimal superoxide dismutase (SOD) mimetic activity (Jensen and Riley, 2002) also improved neutrophil migration and enhanced the survival rate.
Neutrophils are the first cells to migrate to sites of infection, where they kill microorganisms by producing ROS and RNS, and as a result, prevent bacteria dissemination and host death (Yamashiro et al., 2001). In fact, severe experimental sepsis induced by polymicrobial infection (Benjamim et al., 2000; Torres-Duenas et al., 2006) or inoculation with S. aureus (Crosara-Alberto et al., 2002), is associated with the failure of neutrophils to migrate to the site of infection. Furthermore, neutrophils obtained from septic patients have an attenuated migration response to chemotactic mediators, when compared to neutrophils from healthy control subjects (Tavares-Murta et al., 2002). One mediator involved in this process is NO, released systemically by iNOS, whose production has been shown to be stimulated by circulating cytokines and chemokines (Radomski et al., 1990; Tavares-Murta et al., 2002). Pharmacological inhibition or genetic ablation of iNOS re-establishes neutrophil migration (Benjamim et al., 2002; Rios-Santos et al., 2007; Torres-Duenas et al., 2006). Thus, although the production of NO and cytokines/chemokines at the infection site are relevant to the ability of leukocytes to control an infectious insult (Fierro et al., 1999), their systemic overproduction prevents neutrophil migration.
NO has the potential to induce both physiologic and pathologic effects, a dichotomy that is well described in the literature. The ability of NO to induce cellular pathology is largely dependent on its conversion to ONOO−, a more reactive nitrogen intermediate. The formation of ONOO− is also dependent on levels of available O2·− in the cellular microenvironment into which NO is released (Beckman et al., 1990). The formation of ONOO− has already been described in sepsis (Wizemann et al., 1994; Szabó et al., 1995). Furthermore, Gagnon et al. (1998) demonstrated that human neutrophils and monocytes can produce and release significant amounts of ONOO− in response to lipopolysaccharide (LPS). Our data using ONOO− scavengers, demonstrating significant protection from the severe sepsis-induced mortality associated with enhanced neutrophil migration to the infection focus, emphasizes the critical importance of ONOO− as a mediator in these events. The fact that ONOO− quenching, given as a post-treatment, was also able to protect against CLP-induced mortality in severe sepsis, suggests that ONOO− has deleterious effects on both the early and delayed phases of sepsis.
Previously, we have demonstrated that the failure of neutrophil migration in severe sepsis was a consequence of a reduction in endothelium–leukocyte (rolling and adhesion) interactions (Benjamim et al., 2002). Additionally, we have demonstrated these events were prevented by inhibition of iNOS (Benjamim et al., 2002). Moreover, there is evidence that ONOO− attenuates the increase in leukocyte adhesion stimulated by LPS (Lelamali et al., 2001). In this study, mice pretreated with UA, exhibited a recovery in the neutrophil rolling, adhesion and migration and thus presented a marked reduction of bacteria counts in the circulation. The consequence of this was an increase in host survival rate. Thus, ONOO− appears to mediate, at least in part, the inhibitory effect of NO on neutrophil/endothelium interaction in severe sepsis and consequently the failure of neutrophil migration.
Besides neutrophil migration to the site of infection, the phagocytic and microbicidal activities of the migrated cells are fundamental to the restriction of the infection locally (Baker and Huynh, 1995). In this study, we have demonstrated that UA pretreatment did not affect phagocytic ability, but partially inhibited the in vitro microbicidal activity of neutrophils, suggesting that, although ONOO− is not involved in neutrophil phagocytosis, it is important in their microbicidal activity. These data are in accordance to the earlier reports showing that ONOO− mediates the microbicidal effect of NO against different strains of bacteria (Hurst and Lymar, 1997; Umezawa et al., 1997). However, there is an apparent contradiction in that the pretreatment of septic mice with UA leads to a reduction of the infection. A possible explanation for this anomaly is that the increase in the neutrophil numbers sequestered to the peritoneal cavity would counterbalance the reduction of their microbicidal activity.
The production of chemokines/cytokines at infection sites mediates leukocyte recruitment and activation (Huttenlocher et al., 1995; Wagner and Roth, 2000). The levels of TNF-α and MIP-1α in the peritoneal exudates of mice subjected to severe sepsis were higher than those observed in animals subjected to MSI and UA pretreatment did not affect the local release of these chemotactic mediators. Thus, the failure of neutrophil migration in severe sepsis is not due to the deficiency in the production of inflammatory mediators at the site of infection, reinforcing the premise that ONOO− is primarily inhibiting neutrophil rolling and adhesion to endothelial cells.
A SIR is considered to be the central deleterious pathogenic event in severe sepsis. High levels of inflammatory cytokines and chemokines in blood mediate the cardiovascular collapse and multiple organ failure observed in severe sepsis (Walley et al., 1996; Hotchkiss and Karl, 2003). In this context, systemic neutrophil activation by cytokines/chemokines promotes their infiltration into lung tissue, where they play a central role in the pathogenesis of sepsis-related lung injury (Fry et al., 1980; Abraham, 2003; Bhatia and Moochhala, 2004). Consistent with the detrimental effect of ONOO− in severe sepsis, pretreatment with UA reduced the increase of TNF-α and MIP-1α in serum and lung neutrophil infiltration, observed in the mice with SSI. These results reconfirm that an efficient neutrophil recruitment and consequent control of infection reduces the development of systemic inflammatory response syndrome (SIRS).
The pathophysiological effect of ONOO− is a consequence, at least in part, of its ability to promote protein nitration (Beckman et al., 1990). In this study, we observed a significant increase in 3-NT, a marker of protein nitration, in transmigrated leukocytes in mesentery and also in cells present in the peritoneal exudate of mice, after SSI. This was significantly diminished by the pretreatment of the mice with UA, demonstrating that the 3-NT formation in our experimental model is a consequence of UA-sensitive, ONOO−-driven nitration. To confirm the ability of UA to neutralize ONOO− (Whiteman and Halliwell, 1996), we analysed its capacity to prevent the inhibitory effect of SIN-1, a ONOO− donor or SNAP, a specific NO donor (Holm et al., 1998) on neutrophil peritoneal recruitment induced by carrageenan. UA pretreatment blocked the inhibitory effect of SIN-1 upon leukocyte rolling, adhesion and migration, and was ineffective on these inhibitory effects of SNAP. These results, apart from confirming that UA inactivates ONOO−, suggest that NO is able to inhibits neutrophil migration by mechanism(s) independent of ONOO− formation. The fact that in SSI, pretreatment with UA only partially prevented the failure of neutrophil rolling, adhesion and migration (present study), whereas inhibitors of iNOS totally prevent these phenomena (Benjamim et al., 2002) indicate that both ONOO− and NO contribute to the loss of these neutrophil functions.
In summary, the present study demonstrates, to our knowledge for the first time, that ONOO− is involved in a reduction of neutrophil rolling and adhesion and, consequently, to the failure of neutrophils to migrate to the focus of infection during severe sepsis. Importantly, we also show that the inactivation of ONOO− avoids the spread of bacteria and the SIRS and as a consequence, improves the survival rate of mice with sepsis. Therefore, the development of specific ONOO− antagonists has a potential therapeutic value for the treatment of severe sepsis.
Acknowledgments
We thank Fabiola Leslie Mestriner, Giuliana Bertozi, Ana Katia dos Santos, Ieda Regina dos Santos Schivo, Sergio Roberto Rosa, Heleni Tamburus and Monica Azevedo de Abreu for technical assistance and Dr John S Hothersall for the English revision of the manuscript. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq), Programa de Núcleos de Excelência (PRONEX) and Universidad Autonoma de Bucaramanga, UNAB.
Abbreviations
- CLP
cecal ligation and puncture
- EDTA
ethylenediaminetetraacetic acid
- FeTPPs
5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinato iron(III)
- iNOS
inducible nitric oxide synthase
- MPO
myeloperoxidase
- MSI
moderate septic injury
- 3-NT
3-nitrotyrosine
- ONOO−
peroxynitrite
- PBS
phosphate-buffered saline
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SIN-1
3-morpholinosydnonimine
- SIRS
systemic inflammatory response syndrome
- SNAP
S-nitroso-N-acetylpenicillamine
- SSI
severe septic injury
Conflict of interest
The authors state no conflict of interest.
References
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006a;34:461–470. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, Tavares-Murta BM, Barja-Fidalgo C, Benjamim CF, Basile-Filho A, Arraes SM, et al. Neutrophil function in severe sepsis. Endocr Metab Immune Disord Drug Targets. 2006b;6:151–158. doi: 10.2174/187153006777442404. [DOI] [PubMed] [Google Scholar]
- Baker CC, Huynh MD. Sepsis in the critically ill patient. Curr Probl Surg. 1995;32:1013–1083. doi: 10.1016/s0011-3840(05)80018-8. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun. 2002;70:3602–3610. doi: 10.1128/IAI.70.7.3602-3610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Moochhala S. Role of inflammatory mediatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- Clements MK, Siemsen DW, Swain SD, Hanson AJ, Nelson-Overton LK, Rohn TT, et al. Inhibition of actin polymerization by peroxynitrite modulates neutrophil functional responses. J Leukoc Biol. 2003;73:344–355. doi: 10.1189/jlb.0802401. [DOI] [PubMed] [Google Scholar]
- Crosara-Alberto DP, Darini AL, Inoue RY, Silva JS, Ferreira SH, Cunha FQ. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br J Pharmacol. 2002;136:645–658. doi: 10.1038/sj.bjp.0704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro IM, Nascimento-DaSilva V, Arruda MA, Freitas MS, Plotkowski MC, Cunha FQ, et al. Induction of NOS in rat blood PMN in vivo and in vitro: modulation by tyrosine kinase and involvement in bactericidal activity. J Leukoc Biol. 1999;65:508–514. doi: 10.1002/jlb.65.4.508. [DOI] [PubMed] [Google Scholar]
- Fortes ZB, Farsky SP, Oliveira MA, Garcia-Leme J. Direct vital microscopic study of defective leukocyte–endothelial interaction in diabetes mellitus. Diabetes. 1991;40:1267–1273. doi: 10.2337/diab.40.10.1267. [DOI] [PubMed] [Google Scholar]
- Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, et al. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharmacol. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DE, Pearlstein L, Fulton RL, Polk HC., Jr Multiple system organ failure: the role of uncontrolled infection. Arch Surg. 1980;115:136–140. doi: 10.1001/archsurg.1980.01380020006003. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Leblond FA, Filep JG. Peroxynitrite production by human neutrophils, monocytes and lymphocytes challenged with lipopolysaccharide. FEBS Lett. 1998;431:107–110. doi: 10.1016/s0014-5793(98)00741-8. [DOI] [PubMed] [Google Scholar]
- Godshall CJ, Scott MJ, Peyton JC, Gardner SA, Cheadle WG. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- Holm P, Kankaanranta H, Metsa-Ketela T, Moilanen E. Radical releasing properties of nitric oxide donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur J Pharmacol. 1998;346:97–102. doi: 10.1016/s0014-2999(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Hu P, Ischiropoulos H, Beckman JS, Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol. 1994;266:L628–L634. doi: 10.1152/ajplung.1994.266.6.L628. [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Hurst JK, Lymar SV. Toxicity of peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chem Res Toxicol. 1997;10:802–810. doi: 10.1021/tx970008v. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Riley DP. Peroxynitrite decomposition activity of iron porphyrin complexes. Inorg Chem. 2002;41:4788–4797. doi: 10.1021/ic011089s. [DOI] [PubMed] [Google Scholar]
- Lelamali K, Wang W, Gengaro P, Edelstein C, Schrier RW. Effects of nitric oxide and peroxynitrite on endotoxin-induced leukocyte adhesion to endothelium. J Cell Physiol. 2001;188:337–342. doi: 10.1002/jcp.1128. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Moreno JJ, Pryor WA. Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol. 1992;5:425–431. doi: 10.1021/tx00027a017. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Santos F, Alves-Filho JC, Oliveira Souto F, Spiller F, Freitas A, Monteiro C, et al. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by iNOS-derived nitric oxide. Am J Respir Crit Care Med. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- Rios-Santos F, Benjamim CF, Zavery D, Ferreira SH, Cunha Fde Q. A critical role of leukotriene B4 in neutrophil migration to infectious focus in cecal ligaton and puncture sepsis. Shock. 2003;19:61–65. doi: 10.1097/00024382-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- Salgo MG, Bermudez E, Squadrito GL, Pryor WA. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes Arch. Biochem Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Inhibition of MIP-1alpha-induced human neutrophil and monocyte chemotactic activity by reactive oxygen and nitrogen metabolites. J Lab Clin Med. 2000;135:161–169. doi: 10.1067/mlc.2000.104307. [DOI] [PubMed] [Google Scholar]
- Secco DD, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, Cunha Fde Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003;9:153–164. doi: 10.1016/j.niox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Souza DG, Coutinho SF, Silveira MR, Cara DC, Teixeira MM. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur J Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- Szabó C, Salzman AL, Ischiropoulos H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- Tavares-Murta BM, Machado JS, Ferreira SH, Cunha FQ. Nitric oxide mediates the inhibition of neutrophil migration induced by systemic administration of LPS. Inflammation. 2001;25:247–253. doi: 10.1023/a:1010927921018. [DOI] [PubMed] [Google Scholar]
- Tavares-Murta BM, Cunha FQ, Ferreira SH. The intravenous administration of tumor necrosis factor alpha, interleukin 8 and macrophage-derived neutrophil chemotactic factor inhibits neutrophil migration by stimulating nitric oxide production. Br J Pharmacol. 1998;124:1369–1374. doi: 10.1038/sj.bjp.0701965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Murta BM, Zaparoli M, Ferreira RB, Silva-Vergara ML, Oliveira CH, Murta EF, et al. Failure of neutrophil chemotactic function in septic patients. Crit Care Med. 2002;30:1056–1061. doi: 10.1097/00003246-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Torres-Duenas D, Benjamim CF, Ferreira SH, Cunha FQ. Failure of neutrophil migration to infectious focus and cardiovascular changes on sepsis in rats: Effects of the inhibition of nitric oxide production, removal of infectious focus, and antimicrobial treatment. Shock. 2006;25:267–276. doi: 10.1097/01.shk.0000208804.34292.38. [DOI] [PubMed] [Google Scholar]
- Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, et al. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J Leuloc Biol. 2000;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. Effect Of The NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med. 1994;150:1449–1452. doi: 10.1164/ajrccm.150.5.7952574. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic Res. 1996;25:275–283. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock – a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Wizemann TM, Gardner CR, Laskin JD, Quinones S, Durham SK, Goller NL, et al. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukoc Biol. 2001;69:698–704. [PubMed] [Google Scholar]