Abstract
Background:
Anandamide (AEA) activates both cannabinoid CB1 and TRPV1 receptors, which are expressed on cultured dorsal root ganglion neurones. Increased levels of nerve growth factor (NGF) are associated with chronic pain states.
Experimental approach:
The aim of this study was to compare of the effects of AEA on CB1 receptor signalling and TRPV1-CB1 crosstalk in low and high concentrations of NGF, using voltage-clamp electrophysiology and Fura-2 calcium imaging.
Key results:
Chronic exposure to high NGF (200 ng ml−1) as compared to low NGF (20 ng ml−1) increases the proportion of neurones that exhibit an inward current in response to AEA (1 μM), from 7 to 29%. In contrast, inhibition of voltage-gated calcium currents by AEA is not significantly different in low NGF (33±9%, compared to high NGF 28±6%). Crosstalk between CB and TRPV1 receptors is modulated by exposure to high NGF. In low NGF, exposure to the CB1 receptor antagonist, SR141716A, (100 nM) increases the percentage of neurones in which AEA elicits an increase in [Ca2+]i, from 10 to 23%. In high NGF, the antagonist does not alter the percentage of responders (33 to 30%). In low NGF, exposure to the CB receptor agonist, WIN55 (1 μM) reduces capsaicin-mediated increases in [Ca2+]i to 28±8% of control as compared to an enhancement to 172±26% of control observed in high NGF.
Conclusions and implications:
We conclude that cannabinoid-mediated modulation of TRPV1 receptor activation is altered after exposure to high NGF.
Keywords: cannabinoid, CB1, TRPV1, anandamide, nerve growth factor, NGF, DRG, pain
Introduction
Anandamide (N-arachidonoyl-ethanolamide, AEA) is an ‘endocannabinoid', as defined by its ability to bind to and activate cannabinoid receptors, CB1 and CB2 (Pertwee and Ross, 2002). The search for endogenous transient receptor potential vanilloid 1 receptor (TRPV1) activators or ‘endovanilloids' is ongoing and recent advances suggest that AEA may be one such compound (Ross et al., 2001b; Ross, 2003; Evans et al., 2004). Both in culture and in situ, dorsal root ganglion (DRG) neurones express both CB1 and TRPV1 receptors. The colocalization of the CB1 with the TRPV1 receptor is controversial (Rice et al., 2002): some studies suggest a high degree of colocalization (Ahluwalia et al., 2000, 2002) and others suggest little colocalization (Farquhar-Smith et al., 2000; Khasabova et al., 2002, 2004; Bridges et al., 2003). In DRG neurones, cannabinoid agonists including AEA, inhibit voltage-gated Ca2+ currents (Ross et al., 2001a; Evans et al., 2004). AEA has a dual effect on neuropeptide release from cultured DRG neurons; at low concentrations, AEA induces a CB1-receptor-mediated inhibition of electrically stimulated neuropeptide release, while at higher concentrations AEA evokes a TRPV1 receptor-mediated release of neuropeptides (Tognetto et al., 2001). AEA also attenuates capsaicin ((3-methoxy-4-hydroxy)benzyl-8-methyl-6-nonenamide)-evoked neuropeptide release from DRG neurones and isolated paw skin (Richardson et al., 1998; Ellington et al., 2002; Ahluwalia et al., 2003). In the spinal cord, superfusion of a CB1 receptor antagonist significantly enhances the release of neuropeptide evoked by capsaicin (Lever and Malcangio, 2002). Furthermore, in the presence of a CB1 receptor antagonist, AEA becomes equipotent with capsaicin as a TRPV1 receptor agonist (Ahluwalia et al., 2003). The implication of such data is that activation of the CB1 receptor by AEA modulates its TRPV1 receptor-mediated action.
Nerve growth factor (NGF) is a target-derived growth factor on which nociceptive sensory neurones of the DRG depend for their survival during development. In vivo, NGF levels are increased during inflammation, injury or chronic pain states (Hefti et al., 2006). In cultured DRG neurones, increased levels of NGF have been demonstrated to regulate expression and sensitization of TRPV1 receptors (Bevan and Winter, 1995; Price et al., 2005; Zhang et al., 2005; Anand et al., 2006). The majority of studies have used either acute NGF treatments, or NGF-withdrawal-replacement regimes (Winter et al., 1988; Kendall et al., 1994; Bonnington and McNaughton, 2003; Zhang et al., 2005). More recently, two studies have shown that chronic treatment with NGF increases TRPV1 protein levels and enhances capsaicin activation of TRPV1 (Price et al., 2005; Anand et al., 2006) as compared to no NGF.
The aim of the present study was to investigate the consequences of chronically raising levels of NGF in DRG cultures on AEA signalling at both TRPV1 and CB1 receptors. A comparison of the effects of low- and high-NGF concentrations on CB1 signalling and TRPV1-CB1 crosstalk in sensory neurones has not previously been undertaken. These conditions may be physiologically relevant to changes that take place in response to injury, inflammation or chronic pain states in which basal NGF levels are enhanced (Hefti et al., 2006).
Methods
Cell culture
Primary cultures of sensory neurones from dorsal root ganglia of neonatal rats were used in this study and were prepared as reported previously (Ross et al., 2001a). The sensory neurones were plated on to laminin-polyornithine-coated coverslips and bathed in F14 culture medium supplemented with 10% horse serum, penicillin (5000 IU ml−1), streptomycin (50 μg ml−1), NaHCO3 (14 mM) and NGF (NGF-2.5S). The cultures were maintained for up to 7 days and were used between days 1 and 7 in culture. Using a pipette boy and a sterile 25 ml pipette, 1.5 ml of warmed F14/HS containing 10 μl NGF per dish (final concentration 20 ng ml−1) was added to each dish, and the cells replaced in the incubator until required. For cells cultured with high NGF, 100 μl NGF per dish was added, giving a final concentration of 200 ng ml−1. The DRG cultures were re-fed with fresh F14/HS and NGF every 5–7 days.
Electrophysiology
Electrophysiological experiments were conducted at room temperature (18–20°C) using the whole-cell variant of the patch clamp technique. Voltage-activated Ca2+ channel currents carried by Ca2+ and transient inward currents evoked by AEA or capsaicin were recorded from cultured neonatal rat DRG neurones. The patch pipettes with resistances of 3–9 MΩ were made from Pyrex borosilicate glass tubing (1.4/1.6 mm outer diameter, 0.8/1.0 mm bore with 0.15 mm fibre attached to the inside wall, Plowden and Thompson Ltd, Dial Glass Works) using a two-stage vertical microelectrode puller (David Kopf Instruments, Tujunca, USA, Model 730). An Axoclamp 2A switching amplifier or Axopatch 1D amplifier (Axon Instruments, Molecular Devices, Workingham, Berkshire, UK) were used. For all electrophysiological experiments, the patch pipettes were filled with CsCl-based solution containing in mM: 140 CsCl, 0.1 CaCl2, 5 EGTA, 2 MgCl2, 2 ATP, 10 HEPES. The pH and osmolarity of the patch pipette solutions were corrected to 7.2 and 310–320 mosm l−1 with Tris and sucrose. The extracellular bathing solution used contained in mM: 130 choline chloride, 2 CaCl2, 3 KCl, 0.6 MgCl2, 1 NaHCO3, 10 HEPES, 5 glucose, 25 tetrethylammonium chloride, 0.0025 tetrodotoxin (Sigma, Gillingham, Dorset, UK) and 0.01% dimethyl sulphoxide (DMSO; Sigma). The pH and osmolarity of this extracellular bathing solution were corrected to 7.4 and 320 mosml l−1 with NaOH and sucrose, respectively. The recording solutions used in these experiments were designed to isolate voltage-activated Ca2+ currents from other contaminating conductances and improve the solubility of AEA and other drugs. After entering the whole-cell recording configuration, neurones were allowed to equilibrate for 5 min before measurements were made. The DRG neurones were held at a holding potential of −90 mV and high-voltage-activated Ca2+ currents were evoked by 100 ms voltage step commands to 0 mV. Corresponding leak currents were evoked by −30 to −60 mV voltage step commands. Ca2+ currents were activated at frequencies no greater than 0.033 Hz to prevent run down and at least four consistent inward currents were activated before drug application. Stock solutions of AEA (10 mM), capsaicin (10 mM) and SR141716A (10 mM) were made up in 100% DMSO. Control experiments showed that 0.01% DMSO had no acute (3 min; n=6) or chronic (30-180 min; n=11) effects on voltage-activated Ca2+ currents. During the electrophysiological experiments the neurones were continually bathed in extracellular solution containing 0.01% DMSO. Drugs were applied to the extracellular environment by low-pressure ejection from a blunt pipette positioned about 50–100 μM away from the cell being recorded. Data were captured and stored on digital audiotape using a Biologic digital tape recorder (DTR 1200). Analysis of data was performed off-line using Cambridge Electronic Design voltage clamp analysis software (version 6.0). All voltage-activated Ca2+ currents had scaled linear leakage and capacitance currents subtracted to obtain values for the net inward Ca2+ current. Data are given as mean±s.e.m values and statistical significance was determined using a paired or independent Student's t-test as appropriate.
Fura-2 Ca2+ imaging
DRG neurones were incubated for 1 h in NaCl-based extracellular solution containing (in mM): NaCl 130; KCl 3.0; MgCl2 0.6; CaCl2 2.0; NaHCO3 1.0; HEPES 10.0; glucose 5.0 and fura-2AM 0.01 (Sigma, 1 mM stock in dimethylformamide). The pH was adjusted with NaOH to 7.4 and the osmolarity to 310–320 mosm with sucrose. After washing, the neurones were constantly perfused with NaCl-based extracellular solution containing 0.01% DMSO (1–2 ml min−1). When the neurones to be imaged had been selected, a greyscale reference image was captured under a conventional light source (Olympus TH3). This allowed identification of neurones for later analysis, and also measurement of somal area. Images were viewed and analysed using the Ultraview software (version 3), (Merlin Morphometry, Life Science Resources, Cambridge, UK) in temporal mode. Two types of experiment were carried out. In the first series of experiments, increases in intracellular Ca2+ evoked by AEA (1 μM) were compared in DRG neurones identified by their subsequent response to 100 nM capsaicin followed by 30 mM KCl. The amplitude and the duration of the calcium transient measured at 50% of the maximum amplitude (width 50 or W50) were measured. Neurone size was also determined so that response profiles could be correlated with soma areas. Secondly, modulation of capsaicin-evoked Ca2+ influx by WIN55212 was evaluated. Two transient increases in intracellular Ca2+ in response to capsaicin were obtained in a single experiment on cultured DRG neurones. The effect of 1 μM WIN55212 on the response to the second capsaicin stimulus was investigated. In these experiments, the cells were pre-treated with cannabinoid for 2 min before addition of capsaicin (100 nM). The effect of vehicle (0.01% DMSO) or WIN55212 on capsaicin responses was expressed as a percent of the first calcium transient by calculating: ((second peak height)−(first peak height)/(first peak height)) × 100. All experiments were conducted at room temperature.
Data analysis
Results are expressed as means±s.e.m. Statistical significance of differences between means was determined using Student's unpaired or paired t-test, Fishers' exact test and one-way ANOVA followed by Newman–Keuls test. These were carried out using GraphPad Prism 4.
Results
In all subsequent text, a concentration of 20 ng ml−1 NGF will be referred to as ‘low NGF' and a concentration of 200 ng ml−1 NGF as ‘high NGF'.
Neuronal survival
Raising the level of NGF from 20 to 200 ng ml−1 did not to have any effect on the relative numbers of different neuronal subpopulations surviving in culture (see Figure 1). The distribution of DRG neurones according to size was identical to that for neurones cultured in low NGF.
Figure 1.
Frequency distribution curves of the somal area for all DRG neurones cultured in 20 ng ml−1 (low) and 200 ng ml−1 (high) NGF. DRG, dorsal root ganglion; NGF, nerve growth factor.
Electrophysiological studies
The effects of chronic high-NGF treatment on activation of TRPV1-mediated inward currents (ICa) and inhibition voltage-gated calcium currents (VGCCs) by AEA were first investigated using electrophysiology.
AEA- and capsaicin-evoked inward currents
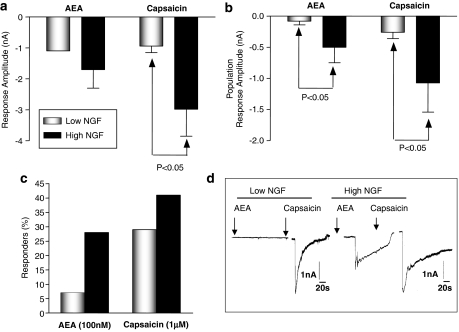
The current amplitudes for individual responders to AEA or to capsaicin, under low- and high-NGF conditions, are shown in Figure 2a. The small number of AEA responders in low-NGF (n=2) precluded statistical analyses of the current amplitude, so mean population response amplitudes (that is, the mean current amplitude taking into account all the zero responses) are presented in Figures 2b and c. From a holding potential of −90 mV, 100 nM AEA evoked inward currents in 5 out of 17 high-NGF neurones (29%), compared to the 7% of low-NGF neurones in which 100 nM AEA elicited an inward current. Also the magnitude of the responses to AEA was greater in neurones exposed to high NGF (Figure 2b). Capsaicin (1 μM) evoked responses in 7 out of 17 (high-NGF) neurones, a response rate of 41%. This was not significantly different (Fisher's exact test) from the 28% (8 from 29) of low-NGF neurones in which 1 μM capsaicin elicited inward currents (Figure 2c). Mean population response amplitudes for 1 μM capsaicin were again markedly higher in neurones exposed to high NGF than in those exposed to low NGF (Figure 2b).
Figure 2.
The effects of alteration in NGF concentration on AEA and capsaicin evoked inward currents was investigated using whole-cell voltage clamp electrophysiology. (a) The inward current response amplitudes in responding neurones cultured in low and high NGF. (b) Mean population response amplitudes which take into account all zero responses, and therefore provide a measure of both the proportion of responding neurones and amplitude of those responses. Population responses for both AEA and capsaicin are increased in neurones cultured with high NGF (n=17) as compared with low NGF (n=29). Bars represent the mean±s.e.m. Statistical significance determined using unpaired Student's t-test. (c) The percentage of DRG neurones in which AEA or capsaicin evoked an inward current in low- and high-NGF neurones and (d) traces recorded from a DRG neurones cultured in low and high NGF. AEA, anandamide; DRG, dorsal root ganglion; NGF, nerve growth factor.
AEA modulation of voltage-activated Ca2+ currents
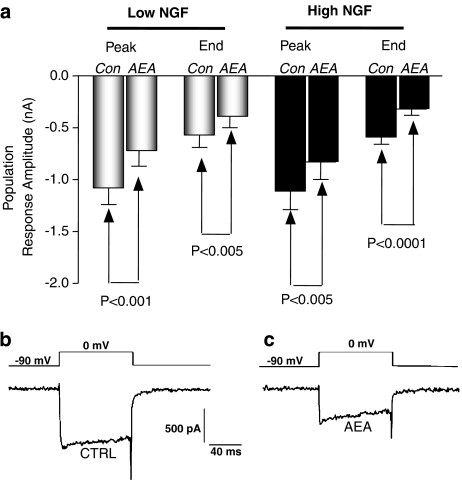
Previous investigations demonstrated that, in low-NGF neurones, AEA inhibits voltage-activated Ca2+ currents (Evans et al., 2004). The same protocol was repeated for high-NGF neurones. Mean control current amplitude was measured at the peak of the current and following application of 100 nM AEA, this amplitude was decreased. This inhibition was partially reversible in two out of the nine neurones following removal of the AEA-containing perfusion pipette. The current measured at the end of the voltage step command showed a similar pattern. These results are illustrated in Figures 3b and c and summarized in Figure 3a. This represented a mean AEA-induced inhibition of peak Ca2+ current by 28±6% in high-NGF neurones, not significantly different from the 33±9% (n=5) inhibition observed in low-NGF neurones (one-way ANOVA).
Figure 3.
AEA-mediated inhibition of voltage-activated Ca2+ currents was investigated using whole-cell voltage clamp electrophysiology in neurones cultured in high NGF. In neurones cultured with high NGF, 100 nM AEA attenuates voltage-activated Ca2+ currents, at both the peak of the current and the end of the voltage step command. (a) The mean current amplitudes under control conditions and following treatment with 100 nM AEA. Bars represent the mean±s.e.m. (b) An example record of a control calcium current from a high-NGF-treated neurone. (c) The Ca2+ current recorded from the same neurone following 4 min application of 100 nM AEA. AEA, anandamide; NGF, nerve growth factor.
The effects of AEA on voltage-activated Ca2+ currents were unaffected by chronic treatment of neurones with high NGF. Control current amplitudes were also compared between high- and low-NGF neurones, and were not found to be significantly different. Mean peak control (with vehicle) Ca2+ current amplitude in low-NGF neurones was −0.97±0.07 nA (n=31), while for high-NGF neurons, peak control current amplitude was −1.11±0.18 nA (n.s., one-way ANOVA).
Calcium imaging studies
The effects of alteration in NGF concentration on AEA-mediated increases in intracellular Ca2+, [Ca2+]i, were also investigated using Fura-2 calcium imaging
AEA effects on [Ca2+]i in neurons cultured in low and high NGF
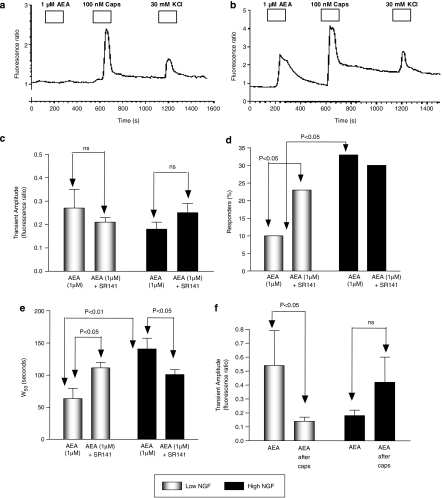
The mean amplitude of the responses to 1 μM AEA in high NGF was not significantly different from the amplitude of responses in low-NGF neurones (Figure 4c; unpaired Student's t-test). Of 154 neurones assayed in high-NGF experiments (19 experimental runs from 5 separate culture preparations), 38 neurones responded to 1 μM AEA (33%). In high-NGF cultures, the proportion of neurones responding to AEA is significantly higher than the 10% of responders in low-NGF cultures (Figure 4d; P<0.009; Fisher's exact test).
Figure 4.
The effects of alteration in NGF concentration on AEA-mediated increases in intracellular Ca2+ in sensory neurones investigated using Fura-2 calcium imaging. (a, b) Examples of the different patterns of responses obtained. (c) The amplitude of Ca2+ transient responses to 1 μM AEA neurones cultured with either low or high NGF in the absence and presence of the CB1 receptor antagonist, SR141716A (100 nM) (unpaired Student's t-tests). (d) The proportion of DRG neurones responding to 1 μM AEA, in neurones cultured in low and high NGF, in the absence and presence of the SR141716A (100 nM) (Fisher's exact test). (e) The width50 (W50) of Ca2+ transient responses to 1 μM AEA in neurones cultured with either low or high NGF (unpaired Student's t-tests), in the absence and presence of SR141716A. (f) The amplitude of the [Ca2+]i response to AEA following pre-exposure of the neurones to capsaicin (100 nM) when cultured in low NGF or high NGF (unpaired Student's t-tests). Bars represent the mean±s.e.m. AEA, anandamide; CB1, cannabinoid receptor; DRG, dorsal root ganglion; NGF, nerve growth factor.
In a very small population of neurones (5 cells in low NGF and 8 cells in high NGF), AEA elicited a Ca2+ transient in cells that did not subsequently respond to 100 nM capsaicin; these responses were not included in the analysis.
Effect of the CB1 receptor antagonist, SR141716A
Previous findings suggest that activation of the CB1 receptor by AEA may modulate activation of the TRPV1 receptor by this endocannabinoid. We therefore investigated the effect of the CB1 receptor antagonist, SR141716A.
In low NGF, in the presence of the CB1 receptor antagonist, SR141716A (100 nM), of 290 neurones assayed in this experiment (six experimental runs, three culture preparations), 1 μM AEA evoked a rise in intracellular calcium in 68 (23%). This represents a significant increase in the proportion of neurones responding to 1 μM AEA when compared with the 10% of neurones that respond to AEA in the absence of antagonist (P<0.005, Fisher's exact test) (Figure 4d). Of DRG neurons, 76 and 75% responded to capsaicin in the absence and presence of SR141716A, respectively (data not shown). The amplitude of the response to 1 μM AEA was not significantly different between the presence (n=68) and absence (n=20) of the antagonist (Figure 4c). In low NGF, W50 for AEA was significantly larger in the presence of the CB1 receptor antagonist indicating a prolonged Ca2+ transient (Figure 4e).
In high NGF, in the presence of the CB1 receptor antagonist SR141716A out of 179 DRG neurones in this sample (eight experimental runs, three culture preparations), 53 (30%) showed a response to 1 μM AEA (Figure 4d). This value was not significantly different from the proportion (33%) of AEA-responsive neurones in the absence of SR141716A (Fisher's exact test). Neither was the amplitude of the response altered by SR141716A (Figure 4c; unpaired Student's t-test). In high NGF, W50 for AEA was significantly smaller in the presence of the CB1 receptor antagonist indicating a shortened Ca2+ transient (Figure 4e).
Desensitization
In low NGF, the amplitude of responses to 1 μM AEA were smaller in neurons previously exposed to capsaicin (100 nM) as compared to naïve neurones (Figure 4f). In contrast, in high NGF, mean amplitudes of AEA responses were not significantly different in naïve and neurons exposed to capsaicin (Figure 4f). There was no significant difference between the proportions of neurones responding to AEA (Fisher's exact test) under each order of drug application. Thus, while in DRG neurones cultured in low NGF, prior exposure to capsaicin appeared to desensitize the response to AEA, attenuating the amplitude of subsequent AEA responses, no evidence for this desensitization was found in neurones cultured with high NGF.
Somal size and response patterns
To investigate whether the NGF treatment had altered the relationship between somal size and response patterns seen in low-NGF neurones, the somal areas of neurones cultured in high NGF were examined. A greater proportion of larger diameter neurones responded to AEA in high-NGF cultured neurones as compared to those cultured in low NGF (Figure 5a). Mean somal areas of neurons that responded to AEA in low NGF (n=19) were significantly smaller than those that responded in high NGF (n=38, Figure 5b).
Figure 5.
The effects of alteration in NGF concentration on the somal area of neurones in which AEA elicits increases in intracellular Ca2+ investigated using Fura-2 calcium imaging. (a) The frequency distribution of DRG neurones of various sizes, which respond to AEA when cultured in low and high NGF. (b) A histogram showing that the mean somal area of neurones in which AEA evokes and increase in [Ca2+]i in low (n=19) and high (n=38) NGF (Student's unpaired t-test). Bars represent the mean±s.e.m. AEA, anandamide; DRG, dorsal root ganglion; NGF, nerve growth factor.
The effect of a synthetic cannabinoid agonist on TRPV1 responses
The next series of experiments were designed to characterize interactions between cannabinoid and TRPV1 receptors. To eliminate the potential complication of AEA simultaneously activating both cannabinoid and TRPV1 receptors, a synthetic cannabinoid was used. Hence, the effect of the CB1/CB2 receptor agonist WIN55212 on responses to capsaicin, in DRG neurones cultured with both low and high NGF was investigated.
Firstly, a set of control experiments was conducted to ensure that consecutive responses to capsaicin could be obtained. Capsaicin (100 nM) was applied twice to the same neurones, with a recovery period (11 min) between applications. A 30 mM KCl stimulus was applied at the end of the experiment. Representative traces are shown in Figures 6a and b.
Figure 6.
The effects the cannabinoid receptor agonist, WIN55212 (1 μM) on capsaicin-evoked Ca2+ transients investigated using Fura-2 calcium imaging. Experimental records are shown of consecutive responses to capsaicin (100 nM) in neurones cultured (a) in low NGF (b) in high-NGF and (c) from low-NGF neurones in which WIN55212 is applied before, and during, the second capsaicin stimulus. (d) A histogram showing that in low-NGF neurones, pre-treatment with WIN55212 attenuated the amplitude of the second response to 100 nM capsaicin, while in high-NGF neurones, WIN55212 potentiated the second capsaicin response (unpaired Student's t-tests). In the presence of SR141 (100 nM), the enhancement by WIN55212 is no longer observed. Bars represent the mean amplitude of the second capsaicin response for each experiment expressed as percent of the initial capsaicin response amplitude for that experiment. NGF, nerve growth factor.
In low-NGF cultures, 42 neurones (six experimental runs from three culture preparations) responded to capsaicin. A significant level of desensitization was evident between the first and second capsaicin responses. Mean evoked transient amplitudes were 2.11±0.27 fluorescence ratio units, and 0.98±0.16 ratio units for the first and second capsaicin responses, respectively (P<0.001, paired Student's t-test). The second response to capsaicin was normalized with respect to the first response from the same neurone. The second response was found to have a mean peak amplitude of 71±12% of the initial capsaicin response (n=42).
In WIN55212-treated neurones, an identical time scale was used. After the first capsaicin response, neurones were pretreated with 1 μM WIN55212 for approximately 3 min before the second capsaicin stimulus was applied in the continued presence of WIN55212. A 30 mM portion of KCl was again applied at the end of the experiment, to confirm the functionality of neurones. Fifty-nine neurones responded to 100 nM capsaicin in this experiment. The mean amplitude of the 1st response was 0.81±0.13 fluorescence ratio units, the amplitude of the response in the presence of WIN55212 being 0.18±0.08 fluorescence ratio units. When normalized with respect to the first capsaicin response, the second response was found to be 28±8% of the control. This value represents a significant attenuation of the second capsaicin response in the presence of WIN55212, compared with the second capsaicin response in control experiments (Figures 6c and d; unpaired Student's t-test).
In high NGF, 56 neurones in the sample (four experimental runs from two culture preparations) responded to 100 nM capsaicin. Mean response amplitudes for the first and second capsaicin responses were not significantly different. The second response was normalized with respect to the first response for each individual neurone and its mean value was 109±14% of the first transient response.
In neurones treated with WIN55212 (six experimental runs from three culture preparations), 58 cells responded to 100 nM capsaicin. Mean response amplitudes were 0.79±0.13 and 0.86±0.13 fluorescence ratio units for the initial capsaicin response, and the capsaicin response following WIN55212 treatment respectively. When normalized with respect to the initial capsaicin responses, the mean response after WIN55212 was significantly greater than the first response (Figure 6d). In the presence of SR141716A (100 nM), WIN55212 no longer enhanced the response to capsaicin (Figure 6d; two experimental runs from two culture preparations, n=62).
Discussion
NGF is known to have effects on TRPV1 expression and sensitization and increased levels are associated with various pain states. The aim of this study was to compare the effects of culture in low- and high-NGF concentrations on endocannabinoid signalling, CB1 signalling and TRPV1-CB1 receptor crosstalk.
Our electrophysiological investigations indicate that, in responsive neurones, capsaicin-evoked inward currents are significantly enhanced in neurones chronically exposed to high NGF, compared to those cultured in low NGF (Figure 2a). In addition, there is a significant enhancement of the population response amplitude for both AEA and capsaicin (Figure 2b), which presumably also reflects the increase in the percentage of TRPV1-responsive neurones (Figure 2c). In calcium imaging studies, in which only responsive neurones are analysed, the W50, but not the peak amplitude of AEA-mediated increases in intracellular Ca2+, is significantly larger in high NGF. Our data suggest that raising the level of NGF from 20 to 200 ng ml−1 does not have any significant effect on the relative numbers of different neuronal subpopulations surviving in culture (see Figure 1). The distribution of DRG neurones according to size was almost identical to that for neurones cultured in low NGF. However, the percentage of cells that responded to AEA both in evoking an inward current and an increase in [Ca2+]i was increased in the presence of high NGF as compared to low NGF. Taken together, the data suggests that NGF influences the phenotype of the neurones, or responsiveness of the receptors, rather than survival. Alternatively, it is possible that the survival of subpopulations, which are indistinguishable by our size analysis, is altered. In a recent study, Anand et al. (2006) demonstrated that culture of human DRG in neurotophic factors (NGF, GDNF and NT3) for 5 days caused an increase in the proportion of large diameter neurones as compared to cultures maintained in the absence of neurotrophic factors. Furthermore, neurotrophic factors caused a significant increase, in particular, of TRPV1-positive large diameter neurones. These findings are in line with our observation that in high NGF, neurones in which AEA evoked an increase in [Ca2+]i were significantly larger in somal area than those that responded in low NGF (Figure 5). Price et al. (2005) also found that 5 days exposure to NGF, compared to no growth factor, had no effect on the number of trigeminal neurons, but increased TRPV1 protein levels by 50%. In line with our findings, this group also found that chronic treatment with NGF significantly increased AEA-evoked CGRP release.
Previous reports support a role for endocannabinoid modulation of NGF-mediated components of the inflammatory process (Farquhar-Smith et al., 2002; Farquhar-Smith and Rice, 2003); AEA significantly attenuates visceral hyperalgesia induced by acute administration of NGF in vivo. In our study, cannabinoid receptor signalling per se did not appear to be affected by levels of NGF; AEA induced a similar level of inhibition of voltage-activated Ca2+ currents in low-NGF- and high-NGF-treated neurones. Ahluwalia et al. (2002) also demonstrated that NGF does not regulate CB1 receptor expression in DRG neurones. On the other hand, we find that cannabinoid-mediated regulation of TRPV1 receptors is affected by treatment of neurones with high NGF. In low-NGF-treated neurones, pre-treatment with the CB1 receptor antagonist, SR141716A significantly increased the proportion of neurones responding to AEA from only 10 to 23%. The presence of the antagonist also increased the duration (W50), but not the amplitude, of AEA-evoked Ca2+ transients. In contrast, in high NGF the CB1 receptor antagonist has no effect on the percentage of neurones in which AEA evoked a Ca2+ transient, but decreased the duration (W50) of AEA-evoked Ca2+ transients. An increase in W50 may be indicative of enhanced Ca2+ flux as a result of slowed repolarization (for example, attenuated K+ conductances), or prolonged activation of Ca2+ permeable channels on the cell membrane or intracellular Ca2+ stores. Additionally, W50 values might also be increased by attenuated Ca2+ homeostatic mechanisms.
These data suggest that high-NGF treatment disrupts tonic regulation of TRPV1 by CB1 receptor signalling pathways. Thus, in low-NGF-treated neurones, the presence of SR141716A allows TRPV1 responses to AEA to become evident in a larger population of neurons; possibly by attenuating CB1 receptor-mediated inhibition in the subpopulation of cells that co-express TRPV1 and CB1. In the high-NGF neurones, AEA exerts either no CB1 receptor-mediated inhibition or an enhancement of TRPV1 responses. This would suggest that there is a significant interaction between CB1- and NGF-mediated pathways regulating TRPV1, and that chronic treatment with high NGF acts to disrupt the CB1 signalling pathway. In line with this, we find that the inhibitory action of the cannabinoid CB1 receptor agonist, WIN55212 on capsaicin responses seen in low-NGF neurones, is converted to an enhancement in high-NGF neurons. The inhibitory effect of WIN55212 is in line with previous findings that the CB1/CB2 receptor agonist, HU210 attenuates capsaicin-evoked increases in [Ca2+]i; an effect that is prevented by the CB1 receptor antagonists (Millns et al., 2001). Thus, whilst DRG cultures are known to express CB2 receptors (Ross et al., 2001a), the inhibitory effect of nonselective agonists appears to be mediated by interaction with the CB1 receptor on these neurones. Notably, in the present study, the enhancement of capsaicin-evoked Ca2+ transients by WIN55212 observed in high NGF was abolished by the CB1 receptor antagonist, SR141716A.
In HEK-293 cells overexpressing both CB1 and TRPV1 receptors, pre-treatment with a cannabinoid receptor agonist significantly enhanced capsaicin-evoked increases of [Ca2+]i (Hermann et al., 2003). This effect was inhibited by SR141716A and was absent from cells that express TRPV1 only, indicating that CB1 receptor activation leads to an enhanced activation of TRPV1. Inhibitors of phosphoinositide-3-kinase (PI-3-K) and phospholipase C (PLC) attenuate the CB1 receptor-mediated enhancement of TRPV1 activation. CB1 receptors have been shown to couple to PLC activation (Ho et al., 1999; Netzeband et al., 1999). However, if cAMP-mediated signalling is activated, CB1 agonists mediate inhibition and not enhancement of TRPV1 receptor activation (Hermann et al., 2003). Ahluwalia et al. (2003) demonstrated that PKA activation significantly enhanced anandamide-evoked neuropeptide release, an effect that was greater in the presence of the CB1 receptor antagonist. Our data suggest that treatment of neurones with high NGF may be altering the dominant signalling pathway activated by cannabinoid receptors. Trk receptor-mediated signalling cascades involve activation of PI-3-K and PLCγ enzymes (Chuang et al., 2001; Bonnington and McNaughton, 2003; Zhang et al., 2005). High-NGF treatment that mirrors chronic pain states, may switch the signalling to a PI-3-K/PLC-dependent pathway, CB1 activation thus potentiating TRPV1 responses.
It is noteworthy that a number of studies demonstrate CB1 receptor expression predominantly located in intermediate sized DRG neurones and little co-expression with TRPV1 receptors (Khasabova et al., 2002; Bridges et al., 2003). In the present study, the ability of the CB1 receptor antagonist to increase the population of AEA-responsive neurones from 10 to 23% suggests that, in low NGF, there is some degree of colocalization. By comparison, this is considerably lower than the ∼70% of neurones that respond to 1 μM capsaicin and this is in line with previous reports of a relatively low level of co-expression of CB1 and TRPV1. It also suggests that, under these conditions, the CB1 receptor has an inhibitory effect on TRPV1 receptor signalling (Figure 7). As discussed above, Anand et al. (2006) have demonstrated that in high-NGF states, TRPV1 receptor expression increases in larger diameter neurones, thus the population of neurones that co-expression with CB1 receptor may increase. This increases the potential for crosstalk between the receptors, but under these conditions, CB1 receptor activation may enhance TRPV1 receptor-mediated events (Figure 7).
Figure 7.
A diagram representing the proposed changes in TRPV1 receptor expression and CB1-TRPV1 receptor crosstalk that take place in low and high NGF in DRG neurones. AEA is released intracellularly and crosses the membranes by diffusion or a putative AEA membrane transporter (AMT). AEA inhibits voltage-activated Ca2+ channels (VACC) in both low- and high-NGF neurones. Crosstalk between CB1 and TRPV1 receptors differs in low and high NGF. In low NGF, the CB1 receptor appears to have an inhibitory effect on TRPV1 receptor signalling as shown by the ability of the CB1 receptor antagonist to increase the population of AEA responsive neurones. In high NGF, TRPV1 receptor expression increases in larger diameter neurones, thus the population of neurones that co-express TRPV1 with CB1 receptors may increase. This increases the potential for crosstalk between the receptors, but under these conditions, CB1 receptor activation either does not inhibit and may enhance TRPV1 receptor-mediated events. AEA, anandamide; CB1, cannabinoid receptor; DRG, dorsal root ganglion; NGF, nerve growth factor; TRPV1, transient receptor potential vanilloid 1 receptor.
It is notable that the present study suggests that in cultures exposed to high NGF, there is less desensitization of the TRPV1 receptor; in calcium imaging experiments, neither AEA nor capsaicin were desensitized by prior application of capsaicin (Figures 4f and 6d). Protein kinase C and phosphatidylinositol bisphosphate, both of which are activated by NGF, have been implicated in modulation of TRPV1 receptor desensitization (Mandadi et al., 2004; Liu et al., 2005). As mentioned above, we find that WIN55212 does not attenuate capsaicin responses in high-NGF neurones. It is possible that this is related to the attenuation of desensitization as a recent report suggests that WIN55212 elicits a calcineurin-mediated desensitization of TRPV1 (Patwardhan et al., 2006). In addition, both WIN55212 and AEA regulate TRPV1 receptor sensitivity by altering receptor phosphorylation, an effect that is mediated via TRPA1 receptors (Jeske et al., 2006). However, in the present study, the CB1 receptor antagonist modulates the effects of AEA. Furthermore, the concentration (25 μM) of cannabinoids used in published studies (Jeske et al., 2006; Patwardhan et al., 2006) is considerably higher than the 1 μM employed in the present study. Furthermore, the enhancement of TRPV1 responses by WIN555212 in high NGF observed in the present study was also shown to be CB1 receptor mediated. Therefore, while a role for TRPA1 cannot be excluded, these observations indicate that the effects of both AEA and WIN55212 on TRPV1 observed in the present study are CB1 receptor mediated. In combination, these studies add a new dimension to our understanding of endocannabinoid-mediated modulation of TRPV1 receptor signalling, which may differ, depending on the concentration of the endocannabinoids and growth factors and on the profile of expression of CB1 and TRPA1 receptors, all of which may be altered in chronic pain states and hyperalgesia.
Here, we reveal that chronic exposure of sensory neurons to high NGF causes a number of key changes in both TRPV1 receptor activation by AEA, and CB1 receptor-mediated modulation of TRPV1 receptor activation. Our data suggest that high NGF increased the proportion of neurons in which AEA elicits inward currents and an increase in [Ca2+]i, which are characteristic of TRPV1 receptor activation. Whilst CB receptor-mediated inhibition of voltage-activated Ca2+ currents was unaltered in neurones cultured in high NGF, crosstalk between CB and TRPV1 receptor was modulated by exposure to high NGF. Our data therefore raise the possibility that during chronic pain states, which are associated with an increase in basal levels of NGF, there may key changes in cannabinoid signalling. Thus, while CB1 receptor agonists retains the ability to inhibit sensory neurone excitability via inhibition of voltage-activated Ca2+ currents; there may be a loss of cannabinoid-mediated attenuation of TRPV1 receptor activation and consequent exacerbation of such conditions. Furthermore, the data presented here indicate that, under these conditions, co-administration of a CB1 receptor agonist with a TRPV1 receptor antagonist may prove an effective strategy for management of chronic pain.
Abbreviations
- AEA
Anandamide, (N-arachidonoyl-ethanolamide)
- capsaicin
((3-methoxy-4-hydroxy)benzyl-8-methyl-6-nonenamide)
- CB1
cannabinoid receptor
- DRG
dorsal root ganglion
- NGF
nerve growth factor
- PKC
protein kinase C
- TRPV1
transient receptor potential vanilloid 1 receptor
Conflict of interest
The authors state no conflict of interest.
References
- Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, et al. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured DRG sensory neurones. Neurosci Lett. 2006;399:51–56. doi: 10.1016/j.neulet.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neurosci. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neurosci. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Ellington HE, Cotter MA, Cameron NE, Ross RA. Effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacol. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Evans RM, Scott RH, Ross RA. Multiple actions of anandamide on neonatal rat cultured sensory neurones. Br J Pharmacol. 2004;141:1223–1233. doi: 10.1038/sj.bjp.0705723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB1 receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Jaggar SI, Rice ASC. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB1 and CB2-like receptors. Pain. 2002;97:11–21. doi: 10.1016/s0304-3959(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Rice AS. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology. 2003;99:1391–1401. doi: 10.1097/00000542-200312000-00024. [DOI] [PubMed] [Google Scholar]
- Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27:85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, Di Marzo V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci. 2003;60:607–616. doi: 10.1007/s000180300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BY, Uezono Y, Takada S, Takase I, Izumi F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Receptors Channels. 1999;6:363–374. [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall G, Ensor E, Brar-Rai A, Winter J, Latchman DS. Nerve growth factor induces expression of immediate-early genes NGFI-A (Egr-1) and NGFI-B (nur 77) in adult rat dorsal root ganglion neurons. Brain Res Mol Brain Res. 1994;25:73–79. doi: 10.1016/0169-328x(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Harding-Rose C, Simone DA, Seybold VS. Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons. J Neurosci. 2004;24:1744–1753. doi: 10.1523/JNEUROSCI.4298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Simone DA, Seybold VS. Cannabinoids attenuate depolarization-dependent Ca2+ influx in intermediate-size primary afferent neurons of adult rats. Neuroscience. 2002;115:613–625. doi: 10.1016/s0306-4522(02)00449-9. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Malcangio M. CB1 receptor antagonist SR141716A increases capsaicin-evoked release of Substance P from the adult mouse spinal cord. Br J Pharmacol. 2002;135:2124. doi: 10.1038/sj.bjp.0704506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang CG, Qin F. Functional Recovery from Desensitization of Vanilloid Receptor TRPV1 Requires Resynthesis of Phosphatidylinositol 4,5-Bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35:471–478. doi: 10.1016/j.ceca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Millns PJ, Chapman V, Kendall DA. Cannabinoid inhibition of the capsaicin-induced calcium response in rat dorsal root ganglion neurones. Br J Pharmacol. 2001;132:969–971. doi: 10.1038/sj.bjp.0703919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzeband JG, Conroy SM, Parsons KL, Gruol DL. Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J Neurosci. 1999;19:8765–8777. doi: 10.1523/JNEUROSCI.19-20-08765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, et al. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rice ASC, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001a;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, et al. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001b;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, et al. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]