Abstract
Background and purpose:
The phenolic compounds isoprenylhydroquinone glucoside (IHG), 3,5-dicaffeoylquinic acid (DCA), and its methyl ester (DCE) have previously been shown to inhibit both contact hypersensitivity (CHS) and peroxynitrite reactivity. The present work seeks to establish a relationship between the anti-inflammatory effect and the release of cytokines and tyrosine nitration in skin.
Experimental approach:
Murine CHS was developed by means of sensitization and challenge with dinitrofluorobenzene (DNFB) or oxazolone. Ear swelling was measured 24 and 96 h after challenge. Interleukin (IL)-1β, IL-4, and tumour necrosis factor (TNF)-α were measured by ELISA; and the expression of inducible nitric oxide synthase (iNOS) was detected by Western blotting. Histological samples were analysed for 3-nitrotyrosine.
Key results:
In the oxazolone model, DCE reduced the 24 h swelling by 54% whereas the effect of DCA was lower (40% inhibition). All the test compounds reduced IL-1β values 24 h after challenge with DNFB or oxazolone, DCE particularly inhibited IL-4 production (74% and 78%, respectively; P<0.01). Tyrosine nitration was also markedly reduced by DCE. In general, the test compounds limited the presence of polymorphonuclear (PMN) leukocytes in the skin.
Conclusions and implications:
These results suggest that the effect of 3,5-dicaffeoylquinic esters on CHS is associated with a decrease in the production of interleukins, but not with the inhibition of iNOS expression. Moreover, esterification of the carboxyl group at C-1 enhanced protection against tyrosine nitration in the skin.
Keywords: contact hypersensitivity, interleukin-1, interleukin-4, tyrosine nitration, skin inflammation
Introduction
Allergic contact dermatitis is one of the most prevalent human skin diseases, causing moderate-to-severe inflammatory damage. This pathological condition arises after contact hypersensitivity (CHS), a well-characterized kind of delayed-type hypersensitivity (DTH), which is in effect an allergic phenomenon based on the interaction between lymphocytes and dendritic antigen-presenting cells (Saint-Mezard et al., 2004). Although the percutaneous penetration of a low-molecular weight hapten gives rise to a marked aetiological feature, no striking differences in the biochemical signalling of CHS and DTH have been reported. In fact, tissue alterations in CHS progress by means of the same events that are recognizable in a number of inflammatory processes. Nevertheless, certain factors, including the presence of regulatory CD4+ helper T cells and the production of both interleukin-4 (IL-4) and monocyte chemotactic protein 1 have prompted researchers to define CHS as a separate entity (Grabbe and Schwarz, 1998). The fact that some transition metals are among the most important contact sensitizers suggests that free radical and redox reactions may be crucial for the process to develop. Furthermore, several studies have associated the inflammatory damage caused by CHS with the genesis, under oxidative stress, of peroxynitrite, an anion which has been found to cause an increase in 3-nitrotyrosine (3-NT) levels in the treated ears of mice (Szabó et al., 2001).
Owing to data on the pharmacological significance of reactive nitrogen species in CHS being scarce, our latest study examines the effects of three natural phenolic compounds that have been previously described as inhibitors of peroxynitrite reactivity (Olmos et al., 2005), and inhibitors of the CHS reaction in mice. These compounds, namely 2-isoprenylhydroquinone-1-glucoside (1-O-glucopyranosyl-1,4-dihydroxy-2-dimethylallylbenzene, IHG), 3,5-dicaffeoylquinic acid ((1S,3R,4S,5R)-1,3,4,5-tetrahydroxy-1-carboxycyclohexane 3,5-di-3-(3,4-dihydroxyphenyl)-propenoate, DCA) and 3,5-dicaffeoylquinic acid methyl ester (DCE), were obtained and identified from the bioactive extracts of the Mediterranean shrub Phagnalon rupestre (Asteraceae) (Góngora et al., 2001, 2002a). To gain insight into the mechanism of action and pharmacological value of this class of compounds as regards both reactive nitrogen species production and the inflammatory mediators involved in CHS, histological and histochemical analyses have been carried out along with an analysis of the cytokines (IL-1β, IL-4 and tumour necrosis factor-α (TNF-α)), 3-NT and inducible NO synthase (iNOS) found in the murine tissue affected by application of dinitrofluorobenzene (DNFB) or oxazolone. Because these two haptens have been used extensively in pharmacological research during the past few decades, there is a great amount of information on their effects.
The phenolic compounds tested effectively reduced both the swelling and the levels of IL-4 and, to a greater degree, of IL-1 in inflamed ears 24 h after challenge with the sensitizers. End-point histological examinations and staining for 3-NT showed that the test compounds also decreased, albeit to a lesser extent, both tyrosine nitration and phagocyte infiltration in inflamed skin.
Materials and methods
CHS reaction
Female CD-1 mice (Harlan Interfauna Ibérica, Sant Feliu de Codines, Spain) weighing 25–30 g were randomly distributed into groups of seven animals, housed at 22±3°C in plastic cages under a 12 h light/darkness cycle and fed with a standard laboratory rodent diet and water ad libitum. The protocol was designed according to the guidelines established by the European Union on Animal Care (Directive 86/609/EEC) and approved by the Ethical Committee of the Faculty of Pharmacy, University of Valencia. At the beginning of the experiment, the animals were sensitized by means of a topical application of 20 μl 0.2% DNFB in acetone on the shaved abdomen for 2 consecutive days. Four days after the first application, the mice were challenged by carefully painting each of their ear surfaces with 10 μl 0.2% DNFB in acetone. Test compounds (0.5 mg per ear, 20 μl) were dissolved in acetone or EtOH:H2O (8:2) and applied to both ears at 1, 24, 48 and 72 h after challenge. The reference drug dexamethasone was administered in acetone solution at a dose of 0.05 and 0.025 mg per ear at 1 and 48 h after challenge, respectively. Ear thickness was measured with a digital micrometer (Series 293 Mitutoyo, Aurora, IL, USA) 24 and 96 h after challenge (Góngora et al., 2000). The oedema was measured as the difference between the thickness of the naive and the inflamed ears. Inhibition percentages were calculated by subtracting the mean oedema value of each group of animals from that of the control group.
After measuring ear thickness at 24 h, two animals from each group were selected for taking ear samples after cervical dislocation and pinnae excision. These samples were homogenized in a buffer solution (10 mm 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES) pH=7.6, 10 mM KCl, 1.5 mM MgCl2, 0.8% Triton X-100, 1 mM dithiothreitol, 2 mM phenylmethanesulphonyl fluoride and protease inhibitor cocktail from Roche Diagnostics, Mannheim, Germany), sonicated (3 × 10 s) and centrifuged at 17 530 g for 15 min at 4°C. Supernatants were analysed for protein content with Bradford reagent, and frozen at −80°C. Ear samples collected at the end of the experiments (96 h) were treated in the same way or preserved whole in 4% aqueous formaldehyde for histological analysis.
A parallel study was carried out with oxazolone as an inducer (Wang et al., 2000). In this case, 150 μl of 3% oxazolone in acetone were administered once on the shaved abdomen. Five days later, the mice were challenged with 20 μl 1% oxazolone applied to their ear surfaces. Pharmacological treatments and end points were the same as those described above for DNFB-induced hypersensitivity.
Cytokine determination
IL-1β, IL-4 and TNF-α were quantified in the homogenates from the excised ears. Each assay was performed in triplicate according to the manufacturer's instructions (eBioscience, San Diego, CA, USA). After incubating the homogenates at 4°C overnight with the appropriate purified anti-mouse antibody in microplate wells, they were treated three times with washing buffer (0.05% Tween 20 in phosphate-buffered saline, pH 7.0). Then, after 1 h of incubation with a diluent, the same operation was repeated. Standards and test solutions (100 μl) were then added and incubated for 2 h at room temperature. Thereafter, two further steps consisting of the addition of biotin conjugate anti-mouse polyclonal antibody (100 μl, 1 h incubation) and avidin–peroxidase (100 μl, 30 min incubation) preceded the termination of the reaction with H2SO4 (1 M). Each incubation was followed by aspiration and repeated washing with the buffer. Final absorbance was read at 450 nm. After a standard curve had been obtained with different cytokine concentrations ranging from 7.81 to 1000 pg ml−1, experimental values were calculated by means of interpolation. Inhibition percentages were defined as the difference between the mean values of control and test value absorbances divided by the control value and multiplied by 100.
Western blot analysis of iNOS protein
After denaturation with dithiothreitol at 100°C for 5 min, electrophoretic separation of the proteins was carried out on sodium dodecylsulphate-10% polyacrylamide gel at 100 V for 90 min. To analyse the amount of protein in the different samples uniformly, protein concentration was measured by Bradford's method. Transference to a nitrocellulose membrane was performed for 90 min at 120 mA. After being blocked with 5% skimmed milk for 2 h, the membrane was incubated with a 1:1000 solution of rabbit polyclonal anti-iNOS antibody (Cayman Chemical Company, Ann Arbor, MI, USA) for 15–18 h at 4°C. The primary complex was bonded to a 1:12 000 solution of peroxidase-labelled rabbit anti-IgG (Sigma, St Louis, MO, USA) after an incubation of 1 h at room temperature. The immunoreactive bands were visualized with the aid of luminol chemiluminescence (ECL plus kit, General Electric Healthcare). A 1:10 000 solution of β-actin served as an internal control to normalize the densitometric data provided by the Scion Image for Windows software.
Histological analysis
Auricular portions fixed in formaldehyde were dehydrated with successive immersions in increasingly enriched (70–100%) aqueous ethanol and then maintained in toluene for 2 × 30 min. The samples were then embedded in paraffin at 56°C and the blocks were subsequently cut in a microtome to give 5 μm sections. These sections were rehydrated sequentially with toluene, ethanol, aqueous ethanol and water. Appropriate staining of the samples was achieved with Mayer's haematoxylin solution and, when a fainter stain was necessary, 1% HCl (10 M) in ethanol was added. The inflammatory pathological scores were graded as follows: 0=no inflammation, no significant findings in comparison with naive ears; 1=slight inflammation, a stage characterized by moderate oedema and influx of inflammatory cells into the dermis; 2=moderate inflammation, a quantitatively increased response and influx of inflammatory cells into the epidermis; 3=severe inflammation, with keratinocyte proliferation, microabscesses and necrosis. Histological examinations were carried out on those groups of mice in which treatment had produced certain level of inhibition of oedema (>30 and >40% in DNFB and oxazolone test; see Results). For immunohistochemical study, paraffin sections were stained following a streptavidin–biotin peroxidase complex technique (Dako, Glostrup, Denmark). Antigen retrieval was obtained using ethylenediaminetetraacetic acid buffer (pH 6) and heating in an autoclave (10 min, 1.5 atm). Incubation of primary mouse anti-3-NT antibody (Zymed clone HM11, Invitrogen, Carlsbad, CA, USA) was performed at room temperature with a dilution of 1/100.
Statistical analysis
Numerical data were expressed as mean±s.e.m. values. Statistical significance was determined with an one way-analysis of variance followed by Newman–Keuls or Dunnett post hoc test for multiple comparisons (*P<0.05, **P<0.01).
Chemicals
The natural test compounds were obtained from aerial parts of Phagnalon rupestre (L.). DC., collected in Serra de Corbera (Valencia, Spain). Plant material was treated with methanol, and the extract was partitioned successively with dichloromethane, ethyl acetate and butanol. The ethyl acetate-soluble fraction was filtered over Sephadex LH-20 with methanol to yield 12 fractions, which were analysed by column chromatography on Si gel 60; then by RP-18 reverse-phase high-performance liquid chromatography (HPLC), to give IHG, DCE and DCA. These compounds, once purified by HPLC, were identified by ultraviolet spectroscopy, 1H- and 13C-nuclear magnetic resonance spectroscopy, and fast atom bombardment–mass spectrometry. For further details see Góngora et al. (2001), (2002a).
Acrylamide, Bradford reagent, dexamethasone, DNFB, dithiothreitol, haematoxylin, HEPES, MgCl2, oxazolone, phenylmethanesulphonyl fluoride, sodium dodecyl sulphate, and Triton X-100 were obtained from Sigma Chemical Co. (St Louis, MO, USA). Ethanol, formaldehyde, HCl and toluene were purchased from JT Baker Chemical Co. (Phillipsburg, NJ, USA; KCl from Panreac Química SA (Castellar del Vallés, Spain); acetone from Merck (Darmstadt, Germany).
Results
Effects on ear swelling
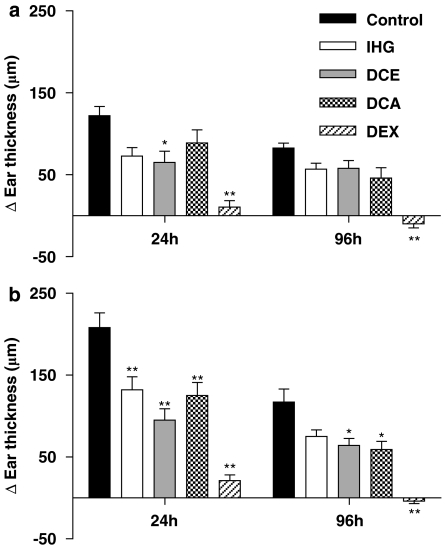
As is the case in every inflammatory process, one of the foremost parameters of contact sensitivity is the swelling of the affected organ. Apart from the initial measuring of the naive mouse ears, two additional measurements were taken within the elicitation, or efferent, phase. The first reading was taken 24 h after challenge with DNFB or oxazolone, when the oedema peaks. The second measurement was taken at 96 h, when, according to many accounts in the literature, the inflammation should be residual. Our observations were that, in the DNFB test, this 96 h oedema was still two-thirds that observed at 24 h (P>0.05, Newman–Keuls multiple comparison test). However, in the oxazolone test, such temporal differences were significant (P<0.01). When DNFB was used as a sensitizer, only DCE showed an inhibition of ear oedema (47%), 24 h after challenge. At 96 h, the absolute level of inflammation faded and no statistically significant effects were found, except for dexamethasone. In the case of the oxazolone model, the effect of each of the agents was higher, especially for the caffeoylquinic derivatives. Thus, DCE inhibited swelling by 54% at 24 h while the free acid (DCA) produced an inhibition of 40%. Both compounds were found to have a more or less sustained effect at 96 h. As was the case in previous studies, dexamethasone had a marked action and reduced the oedema values roughly to basal values (Figure 1).
Figure 1.
Effect of the test compounds on CHS ear swelling induced by DNFB (a) or oxazolone (b), measured 24 and 96 h after challenge. Increase in ear thickness is expressed as mean±s.e.m. of at least five different animals. **P<0.01, *P<0.05 after Newman–Keuls test as compared with control group. CHS, contact hypersensitivity; DCA, 3,5-dicaffeoylquinic acid; DCE, 3,5-dicaffeoylquinic acid methyl ester; DEX, dexamethasone; DNFB, dinitrofluorobenzene; IHG, isoprenylhydroquinone glucoside.
Histological analysis
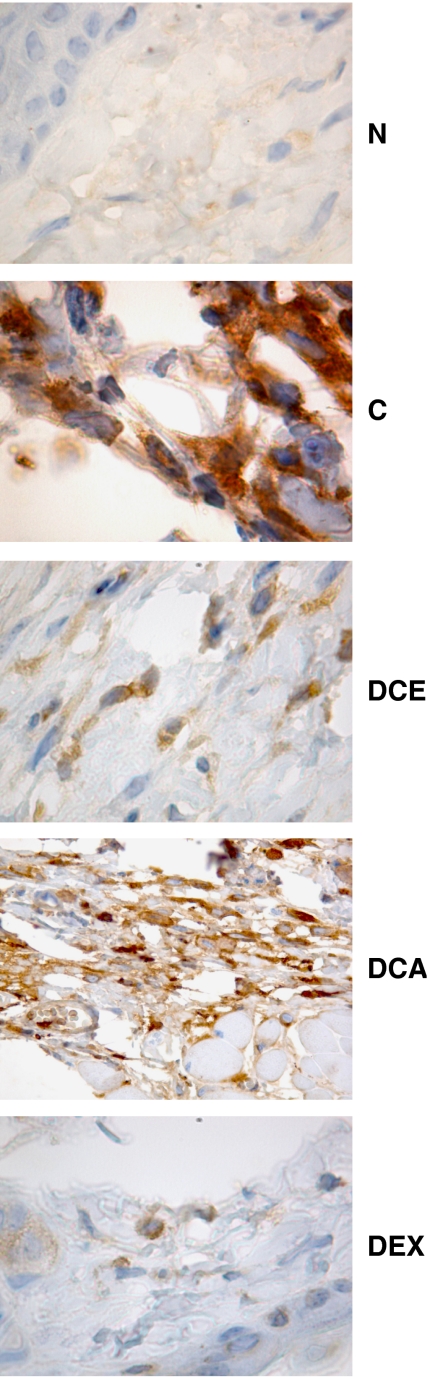
Ear sections from mice treated only with DNFB (DNFB control) showed moderate focal inflammation with a cellular infiltrate, predominantly polymorphonuclear (PMN) leukocytes, in the dermis and epidermis. Immunostaining for 3-NT was abundant. The reference drug dexamethasone suppressed various signs of inflammation and strongly diminished 3-NT staining. The effects of IHG were not noticeable, although a change occurred in the cellular infiltrate, with the predominant leukocytes being mononuclear in this case. DCA moderately reduced tyrosine nitration and the proportion of PMN leukocytes (Table 1). In regard to the pathological features of oxazolone-treated ears, they differed from those treated with DNFB in three main aspects: mononuclear and PMN leukocytes were roughly equipresent in the cutaneous infiltrate, inflammation was diffuse rather than focal and 3-NT staining was observed to a greater extent. The effects of DCE and DCA were mild, but DCE was found to produce a clear reduction in the staining for tyrosine nitration. Finally, dexamethasone suppressed inflammatory morphological parameters and abolished tyrosine nitration (see Table 2 and Figure 2).
Table 1.
Main histological features of mouse ears exhibiting contact hypersensitivity to DNFB
| Intensity | Character | Cellular dominance | 3-NT | |
|---|---|---|---|---|
| Control | 2 | Focal | PMN | ++ |
| IHG | 2 | Focal | MN | ++ |
| DCA | 2 | Focal | PMN/MN | + |
| DEX | 0 | — | — | − |
Abbreviations: DCA, 3,5-dicaffeoylquinic acid; DEX, dexamethasone; IHG, isoprenylhydroquinone glucoside; MN, mononuclear. 3-NT, 3-nitrotyrosine; PMN, polymorphonuclear.
Gradation of inflammation: 2=moderate; 1=slight; 0=not significant. Further details for numerical scores are explained in Materials and methods section, under the heading ‘Contact hypersensitivity reaction'. Symbols (−) to (++) represent increased 3-NT staining. Histological analyses were applied when drug treatment inhibited oedema more than 30% after 96 h.
Control=animals treated only with DNFB.
Table 2.
Main histological features of mouse ears exhibiting contact hypersensitivity to oxazolone
| Intensity | Character | Cellular dominance | 3-NT | |
|---|---|---|---|---|
| Control | 3 | Diffuse | PMN/MN | +++ |
| DCE | 2 | Diffuse | MN | + |
| DCA | 2 | Diffuse | MN | +++ |
| DEX | 1 | — | — | − |
Abbreviations: DCA, 3,5-dicaffeoylquinic acid; DCE, 3,5-dicaffeoylquinic acid methyl ester; DEX, dexamethasone; MN, mononuclear; 3-NT, 3-nitrotyrosine; PMN, polymorphonuclear.
Gradation of inflammation: 3=severe; 2=moderate; 1=slight. Further details for numerical scores are explained in Materials and methods section, under the heading ‘Contact hypersensitivity reaction'. Symbols (−) to (+++) represent increased 3-NT staining. Histological analyses were performed when drug treatment inhibited oedema more than 40% after 96 h.
Control=animals treated only with DNFB.
Figure 2.
Immunohistochemical detection of 3-NT in CHS induced by oxazolone. C, oxazolone-only control; CHS, contact hypersensitivity; DCA, 3,5-dicaffeoylquinic acid; DCE, treatment with 3,5-dicaffeoylquinic acid methyl ester; DEX, treatment with dexamethasone; N, naive ear section; 3-NT, 3-nitrotyrosine.
Cytokine profile in tissue homogenates
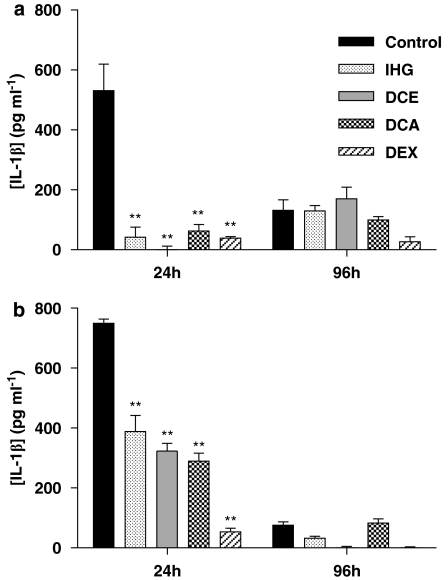
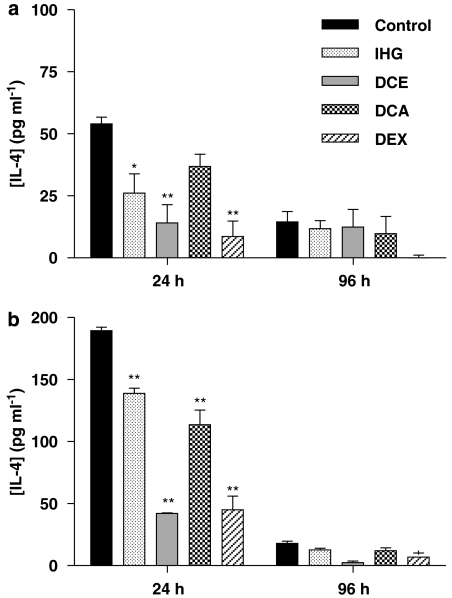
The levels of IL-1β in inflamed ears from the control group more or less paralleled the time course observed for swelling, though the decay at 96 h was more pronounced (Figure 3). This was very marked in the case of induction with oxazolone, in which the IL-1β levels fell to one-tenth of the level at 24 h. Although levels of IL-4 were consistently lower that those of IL-1β (Figure 4), this cytokine followed a time course quite similar to that of IL-1β. It is clear that while the ratio of cytokine concentrations measured at 96 and 24 h was almost exactly the same for both IL-1β and IL-4, it varied depending on the hapten: 1:10 for oxazolone and 1:4 for DNFB. Each of the compounds assayed drastically reduced the presence of IL-1β found in ear homogenates 24 h after challenge with DNFB, but no significant effects appeared after 96 h. Levels of IL-4 induced by oxazolone were equally inhibited by IHG or DCA, whereas DCE was clearly more potent, approximating to the effect of dexamethasone.
Figure 3.
Effect of the test compounds on IL-1β production in CHS induced by DNFB (a) or oxazolone (b) 24 and 96 h after challenge. Data represent mean±s.e.m. of at least four different animals. **P<0.01 after Newman–Keuls test as compared with control group. CHS, contact hypersensitivity; DNFB, dinitrofluorobenzene; IL, interleukin.
Figure 4.
Effect of the test compounds on IL-4 production in CHS induced by DNFB (a) or oxazolone (b) 24 and 96 h after challenge. Data represent mean±s.e.m. of at least four different animals. **P<0.01, *P<0.05 after Newman–Keuls test as compared with control group. CHS, contact hypersensitivity; DNFB, dinitrofluorobenzene; IL, interleukin.
The concentration of TNF-α in ear homogenates was very low in DNFB-challenged mice (data not shown) whereas when oxazolone was used, measurable values could only be obtained 24 h after challenge. The levels, which were 26±4 pg ml−1 (n=3) for the control group, were markedly reduced by each of the phenolic compounds to values below 5 pg ml−1.
Expression of iNOS protein
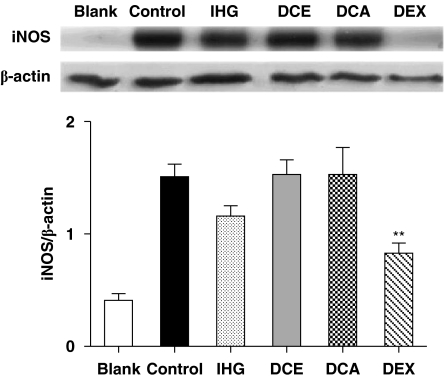
Western blot analyses of ear homogenates from the oxazolone group showed a marked increase in the expression of iNOS, which was reversed by the reference drug, dexamethasone. None of the test compounds (IHG, DCA and DCE) significantly inhibited the increased iNOS expression (Figure 5). Similar results were obtained in the DNFB test, although dexamethasone was less active.
Figure 5.
Western blotting analysis for iNOS protein in homogenates of mouse ear with CHS and challenge with oxazolone. The y axis scale represents mean±s.e.m values for the quotient between i-NOS and β-actin spot densities from three independent experiments. B, blank; C, oxazolone-only control; CHS, contact hypersensitivity; DCA, 3,5-dicaffeoylquinic acid; DCE, 3,5-dicaffeoylquinic acid methyl ester; DEX, dexamethasone; IHG, isoprenylhydroquinone glucoside; iNOS, inducible nitric oxide synthase. **P<0.01 after Dunnett test as compared with control.
Discussion
The new findings in this research shed light not only on the effect of naturally occurring phenolic compounds on CHS, but also on the temporal evolution of several critical cytokines and the histological analysis of tissue samples taken in the late period after elicitation. The fact that 96 h after challenge both ear swelling and histological damage were sustained in the DNFB and oxazolone controls demonstrated that self-healing mechanisms in CHS failed to suppress long-lasting, morphologically measurable inflammation, even though only a single challenge was applied. This was corroborated by the fact that in the mice treated with the phenolic test compounds, we found a partial alleviation of the histological damage, as signalled by a reduction of infiltrating PMN cells. This left a preponderance of mononuclear cells, mainly lymphocytes and monocytes, which are characteristic triggers of chronic inflammatory diseases once they escape from the microvasculature (Schön et al., 2003). In the case of CHS, it has been reported that the massive mixed infiltration of PMN leukocytes and inflammatory monocytes is in fact caused by cytotoxic CD8+ T lymphocytes. These cells have been found to release interferon-γ and other Th type-1 cytokines, which then stimulate the production of interferon inducible proteins 9 and 10, macrophage inflammatory protein-2, granulocyte/macrophage colony-stimulating factor, and IL-6, along with IL-1 and TNF-α, resulting in cell death and patent skin tissue degradation (Saint-Mezard et al., 2004). Moreover, the intensity of the inflammatory alterations was found to be parallel with tyrosine nitration, a reaction that we have now localized, at least for the most part, in these skin-infiltrating mononuclear cells. It should be noted that a previous histological analysis of ear samples taken 24 h after challenge showed that 3-NT was located mainly in the zones of keratinocyte necrosis (Szabó et al., 2001). Our results thus raise the possibility that a dominance of tyrosine nitration in mononuclear cells is a characteristic signal of the last stages of the CHS process.
The decrease of ILs throughout the elicitation phase highlighted certain differences between DNFB and oxazolone as inducers of inflammation. Thus, although 24 h after challenge, oxazolone induced higher levels of IL-1β and IL-4 than DNFB, in the case of the latter, the response faded more slowly. While a comparison between the inflammatory effects of the sensitizers is beyond the scope of the present paper, our results suggest that the DNFB reaction is characterized by the duration of the response rather than by peak intensity. To prove this, however, a time course analysis of the process after the application of different dose regimes would be necessary.
The three cytokines evaluated have different cellular origins and distinct roles in the development of CHS. IL-1β is rapidly secreted in huge amounts from Langerhans cells and keratinocytes at the beginning of the sensitization phase, but it also performs important functions in the spreading of inflammation during the elicitation phase (Nakae et al., 2003). IL-4 is possibly one of the most specific, as well as one of the most controversial cytokines in CHS. Although it has previously been characterized as a T helper lymphocyte subtype 2 (Th2) limiting or repressor agent, its function seems, in fact, to be critical. Indeed, it has been reported that within the elicitation phase there is a short, initial IL-4-insensitive period, but that from 48 h onward, this cytokine is necessary for the full development of the process (Weigmann et al., 1997). Recently, Campos et al. (2006) demonstrated that IL-4 is secreted from hepatic natural killer T cells very early after sensitization with the hapten. This secretion stimulates IgM synthesis by B-1 lymphocytes, which is a requisite for the elicitation of CHS. Thus, as early as 2 h after challenge with picryl chloride, a reduction of the inflammatory reaction was observed in IL-4−/− mice. Unfortunately, as is often the case in this field of research, differences in murine strain, sensitizer class and dosage make comparative time course analyses difficult. According to our results, both IL-1β and IL-4 are first-line cytokines in the elicitation and should not have a special role in the maintenance of the inflammatory reaction. Nevertheless, the relatively higher effect of DCE on both the production of IL-4 and on very late 3-NT staining in mouse ears suggests a possible link between the two events. A hypothetical positive relationship between IL-4 and the nitration of tyrosine residues in CHS definitely requires a more extensive analysis, but seems appealing due to the existence of negative evidence in other, quite different inflammatory processes, such as neuronal injury (Koeberle et al., 2004) or endotoxaemic heart damage (Scumpia et al., 2004).
As is the case with IL-1β, the role of TNF-α, a Th1 cytokine, is directly related to the migration of Langerhans cells to subcutaneous lymph nodes and the expression of major histocompatibility II complex protein. Additionally, TNF is important in the elicitation phase as it recruits PMN cells (Biedermann et al., 2000) and mediates the production of vascular endothelial growth factor, the levels of which have been found to correspond directly to ear swelling (Shibata et al., 2005). One of the best-characterized mechanisms of the way in which TNF-α favours leukocyte infiltration in the first hours after challenge is the upregulation of surface cellular adhesion molecules on the endothelium (McHale et al., 1999). Although TNF-α is released from different cutaneous cells, there is a strong consensus on the pivotal role of mast cells since they co-localize with lymphocytes in CHS inflamed tissues and activate the proliferation of different subsets of T cells. The enhancement of T-cell proliferation and cytokine release is thus significantly reduced by co-culture with TNF-α−/− bone marrow-derived mast cells compared to the wild-type mast cells (Nakae et al., 2005). In spite of all these reports, however, our results do not allow for a definitive discussion of the effects of the test compounds, as only very low levels of TNF-α were found at CHS sites.
In accordance with previously published results, the increased expression of iNOS was one of the hallmarks of CHS induced either by DNFB or by other nitrohalobenzenes in rodents (Ross et al., 1998; Hartmann et al., 2006). In contrast, there was no data available on oxazolone-based experimental models with which to compare our findings. The genesis of NO by iNOS has a definite, although perhaps non-specific, inflammatory character, not only in the skin, but also in many other organs. It increases blood flow and is transformed into peroxynitrite, which seems to be responsible for cytotoxicity in dermal tissues. However, NO may also be considered a downregulator in CHS because of its mediation in depleting epidermal Langerhans cells (Yuen et al., 2002) and its inhibition of the synthesis of adhesion molecules in vitro (De Caterina et al., 1995). What is clear is that the effect of different inflammatory agents is reduced both in iNOS−/− mice and upon treatment with NOS inhibitors (Cals-Grierson and Ormerod, 2004). As regards our results in inflamed ears, none of the test phenolic compounds caused a significant reduction in iNOS expression.
We must therefore conclude that the efficacy of caffeoyl derivatives in inhibiting most of the inflammation parameters was related to their antioxidant activity. This is best illustrated by comparing several potency values. In contrast to IHG, which was inactive, DCE and DCA strongly inhibited the microsomal lipid peroxidation induced by CCl4/NADPH, with IC50 values of 4.8 and 8.2 μM, respectively. The IC50 of the reference drug, butylhydroxytoluene was 5.5 μM. Both DCE and DCA were potent scavengers of the superoxide anion generated by xanthine oxidase, with IC50 values of 2.3 and 1.9 μM, respectively, while the standard (pyrogallol) was slightly less potent (17.7 μM) (Góngora et al., 2003). These caffeoylquinic derivatives were also effective against the oxidative burst in human neutrophils. They inhibited the superoxide generation induced by TPA in human PMN leukocytes with IC50 values of 32 μM for DCE and 43 μM for DCA (Góngora et al., 2002b) while under the same conditions, N-acetylcysteine showed an IC50 of 23 μM (Villagrasa et al., 1997). With respect to the oxidation driven by reactive nitrogen species, the IC50 values of DCE, DCA and pyrogallol in inhibiting the oxidation of dihydrorhodamine 123 by peroxynitrite were 3.0, 2.1 and 9.1 μM, respectively. In this experiment, IHG was much less active, with an IC50 of 55 μM (Olmos et al., 2005).
These results suggest that although oxidative stress is a co-stimulating factor of cytokine-induced iNOS expression, antioxidant activity is not the sole condition for restricting such expression. Moreover, for certain spin-trapping agents such as α-phenyl-tert-butylnitrone, the effect on iNOS induction is not dependent on antioxidant capacity (Floyd, 1999). However, it must be noted that the antioxidant activity of these caffeoylquinic derivatives (see above) did, in fact, correlate with their reduction of tyrosine nitration observed in our protocol of murine CHS, as well as in other models of protein nitration in vitro (Olmos et al., 2007).
In summary, we can confirm that the major phenolic constituents of Phagnalon rupestre exerted an anti-inflammatory activity in murine CHS. The effects of caffeoylquinic acid derivatives (DCA and DCE) were generally higher than those of IHG for both IL production and ear swelling, whereas no such correlation was found for inhibition of iNOS expression. In the case of oxazolone-induced CHS, DCE protected against tyrosine nitration in the skin. The cutaneous anti-inflammatory effect of caffeoylquinic derivatives corroborates previous data concerning their inhibition of reactive nitrogen/oxygen species generation and biological activity.
Acknowledgments
This research is funded by the Generalitat Valenciana (Project GV/06 353). Ana Olmos was a recipient of a predoctoral fellowship from the same Institution (CTBPRA 2002/56).
Abbreviations
- CHS
contact hypersensitivity
- DCA
3,5-dicaffeoylquinic acid
- DCE
3,5-dicaffeoylquinic acid methyl ester
- DNFB
dinitrofluorobenzene
- DTH
delayed-type hypersensitivity
- IHG
isoprenylhydroquinone glucoside
- IL
interleukin
- NOS
nitric oxide synthase
- 3-NT
3-nitrotyrosine
- PMN
polymorphonuclear
- Th
T helper lymphocyte subtype
- TNF
tumour necrosis factor
Conflict of interest
The authors state no conflict of interest.
References
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Campos RA, Szczepanik M, Itakura A, Lisbonne M, Dey N, Leite-de-Moraes MC, et al. Interleukin-4-dependent innate collaboration between iNKT cells and B-1 B cells controls adaptative contact sensitivity. Immunology. 2006;117:536–547. doi: 10.1111/j.1365-2567.2006.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Góngora L, Giner RM, Máñez S, Recio MC, Rios JL. New prenylhydroquinone glycosides from Phagnalon rupestre. J Nat Prod. 2001;64:1111–1113. doi: 10.1021/np010166t. [DOI] [PubMed] [Google Scholar]
- Góngora L, Giner RM, Máñez S, Recio MC, Ríos JL. Phagnalon rupestre as a source of compounds active on contact hypersensitivity. Planta Med. 2002a;68:561–564. doi: 10.1055/s-2002-32566. [DOI] [PubMed] [Google Scholar]
- Góngora L, Giner RM, Máñez S, Recio MC, Schinella G, Ríos JL. Effects of caffeoyl conjugates of isoprenyl-hydroquinone glucoside and quinic acid on leukocyte function. Life Sci. 2002b;71:2995–3004. doi: 10.1016/s0024-3205(02)02167-7. [DOI] [PubMed] [Google Scholar]
- Góngora L, Máñez S, Giner RM, Recio MC, Rios JL. On the activity of trifluoperazine and palmitoylcarnitine in mice: delayed hypersensitivity models. Life Sci. 2000;66:PL183–PL188. doi: 10.1016/s0024-3205(00)00447-1. [DOI] [PubMed] [Google Scholar]
- Góngora L, Máñez S, Giner RM, Recio MC, Schinella G, Rios JL. Inhibition of xanthine oxidase by phenolic conjugates of methylated quinic acid. Planta Med. 2003;69:396–401. doi: 10.1055/s-2003-39715. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- Hartmann B, Staedtler F, Hartmann N, Meingassner J, Firat H. Gene expression profiling of skin and draining lymph nodes of rats affected with cutaneous contact hypersensitivity. Inflamm Res. 2006;55:322–334. doi: 10.1007/s00011-006-5141-z. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Gauldie J, Ball AK. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-beta on the survival of axotomized retinal ganglion cells. Neuroscience. 2004;125:903–920. doi: 10.1016/S0306-4522(03)00398-1. [DOI] [PubMed] [Google Scholar]
- McHale J, Harari OA, Marshall D, Haskard D. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-α. J Immunol. 1999;162:1648–1655. [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Narumi S, Sudo K, Horai R, Tagawa Y, et al. IL-1-induced tumor necrosis factor-alpha elicits inflammatory cell infiltration in the skin by inducing IFN-gamma-inducible protein 10 in the elicitation phase of the contact hypersensitivity response. Int Immunol. 2003;15:251–260. doi: 10.1093/intimm/dxg028. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos A, Máñez S, Giner RM, Recio MC, Ríos JL. Isoprenylhydroquinone glucoside: a new non-antioxidant inhibitor of peroxynitrite-mediated tyrosine nitration. Nitric Oxide. 2005;12:54–60. doi: 10.1016/j.niox.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Olmos A, Mánez S, Giner RM, Recio MC, Ríos JL. Protein tyrosine nitration induced by heme/hydrogen peroxide: inhibitory effect of hydroxycinnamoyl conjugates. Planta Med. 2007;73:20–26. doi: 10.1055/s-2006-951744. [DOI] [PubMed] [Google Scholar]
- Ross R, Gillitzer C, Kleinz R, Schwing J, Kleinert H, Förstermann U, et al. Involvement of NO in contact hypersensitivity. Int Immunol. 1998;10:61–69. doi: 10.1093/intimm/10.1.61. [DOI] [PubMed] [Google Scholar]
- Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, et al. Allergic contact dermatitis. Eur J Dermatol. 2004;14:284–295. [PubMed] [Google Scholar]
- Schön MP, Zollner TM, Boehncke WH. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J Invest Dermatol. 2003;121:951–962. doi: 10.1046/j.1523-1747.2003.12563.x. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, Sarcia PJ, Kelly KM, DeMarco VG, Skimming JW. Hypothermia induces anti-inflammatory cytokines and inhibits nitric oxide and myeloperoxidase-mediated damage in the hearts of endotoxemic rats. Chest. 2004;125:1483–1491. doi: 10.1378/chest.125.4.1483. [DOI] [PubMed] [Google Scholar]
- Shibata M, Sueki H, Suzuki H, Watanabe H, Ohtaki H, Shioda S, et al. Impaired contact hypersensitivity reaction and reduced production of vascular endothelial growth factor in tumor necrosis factor-alpha gene-deficient mice. J Dermatol. 2005;32:523–533. doi: 10.1111/j.1346-8138.2005.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Szabó E, Virág L, Bakondi E, Gyüre L, Haskó G, Bai P, et al. Peroxynitrite production, DNA breakage, and poly(ADP-ribose) polymerase activation in a mouse model of oxazolone-induced contact hypersensitivity. J Invest Dermatol. 2001;117:74–80. doi: 10.1046/j.0022-202x.2001.01388.x. [DOI] [PubMed] [Google Scholar]
- Villagrasa V, Cortijo J, Marti-Cabrera M, Ortiz JL, Berto L, Esteras A, et al. Inhibitory effects of N-acetylcysteine on superoxide anion generation in human polymorphonuclear leukocytes. J Pharm Pharmacol. 1997;49:525–529. doi: 10.1111/j.2042-7158.1997.tb06836.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Fujisawa H, Zhuang L, Freed I, Howell BG, Shahid S, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–6790. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- Weigmann B, Schwing J, Huber H, Ross R, Mossmann H, Knop J, et al. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase of the elicitation reaction. Scand J Immunol. 1997;45:308–314. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]
- Yuen KS, Nearn MR, Halliday GM. Nitric oxide-mediated depletion of Langerhans cells from the epidermis may be involved in UVA radiation-induced immunosuppression. Nitric Oxide. 2002;6:313–318. doi: 10.1006/niox.2001.0414. [DOI] [PubMed] [Google Scholar]