Abstract
Background and purpose:
Avarol is a marine sesquiterpenoid hydroquinone with anti-inflammatory and antipsoriatic properties. The aim of this study was to evaluate the in vitro and in vivo pharmacological behaviour of the derivative avarol-3′-thiosalicylate (TA) on some inflammatory parameters related to the pathogenesis of psoriasis.
Experimental approach:
Human neutrophils and monocytes as well as the human keratinocyte cell line HaCaT were used to study the effect of TA on oxidative stress, the arachidonic acid pathway, tumour necrosis factor-α (TNF-α) release and nuclear factor-κB (NF-κB) activation. All these parameters were also determined in vivo using the zymosan induced mouse air pouch model and the 12-O-tetradecanoylphorbol-13-acetate (TPA) induced mouse epidermal hyperplasia model.
Key results:
TA showed antioxidant properties in human neutrophils and in the hypoxanthine/xanthine oxidase assay. This compound reduced, in a concentration-dependent manner, leukotriene B4, prostaglandin E2 and TNF-α production in activated leukocytes. Oral and intrapouch administration of TA in the mouse air pouch model produced a dose-dependent reduction of all these inflammatory mediators. TA also inhibited secretory phospholipase A2 activity and NF-κB DNA-binding in HaCaT keratinocytes. In TPA-induced mouse epidermal hyperplasia, topical administration of TA reduced oedema, leukocyte infiltration, eicosanoid levels and TNF-α in skin. In addition, interleukin (IL)-1β and IL-2 production were also inhibited. Finally, TA was also capable of suppressing NF-κB nuclear translocation in vivo.
Conclusions and implications:
TA inhibited several key biomarkers up-regulated in the inflammatory response of psoriatic skin and this compound could be a promising antipsoriatic agent.
Keywords: avarol 3′-thiosalicylate, inflammation, keratinocyte, monocyte, mouse air pouch, neutrophil, nuclear factor-κB, psoriasis, tumour necrosis factor-α
Introduction
Psoriasis is a chronic inflammatory skin disorder characterized by inflammation in dermis and epidermis, keratinocyte hyperproliferation and leukocyte infiltration (Lizzul et al., 2005). Immune-mediated inflammatory processes, involving both innate and adaptative immunity effector mechanisms drive this pathology (Gaspari, 2006). It has recently been suggested that innate immune responses driven by neutrophils, macrophages or keratinocytes play an important role in the pathogenesis of psoriasis (Bos et al., 2005). Early and active psoriatic lesions are characterized by intra-epidermal penetration of activated polymorphonuclear leukocytes, which cause uncontrolled production of reactive oxygen species (ROS), leading to peroxidative damage to membranes of the skin and contributing to the exacerbation of lesions (Briganti and Picardo, 2003; Yildirim et al., 2003; Okayama, 2005). ROS may also activate phospholipase A2 (PLA2) and thus increase the release of mediators of arachidonic acid (AA) (Yildirim et al., 2003). Psoriatic patients present elevated levels of leukotriene B4 (LTB4) (Ikai, 1999), a potent chemoattractant molecule formed by 5-lipoxygenase (5-LO)-dependent metabolism. Prostaglandin E2 (PGE2) produced by the cyclooxygenase (COX) pathway also contributes by dilating capillaries in the dermis, increasing leukocyte infiltration and stimulating keratinocyte cell growth (Ikai, 1999; Rys-Sikora et al., 2000).
Mammalian PLA2 are now classified into 12 groups (I–XII) that are further subdivided in terms of their substrate specificities, calcium sensitivity and cellular location (Murakami and Kudo, 2004). In psoriatic skin, an increased expression of the secretory phospholipase, sPLA2-IIA, in the basal epidermal layer and in the dermis has been observed (Andersen et al., 1994; Haas et al., 2005), contributing to the sustained activation of mitogen-activated protein (MAP) kinases, as well as nuclear factor-kappa B (NF-κB) in keratinocytes (Thommesen et al., 1998; Anthonsen et al., 2001; Haase et al., 2001).
NF-κB is a crucial factor for the immunoinflammatory responses implicated in various skin diseases including psoriasis (Banno et al., 2005). In fact, several antipsoriatic drugs such as tacrolimus, dimethylfumarate, calcipotriol or corticosteroids act in part by inhibition of this nuclear factor (Bell et al., 2002; Lan et al., 2005; Mrowietz and Asadullah, 2005; Norris, 2005). Activation of the NF-κB pathway leads to the transcription of numerous genes, regulating the production of cytokines, chemokines and growth factors, which are involved in the initiation of the inflammatory response (Courtois, 2005; De Vry et al., 2005). Remarkably, activation of NF-κB induces the production of proteins, such as TNF-α, which are, in turn, able to stimulate the signal transduction pathway to activate NF-κB, thus constituting a proinflammatory positive feedback (Quivy and Van Lint, 2004). In this way, a crucial link between high levels of TNF-α and NF-κB activation has been found in the skin of psoriatic patients and a potential mechanism of action for TNF-targeting agents is the downregulation of NF-κB transcriptional activity (Bos et al., 2005; Gottlieb, 2005; Lizzul et al., 2005). Recently, anti-inflammatory therapies based on blocking TNF-α signalling were shown to be effective in the treatment of psoriasis and could become a highly promising option for the treatment of this skin condition (Asadullah et al., 2002; Gottlieb, 2005).
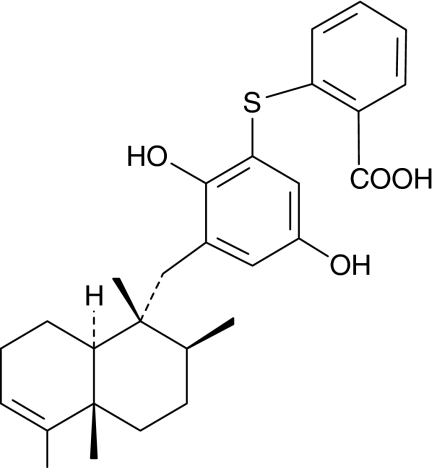
Avarol is a marine sesquiterpenoid hydroquinone with interesting pharmacological properties including anti-inflammatory and antipsoriatic effects (Ferrándiz et al., 1994; De Rosa, 2002; Sipkema et al., 2005). In a recent study, we reported that its derivative avarol-3′-thiosalicylate (TA) (Figure 1) inhibited superoxide anion generation in stimulated human neutrophils and PGE2 release in the human HaCaT keratinocyte cell line (Amigó et al., 2004).
Figure 1.
Chemical structure of avarol-3′-thiosalicylate.
In the present study, we investigate the effect of TA on some inflammatory parameters upregulated during the innate immune response in psoriasis. In vitro studies performed in human neutrophils, monocytes and keratinocytes have been corroborated in vivo using the zymosan-stimulated mouse air pouch model and the TPA-induced murine hyperplasia skin model. The ability of TA to inhibit NF-κB activation and TNF-α generation in vitro and in vivo has been demonstrated.
Methods
Animals
Female Swiss CD-1 mice (25–30 g) were obtained from Harlan (Spain). The mice were kept at room temperature and had free access to food and water. All studies were performed in accordance with European Union regulations for the handling and use of laboratory animals. The protocols were approved by the Institutional Animal Care and Use Committee of the University of Valencia.
Chemiluminescence by human neutrophils
A suspension of human neutrophils was obtained from the citrated blood of healthy volunteers after sequential centrifugation as described previously (Bustos et al., 1995). Neutrophils (2.5 × 106 cells ml−1) were incubated with luminol (40 μM) and stimulated with 1 μM 12-O-tetradecanoyl phorbol 13-acetate (TPA). Chemiluminescence was recorded with a Microbeta trilux counter (Wallac, Turku, Finland). The mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan (Borenfreund et al., 1988) was used to assess the possible cytotoxic effects of compounds.
Chemiluminescence by the hypoxanthine/xanthine oxidase system
Superoxide anions were generated by the hypoxanthine/xanthine oxidase system to assess a possible direct scavenging activity of the test compound (Betts, 1985). The reaction mixture contained 50 mM KH2PO4–KOH (pH 7.4), 1 mM ethylenediaminetetraacetic acid (EDTA), 100 μM hypoxanthine and luminol (40 μM) in a total volume of 250 μl. The reaction was started by adding 76.8 μU μl−1 of xanthine oxidase. Chemiluminescence was recorded with a Microbeta trilux counter (Wallac, Turku, Finland). We had previously found that this compound did not inhibit xanthine oxidase activity, by following the formation of uric acid (Paya et al., 1992).
Synthesis and release of LTB4 by human neutrophils
A suspension of human neutrophils obtained as above, was resuspended at 5 × 106 cells ml−1. Cells were preincubated with test compound or vehicle for 5 min and then stimulated with calcium ionophore A23187 (1 μM) for 10 min at 37°C. LTB4 levels in supernatants were measured by radioimmunoassay (Moroney et al., 1988). To assess 5-LO activity, high-speed (100 000 × g) supernatants from sonicated human neutrophils were obtained. Aliquots (50 μg of protein/tube) in PBS containing 2 mM CaCl2 were incubated with 5 μM AA at 37°C for 5 min in the presence of test compounds or vehicle. The samples were then heated at 90°C and centrifuged at 10 000 × g at 4°C for 10 min (Tateson et al., 1988). LTB4 levels in supernatants were measured by radioimmunoassay.
TNF-α and PGE2 production in intact human monocytes
Human monocytes were obtained from peripheral blood taken from healthy volunteers, as described previously (Bustos et al., 1995; Rioja et al., 2002). Cells (2 × 106 cells ml−1) were coincubated in 96-well culture plate (200 μl) with 1 μg ml−1 of Escherichia coli (serotype 0111:B4) lipopolysaccharide (LPS) at 37°C for 20 h in the presence of test compounds or vehicle. PGE2 levels were determined in culture supernatants by radioimmunoassay (Moroney et al., 1988). The MTT assay (Borenfreund et al., 1988) was also used to assess the possible cytotoxic effects of compounds on monocytes. In a parallel experiment, TNF-α levels were determined by time-resolved fluoroimmunoassay (Pennanen et al., 1995) in supernatants of zymosan-stimulated monocytes during 3 h (0.1 mg ml−1).
COX-1/COX-2 activity in intact human monocytes
To assess the effects of compounds on COX-2 activity, aspirin-treated human monocytes were incubated with LPS (1 μg ml−1) for 24 h to induce COX-2. Cultured medium was changed and test compounds, indomethacin, NS398 or vehicle were added for 15 min preincubation at 37°C. Arachidonic acid (10 μM) was then added and the cells were incubated for further 2 h. In parallel experiments, untreated monocytes (without aspirin) were preincubated for 15 min with test compounds or vehicle. Afterwards, arachidonic acid (10 μM) was then added and the cells were incubated for 2 h at 37°C to assess the effects of compounds on COX-1 activity. After the incubation period, supernatants were collected for the measurement of PGE2 levels as above.
Western blot assay of COX-2
Cellular lysates from human monocytes (10 × 106 cells ml−1 in 6-cm Petri dishes) incubated for 20 h with LPS were obtained with lysis buffer (1% Triton X-100, 1% deoxycholic acid, 20 mM NaCl and 25 mM Tris, pH 7.4). Following centrifugation (10 000 × g, 15 min), supernatant protein was determined by the DC Bio-Rad protein reagent (Richmond, CA, USA). Equal amounts of protein (30 μg) were loaded on 12.5% SDS–PAGE and transferred onto polyvinylidene difluoride membranes (Amersham Biosciences, Barcelona, Spain) for 90 min at 125 mA. Membranes were blocked in PBS (0.02 M, pH 7.0)-Tween-20 (0.1%), containing 3%, wv−1, defatted milk and incubated with specific polyclonal antibody against COX-2 (1/1,000 Cayman Chemical Company, Ann Arbor, MI, USA) or anti-β-actin (1:1,000 Sigma Chemical Co., St Louis, MO, USA). Finally, membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:10 000 DakoCytomation, Glostrup, Denmark). The immunoreactive bands were visualized using an enhanced chemiluminescence system (UV Transilluminator, AutoChemi System, UVP Bioimaging System).
Human recombinant sPLA2-IIA assay
Secretory phospholipase A2 was assayed by using a modification of the method of Franson (Franson et al., 1974). Human recombinant enzyme was diluted in 10 μl of 100 mM Tris-HCl, 1 mM CaCl2 buffer, pH 7.5, and preincubated at 37°C for 5 min with test compound or vehicle in a final volume of 250 μl. Incubation proceeded for 15 min in the presence of 10 μl of [3H]oleic-Escherichia coli membranes and was terminated by addition of 100 μl ice-cold solution of 0.25% BSA in saline to a final concentration of 0.07%, wv−1. After centrifugation at 2500 g for 10 min at 4°C, the radioactivity in the supernatants was determined by liquid scintillation counting.
cPLA2 assay
Cytosolic phospholipase A2 (cPLA2) was determined from cytosolic fraction of sonicated RAW 264.7. macrophages. Enzymatic activity was measured as the release of radiolabelled arachidonic acid (Clark et al., 1990). The substrate consisted of 5 μl of micelles (104 c.p.m.) containing 1-palmitoyl-2-[14C]arachidonyl-sn-glycero-3-phosphocholine.
HaCaT cell culture
The human keratinocyte cell line HaCaT was provided by Dr N E Fusenig (Heidelberg, Germany) (Boukamp et al., 1988). The cells were cultured in modified Eagle's medium with 10% fetal calf serum (FCS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, in a humidified incubator (5% CO2 at 37°C).
For sPLA2 activity, keratinocytes (2 × 105 cells/well) were grown for 24 h on six-well culture plates. The medium was replaced (5% FCS) and test compounds or vehicle (1% EtOH) were added for a further 24 h incubation. Finally, supernatants were collected for the determination of sPLA2 activity as above (Franson et al., 1974).
For assessment of NF-κB activation, HaCaT cells (106 cells/well) were grown for 24 h on six-well culture plates. Cells were preincubated with test compound or vehicle for 30 min and then stimulated with TNF-α (2 ng ml−1) for 45 min (Moustafa et al., 2002).
Electrophoretic mobility shift assay
HaCaT cells were preincubated with test compound or vehicle for 30 min and then stimulated with TNF-α (2 ng ml−1) for 45 min (Moustafa et al., 2002). Cells were washed twice with ice-cold phosphate-buffered saline, and then treated with 0.2 ml of buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 8, 1 mM EDTA, 10 mM KCl, 1 mM ethylene glycol bis(β-aminoethylether)-N,N,N′,N′,-tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), 0.5 mM phenylmethyl sulphonyl fluoride (PMSF) and a protease inhibitor cocktail) for 15 min followed by addition of Nonidet P-40 (0.5%, w v−1). The tubes were vortexed for 5 s, and nuclei were sedimented by centrifugation at 15 500 g for 1 min. Aliquots of the supernatant were stored at −80°C (cytoplasmic extract), and the pellet was resuspended in 50 μl of buffer C (20 mM HEPES, pH 8, 1 mM EDTA, 0.4 M NaCl, 1 mM EGTA, 1 mM DTT, 0,5 mM PMSF and protease inhibitors cocktails) and incubated at 4°C for 30 min with constant shaking. After centrifugation at 15 500 g for 5 min, aliquots of the supernatant (nuclear extract) were stored at −80°C. Protein was determined by the DC Bio-Rad protein reagent (Richmond, CA, USA). The double-stranded oligonucleotide containing the consensus NF-κB sequence (Promega Corp., Madison, WI, USA) was end-labelled using T4 polynucleotide kinase (Amersham Biosciences, Barcelona, Spain) and [γ-32P]ATP, followed by purification using G-50 microcolumns (Amersham Biosciences). The binding reaction was carried out in buffer C containing 10 μg of nuclear extracts, 1 μl of 2 μg μl−1 poly(dI-dC) (Amersham Biosciences, NJ, USA), 4 μl of 0.1% bromophenol blue and 200 000 c.p.m. of the end-labelled oligonucleotide probe. After 20 min of incubation at room temperature, samples were loaded onto a 6% polyacrylamide gel (37.5:1) in 0.5 × TBE (prerun for 30 min) and run at 150 V for 2 h. Finally, complexes were analysed by autoradiography of the dried gel using a Typhoon Imager-9400. The identity of the NF-κB complex was confirmed by the disappearance of the band upon competition with 50-fold unlabelled NF-κB consensus probes.
Mouse air pouch model
The air pouch model was in female Swiss mice (25–30 g), as described previously (Posadas et al., 2000). Six days after the initial air injection, 1 ml of sterile saline or 1 ml of 1%, w v−1, zymosan in saline was injected into the air pouch. Products were administered at the same time as zymosan or orally 1 h before zymosan injection. After 4 h, the animals were killed by cervical dislocation and the exudate in the pouch was collected with 1 ml of saline. After centrifugation of the exudates, the supernatants were used to determine LTB4 and PGE2 by radioimmunoassay and TNF-α and IL-1 β by time-resolved fluoroimmunoassay.
TPA-induced epidermal hyperplasia model
We evaluated the effect of TA on TPA-induced hyperplasia in murine skin used as a possible psoriatic model (Sawa et al., 2002; Sato et al., 2004). The back of female Swiss mice were shaved by an electric clipper and treated with depilatory cream (Deliplus, Barcelona, Spain). One day later, the animals that displayed no evidence of hair regrowth were used for the experiment. A total of 20 μl of TPA 100 μM (2 nmol per site) or vehicle (acetone) was applied to the skin surface of the backs in an area of 1 cm2 using a micropipette. One hour before, test compound or vehicle (acetone) was topically applied to the area to be treated with TPA (day 0). The treatment of the test compound or vehicle was repeated on days 1 and 2. On day 3, the mice were killed by cervical dislocation and 1 cm2 punch biopsies were taken from the treated dorsal skin and weighed. Skin sections were homogenized in 750 μl saline, and after centrifugation at 1372 g for 8 min at 4°C, supernatants were used for determination of PGE2 and LTB4 content by radioimmunoassay, and for determination of IL-1β and IL-2 by ELISA. To measure myeloperoxidase (MPO) activity, skin punches were homogenized in 750 μl of 80 mM sodium phosphate buffer (pH 5.4) containing 0.5% of hexadecyltrimethylammonium bromide (HTAB) (Sato et al., 2004).
For assessment of NF-κB activity, animals were killed 1.5 h after TPA treatment. The treated area of skin was excised and placed in chilled Petri dishes with the epidermis side up (Han et al., 2001). The epidermis was removed by gentle scraping with the razor blade and placed in 1 ml of hypotonic buffer A (as above) and homogenized in an ice bath using a Polytron tissuemizer. To the homogenates was added 63 μl of 10% Nonidet P-40 (NP-40) solution and the mixture was then centrifuged for 30 s at 14 800 g. The pelleted nuclei were washed once with 400 μl of buffer A plus 25 μl of 10% NP-40, centrifuged, resuspended in 50 μl of a high-salt buffer consisting of 50 mM HEPES (pH 8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, protease inhibitors cocktails and 10% glycerol, mixed for 30 min and centrifuged for 5 min at 4°C. The supernatant containing nuclear proteins was collected and stored at −80°C after determination of the protein concentration. Electrophoretic mobility shift assay (EMSA) was performed as above.
Histochemical study
Tissue samples from murine skin were fixed in 4% neutral-buffered formalin and embedded in paraffin. The formalin-fixed, paraffin-embedded, tissue sections (4–6 μm) were mounted on slides, deparaffinized with xylene, rehydrated through graded alcohols, and stained with hematoxylin and eosin. Immunohistochemical detection of TNF-α in skin, was carried out in deparaffinized and rehydrated sections using the HRP-DAB Cell & Tissue Staining Kit (R&D Systems, Minneapolis, USA) according to the manufacturer's protocol. Samples were incubated overnight at 4°C with 20 μg ml−1 anti-mouse TNF-α antibody (R&D Systems). Finally, samples were counterstained with hematoxylin, mounted with Dako paramount aqueous mounting medium (DakoCytomation) and observed with a Nikon Eclipse E600FN microscope (Nikon Instruments Inc., Melville, USA).
Statistical analysis
Results are presented as mean±s.e.mean of n separate experiments. IC50 values and their 95% confidence limits were calculated from at least four concentrations (n=6). Statistical analyses were performed using one-way ANOVA followed by Dunnett's t-test for multiple comparisons.
Chemicals and reagents
Avarol-3′-thiosalicylate was synthesized following published procedures (Amigó et al., 2004). [5,6,8,11,12,14,15(n)-3H]PGE2 was from Amersham Biosciences and [5,6,8,9,11,12,14,15(n)-3H]LTB4 was from PerkinElmer (Boston, USA). [9,10–3H]oleic acid and 1-palmitoyl-2-[14C]arachidonyl-sn-glycero-3-phosphocholine were purchased from Du Pont, (Itisa, Madrid, Spain). Escherichia coli strain CECT 101 was a gift from Professor Uruburu, Department of Microbiology, University of Valencia, Spain. N-(2-cyclohexyloxy-4-nitrophenyl) methanesulphonamide (NS398) and COX-2 polyclonal antiserum were purchased from Cayman Chemical Co. The peroxidase-conjugated goat anti-rabbit IgG was purchased from Dako Co. (Denmark). Anti-mouse IL-1β and TNF-α kits for ELISA and anti-mouse TNF-α antibody for immunohistochemistry were from R&D Systems. ELISA kit for IL-2 was obtained from eBioscience (San Diego, CA, USA). Antibody against LTB4 and ZM 230,487 were kindly provided by Zeneca Pharmaceuticals (Macclesfield, Cheshire, UK). The other reagents were from Sigma Chem.
Results
Effect on chemiluminescence generated by human neutrophils and by the hypoxanthine–xanthine oxidase system
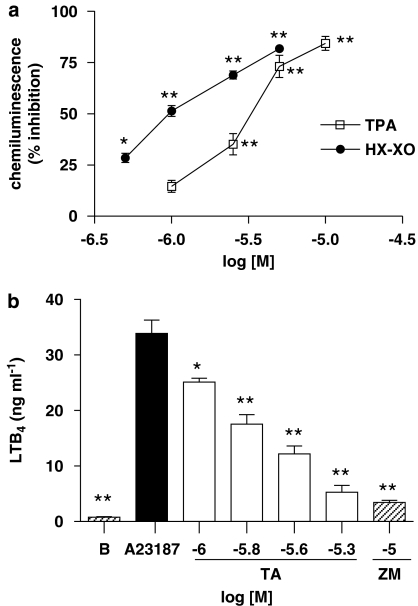
No cytotoxic effects of TA were observed on neutrophils at the concentrations used in our study, as assessed by mitochondrial reduction of MTT after 30 min challenge (data not shown). Figure 2a shows the inhibitory effect of TA on chemiluminescence responses induced by TPA-stimulated human neutrophils in comparison to the cell-free hypoxanthine–xanthine oxidase system. IC50 values were 3.40 (2.70–4.30) μM for intact cells and 1.00 (0.60–1.80) μM for the cell-free assay, indicating a scavenging effect of TA on reactive oxygen species. Fraxetin was tested as a reference scavenger compound, showing IC50 values of 1.00 (0.70–1.80) μM for stimulated neutrophils and 0.97 (0.57–1.78) μM for the hypoxanthine–xanthine oxidase system. A possible direct inhibition of xanthine oxidase activity by TA was discounted (data not shown).
Figure 2.
Effect of avarol-3′-thiosalicylate on stimulated human neutrophils. (a) Inhibitory effect on chemiluminescence generated by human neutrophils stimulated with 12-O-tetradecanoylphorbol-13-acetate and by the cell-free hypoxanthine–xanthine oxidase system. Control chemiluminescence values were 4893±174 units min−1106 cells for stimulated neutrophils and 1047±69 units min−1 for the hypoxanthine–xanthine oxidase assay. (b) Effect on leukotriene B4 generation by human neutrophils stimulated with calcium ionophore A23187. B: non stimulated cells. ZM: ZM230,487. All data represent mean±s.e.mean., n=6–8. *P<0.05, **P<0.01 significantly different from control values.
Effect on LTB4 release by human neutrophils
Figure 2b shows the concentration-dependent inhibition of LTB4 generation produced by TA in A23187-stimulated human neutrophils. IC50 value was 1.79 (1.43–2.16) μM. The compound ZM 230487 was also tested as a specific inhibitor of 5-lipoxygenase activity, showing an IC50 value of 0.06 (0.03–0.09) μM. To evaluate whether TA directly inhibited 5-LO activity, neutrophil cytosol was prepared to study the synthesis of LTB4. Results indicated that TA did not exert significant inhibitory effects in this cell-free system assay (Table 1).
Table 1.
Effect of TA on COX-1, COX-2, 5-LO and cPLA2 activities
| COX-1 PGE2 (ng ml−1) | COX-2 PGE2 (ng ml −1) | 5-LO LTB4 (ng ml−1) | cPLA2 AA (pmol mg−1 min−1) | |
|---|---|---|---|---|
| Basal | 0.86±0.10** | 0.24±0.02 ** | 0.97±0.09** | 4.51±0.12** |
| Control | 5.10±0.38 | 2.38±0.22 | 8.94±0.8 | 12.91±1.42 |
| TA (5 μM) | 4.82±0.43 | 2.56±0.12 | 7.08±1.6 | 10.34±0.38 |
| NS-398 (1 μM) | ND | 0.32±0.01** | ND | ND |
| Indomethacin (1 μM) | 1.27±0.11** | ND | ND | ND |
| ZM 230,487 (1 μM) | ND | ND | 1.21±0.03** | ND |
| PTK (10 μM) | ND | ND | ND | 3.4 ± 0.7** |
Abbreviations: AA, arachidonic acid; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; cPLA2, secretory phospholipase A2; ND, not determined; PGE2, prostaglandin E2; TA, avarol-3′-thiosalicylate.
PGE2 levels were measured in supernatants of intact human monocytes as index of COX-1 and COX-2 activity. LTB4 generation was determined from high-speed supernatants from sonicated human neutrophils. cPLA2 activity was determined from cytosolic fraction of sonicated RAW 264.7 macrophages. Indomethacin, NS398, palmityl trifluoromethyl ketone (PTK) and ZM 230487 were used as reference inhibitors of COX-1, COX-2, cPLA2 and 5-LO respectively.
Values are represented as mean±s.e.mean; n=8.
**P<0.01 compared with control values.
Effect on PGE2 production in LPS-stimulated human monocytes
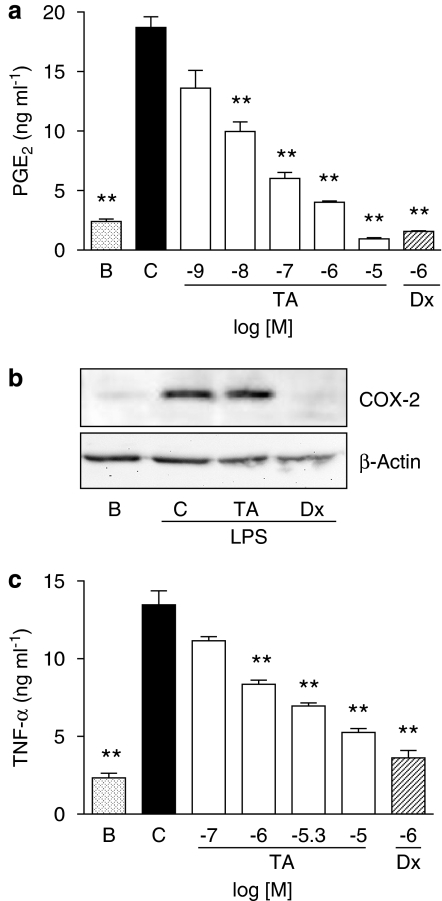
As shown in Figure 3a, TA inhibited in a concentration-dependent manner the generation of PGE2 during 20 h of coincubation with LPS-stimulated monocytes. The IC50 value was 17.30 (11.70–23.10) nM. TA did not exert cytotoxic effects at the concentrations tested during the 20 h incubation period with human monocytes, as indicated by the MTT reduction assay (data not shown).
Figure 3.
Effect of avarol-3′-thiosalicylate (TA) on prostaglandin E2 (PGE2) generation, cyclooxygenase-2 (COX-2) protein expression and tumour necrosis factor-α (TNF-α) production in stimulated human monocytes. (a) Concentration–effect relationship for the inhibition of PGE2 release in 20 h-LPS stimulated cells. Data represents mean±s.e.mean, n=6. **P<0.01 significantly different from stimulated cells (C). B: basal (non stimulated cells). Dx: dexamethasone. (b) Western blot analysis of COX-2 and β-actin protein expression for TA (5 μM) and Dx 1 μM after 20 h of coincubation with LPS-stimulated monocytes. The figure is representative of three similar experiments. B: non-stimulated cells. (c) Concentration-effect relationship for the inhibition of TNF-α generation in 3 h-zymosan-stimulated monocytes. Data represents mean±s.e.mean, n=6–12. **P<0.01 significantly different from TNF-α production by stimulated cells.
Effect on COX-2 protein expression in human monocytes
Western blot analysis for COX-2 was carried out on lysates of monocytes to evaluate if the inhibition of PGE2 observed in cultured cells was related to the reduction in protein expression. As expected, the reference inhibitor dexamethasone (1 μM) powerfully inhibited the induction of this enzyme, whereas in cells treated with TA (5 μM), protein expression was unaffected (Figure 3b).
Effect on COX-1/COX-2 activity in human monocytes
The following experiments were designed to determine if the reduction of PGE2 production in monocytes was due to a direct inhibition of enzyme activities. COX-2 was induced in aspirin-treated monocytes by 24 h LPS stimulation. Cultured medium was then changed and test products were added and incubated during 2 h in presence of arachidonic acid. In a parallel experiment, COX-1 activity was studied in non-induced cells after 2 h incubation with arachidonic acid. As expected, the reference compounds indomethacin and NS-398 reduced PGE2 levels generated by COX-1 and COX-2 activities. Nevertheless, no significant inhibition was observed for TA after this 2 h period (Table 1).
Effect on TNF-α generation in human monocytes
Monocytes were stimulated with zymosan during 3 h and TNF-α levels were determined in supernatants by time-resolved fluoroimmunoassay. As shown in Figure 3c, coincubation of stimulated cells with TA reduced in a concentration-dependent manner the generation of this cytokine, with an IC50 value of 4.18 (2.42–8.86) μM.
Effect on sPLA2 and cPLA2 activities
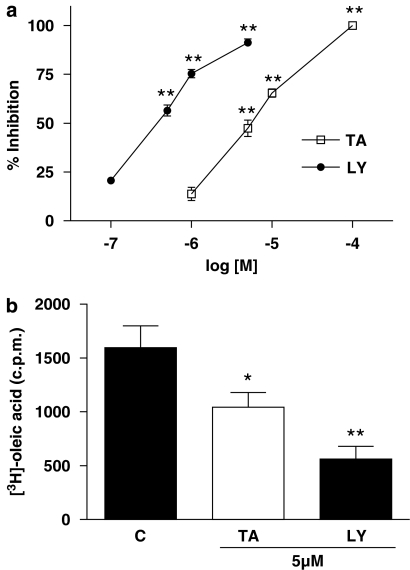
To study if the inhibitory response on PGE2 and LTB4 production in intact cells could be related to interference with the release of arachidonic acid, we assayed the effect of TA on sPLA2 and cPLA2 activities. As shown in Figure 4a, TA and the reference inhibitor of sPLA2 LY311727, reduced the amount of [3H]oleic acid released from E. coli membranes by human synovial recombinant sPLA2 activity. IC50 values were 5.90 (2.57–12.59) μM and 0.30 (0.10–0.80) μM respectively. The inhibition of sPLA2 activity was also determined in intact cells using the HaCaT human keratinocyte cell line. After 24 h of coincubation with cells, TA and LY311727 (5 μM) significantly reduced sPLA2 activity determined in cell supernatants (Figure 4b). No inhibition by TA was observed on cPLA2 activity obtained from the RAW 264.7 cell line (Table 1).
Figure 4.
Effect of avarol-3′-thiosalicylate and the reference inhibitor LY311727 (LY) on sPLA2 activity. (a) Concentration-effect relationship for the inhibition of synovial recombinant sPLA2 activity. Data represents mean±s.e.mean, n=6. *P<0.05, **P<0.01 significantly different from control sPLA2 activity (3144.0±120.7 pmol of oleic acid mg−1 min−1). (b) Effect on sPLA2 activity determined in supernatants from HaCaT cells incubated during 24 h. *P<0.05, **P<0.01 significantly different from untreated cells (C).
Effect on NF-κB binding to DNA in HaCaT keratinocytes
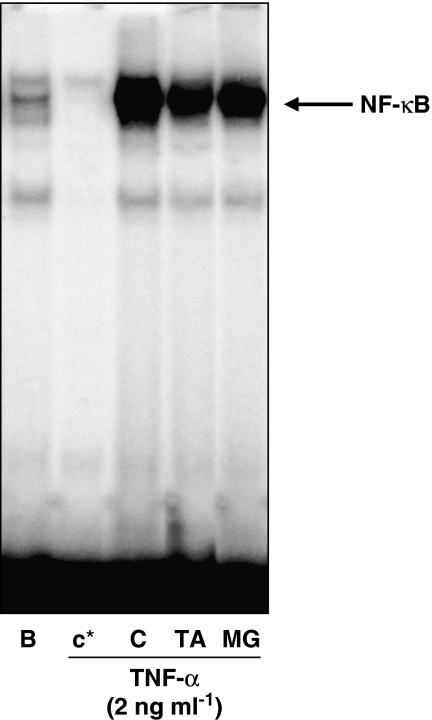
Analysis of DNA binding was performed by EMSA in nuclear extracts from HaCaT human keratinocytes stimulated with 2 ng ml−1 TNF-α for 45 min in the absence or presence of compounds. As expected, the proteasome inhibitor MG132 reduced the TNF-α -induced NF-κB binding activity (Figure 5). TA also inhibited the binding of NF-κB to DNA at 5 μM.
Figure 5.
Effect of avarol-3′-thiosalicylate (TA) on NF-κB-DNA binding in nuclear extracts of HaCaT keratinocytes. Cells were preincubated with TA (5 μM) or the reference compound MG132 (MG 10 μM) for 30 min before tumour necrosis factor-α stimulation (2 ng ml−1) for 45 min. NF-κB-DNA-binding activity was assayed by electrophoretic mobility shift assay. Results are representative of three independent experiments. B: non stimulated cells. C: stimulated cells. In lane c*, a 50-fold excess of unlabelled oligonucleotide was added to the reaction mixture.
Effect on mouse air pouch
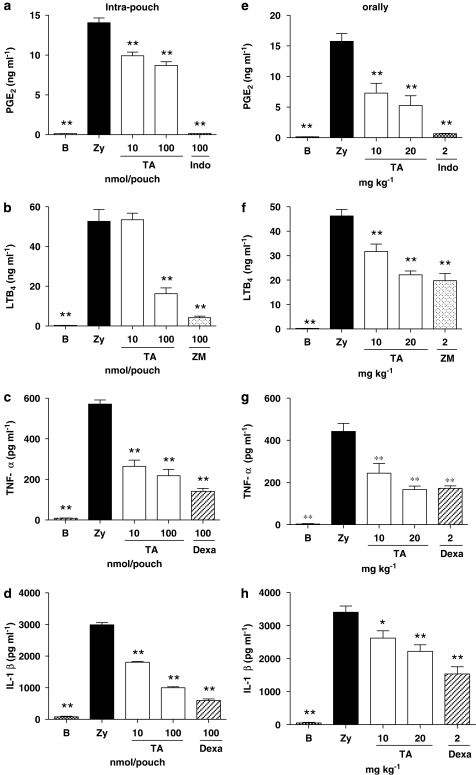
We assessed the in vivo effect of TA on PGE2, LTB4, TNF-α and IL-1β generation in the 4 h zymosan-stimulated mouse air pouch. Indomethacin, ZM230.487 and dexamethasone, were used as reference inhibitors of PGE2, LTB4 and cytokine levels respectively. All compounds were given locally (intra-pouch) or orally. As shown in Figure 6a, c and d, PGE2, TNF-α and IL-1β release in pouch exudates was significantly reduced by TA at 100 and 10 nmol per pouch. The dose-dependent study at 1000, 100, 10 and 1 nmol per pouch showed ED50 values of 136.1 nmol per pouch, 4.1 nmol per pouch and 89,9 nmol per pouch for PGE2, TNF-α and IL-1β respectively. LTB4 generation in exudates was also reduced by TA at 100 nmol per pouch (Figure 6b).
Figure 6.
Effect of avarol-3′-thiosalicylate (TA) on prostaglandin E2, leukotriene B4, tumour necrosis factor-α and IL-1β release in the 4 h zymosan injected air pouch. (a–d) TA (10–100 nmol per pouch) and reference compounds (100 nmol per pouch) were injected in the air pouch at the same time of zymosan. (e-h) TA (10–20 mg kg−1) and reference compounds (2 mg kg−1) were administered orally 1 h before injection of zymosan. Data represent means±s.e.mean (n=6–12 animals). *P<0.05, **P<0.01 significantly different from control group (Zy). B: untreated animals. Indo:indomethacin. ZM: ZM230,487. Dexa: dexamethasone.
In a parallel experiment, TA was given orally (10 or 20 mg kg−1) 1 h before zymosan injection and the levels of the same proinflammatory mediators were determined in pouch exudates 4 h after stimulation. As shown in Figure 6e–h, TA significantly reduced PGE2, LTB4, TNF-α and IL-1β production at both doses.
Effect on the TPA-induced epidermal hyperplasia model
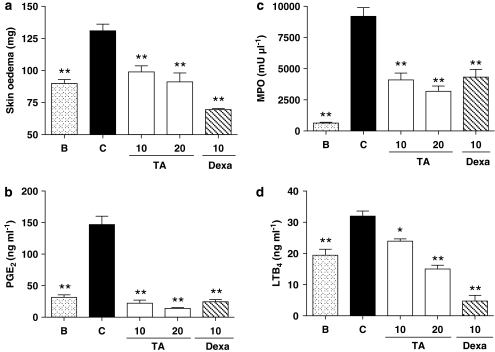
As determined by the weight of a 1 cm2 punch biopsy of dorsal skin, application of TPA to mouse skin resulted in a development of skin oedema, measured 72 h after the first application of TPA. Topical application of 20 μl of TA (10 and 20 μg μl−1) or the reference compound dexamethasone (10 μg μl−1), 30 min before TPA, significantly reduced the skin punch weight (Figure 7a). This effect was accompanied by a clear inhibition of MPO activity and LTB4 levels in punch homogenates (Figure 7c–d). In addition, PGE2 levels were also strongly inhibited by TA at both assayed doses (Figure 7b).
Figure 7.
Effect of avarol-3′-thiosalicylate (TA) on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced epidermal hyperplasia model. TA (10–20 μg μl−1), dexamethasone (Dexa) (10 μg μl−1) or vehicle (acetone) were topically applied 1 h before TPA (2 nmol per site) during three consecutive days. (a) skin oedema, assayed as punch weight. (b) Prostaglandin E2 levels in homogenates. (c) MPO activity determined in punch homogenate. (d) Leukotriene B4 release. Data represent means±s.e.mean (n=6 animals). *P<0.05, **P<0.01 significantly different from TPA-treated group (C). B: animals only treated with acetone.
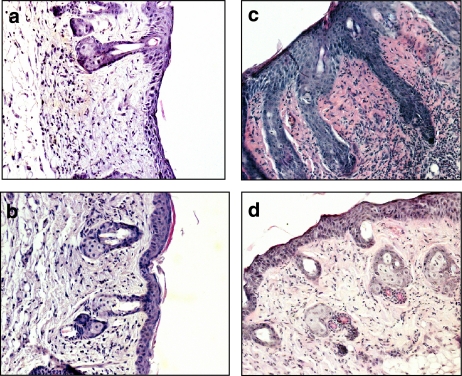
We examined hematoxylin and eosin-stained sections of mouse skin (Figure 8). TPA application results in a marked increase in skin thickness, with clear evidence of oedema, epidermal hyperplasia and large numbers of infiltrating inflammatory cells, compared with the effects of acetone treatment. The skin of mice treated with TA and TPA presented significantly less epidermal hyperplasia and dermal inflammation than TPA-treated animals. In addition, a marked decrease in the number of cells infiltrating the epidermal and dermal microenvironment was observed microscopically. The reference compound dexamethasone also significantly reduced hyperplasia and the degree of TPA-induced epidermal and dermal infiltrate. IL-1β and IL-2 levels in tissue homogenates were also determined by ELISA in this model. As shown in Table 2, administration of TA and dexamethasone at 10 μg μl−1 significantly reduced the levels of both cytokines with respect to the TPA-treated animals.
Figure 8.
Photomicrographs of eosin-haematoxylin staining of skin biopsies from 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced hyperplasia in mice. (a) Acetone treatment alone; (b) TPA (2 nmol per site); (c) TPA+ avarol-3′-thiosalicylate 10 μg μl−1; (d) TPA+ dexamethasone 10 μg μl−1. Original magnification × 200.
Table 2.
Effect of TA on IL-1β and IL-2 levels in skin homogenates from the TPA-induced epidermal hyperplasia model
| Acetone | TPA | TPA+TA | TPA+Dexa | |
|---|---|---|---|---|
| IL-1β (pg ml−1) | 955±18** | 4194±320 | 2303±235** | 1928±285** |
| IL-2 (pg ml−1) | 120±14** | 403±76 | 121±13** | 148±25** |
Abbreviations: IL-1β, interleukin-1β; TA, avarol-3′-thiosalicylate; TPA, 12-O-tetradecanoylphorbol-13-acetate.
TA and dexamethasone (Dexa) were topically applied at 10 μg μl−1 1 h before TPA (2 nmol per site) during three consecutive days.
Data represent means±s.e.mean (n=6 animals).
**P<0.01, significantly different from TPA group.
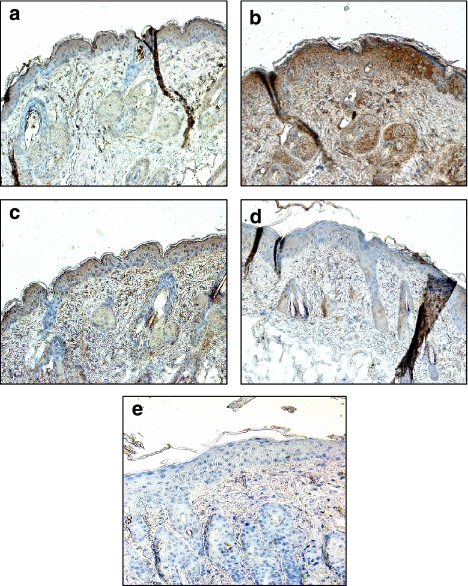
Immunohistochemical analysis was used to determine the possible effect of compounds on TNF-α release in skin. Representative photographs depicting the TNF-α staining pattern observed for the different treatment groups are shown in Figure 9. TNF-α protein was strongly increased in TPA-treated skin mainly around epidermal keratinocytes, while little protein was detected in the normal skin sample. Animals treated with TA presented a clear reduction of TNF-α protein levels within the epidermis as compared with TPA-treated group. Similar results were obtained with dexamethasone, which also reduced the levels of this cytokine in skin.
Figure 9.
Immunohistochemical detection of tumour necrosis factor-α (TNF-α) protein in mouse skin biopsies from 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced hyperplasia in mice. TNF-α staining gives brown reaction products. Sections were counterstained with haematoxylin. (a) acetone treatment; (b) TPA (2 nmol per site); (c) TPA+avarol-3′-thiosalicylate 10 μg μl−1; (d) TPA+Dexamethasone 10 μg μl−1 (e) TPA-treated animals with second antibody only. Original magnification × 200.
Effect on TPA-induced NF-κB activation in the epidermal hyperplasia model
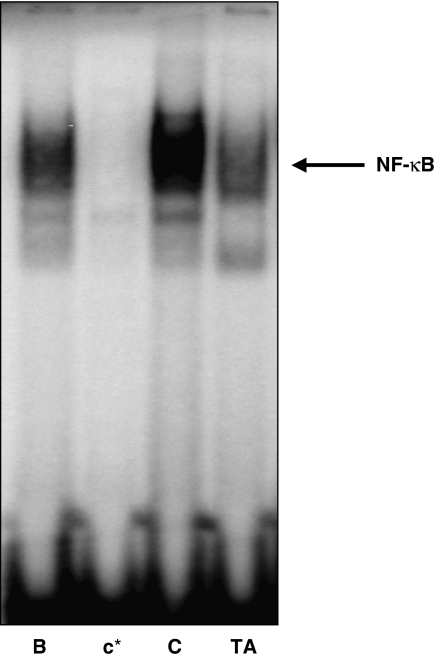
In a parallel experiment, the animals were killed 1 h after a single application of TPA (10 nmol per site) and epidermal nuclear lysates were prepared to determine the activation of NF-κB. As shown in Figure 10, there was relatively low nuclear NF-κB binding activity detected in the epidermal extracts from acetone-treated animals, whereas treatment with TPA caused a marked increase of NF-κB binding to DNA. This effect was clearly reduced by topical application of TA (10 μg μl−1) 30 min before TPA.
Figure 10.
Inhibitory effect of avarol-3′-thiosalicylate (TA) on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced nuclear factor-kappa B (NF-κB) DNA binding activity in mouse skin. Nuclear extracts were isolated from skin topically treated with acetone (B), TPA (C) (10 nmol per site), or TA (10 μg μl−1) 30 min before application of TPA. NF-κB-DNA binding activity was assayed by electrophoretic mobility shift assay. In lane c*, a 50-fold excess of unlabelled oligonucleotide was added to the reaction mixture.
Discussion
The psoriatic process is driven by a complex interplay between innate immunity (neutrophils, macrophages, dendritic cells and keratinocytes) and activated T lymphocytes (Arthur and Darragh, 2006; Gaspari, 2006). In addition to the recruitment of T cells, one of the primary abnormalities in developing lesions of psoriasis is the perivascular accumulation of neutrophils and their influx into the epidermis, leading to microscopically detectable microabcesses (Bos et al., 2005). The phagocytic reaction of neutrophils causes an abundant superoxide anion production, which induces oxidative damage as well as the expression of redox sensitive transcription factors implicated in inflammatory skin diseases (Briganti and Picardo, 2003; Yildirim et al., 2003). ROS may also activate PLA2 and thus cause overproduction of many mediators of arachidonic acid metabolism. In fact, leukotrienes are elevated in psoriatic epidermis and patient serum, stimulating chemokinesis, aggregation and degranulation (Ikai, 1999; Yildirim et al., 2003). In the present study, we have demonstrated that TA inhibits LTB4 synthesis as well as the respiratory burst elicited in human neutrophils, exerting at the same time, potent scavenging effects in the hypoxanthine–xanthine oxidase system. As a consequence, TA could contribute to the attenuation of the formation, or the exacerbation of neutrophil-mediated psoriatic lesions. Blocking neutrophil functions has already been suggested as having therapeutic benefit in psoriatic disease (Rocha-Pereira et al., 2004; Bos et al., 2005).
PGs also play an important role in the pathophysiology of psoriasis, affecting blood vessels and inflammatory cells, contributing to vasodilatation in the dermis and increased leukocyte infiltration and epidermal cell growth (Ikai, 1999). Our previous results indicated that TA inhibited the generation of PGE2 in human HaCaT keratinocytes (Amigó et al., 2004). Now, we have shown that TA also strongly reduced the production of PGE2 in activated human monocytes, without affecting either COX-1/COX-2 activity or COX-2 protein expression. Because TA also reduced LTB4 release in neutrophils without affecting 5-LO activity, we studied a possible effect of this compound on the release of arachidonic acid, showing a direct blockade of human sPLA2-IIA activity by TA. This effect was also observed in HaCaT keratinocytes, where inhibition of sPLA2 activity by this avarol derivative could explain the reduction of PGE2 release by TA, reported previously in these cells (Amigó et al., 2004).
It is interesting to note that a marked upregulation of sPLA2-IIA has been observed in keratinocytes of the hyperproliferative psoriatic epidermis (Andersen et al., 1994; Haas et al., 2005). sPLA2-IIA activity participates in keratinocyte motility and migration (Rys-Sikora et al., 2003), contributes to the sustained activation of MAP kinase in keratinocytes (Haase et al., 2001), activates phosphorylation of cPLA2, and amplifies cytokine-stimulated NF-κB activation in HaCaT cells (Thommesen et al., 1998; Anthonsen et al., 2001). In this regard, the inhibition of sPLA2-IIA activity by TA could also play a beneficial role in the treatment of psoriasis, as already suggested for other sPLA2 inhibitors (Sjursen et al., 2000).
In this study, TA also reduced TNF-α release in stimulated human monocytes. TNF-α is one of the most important cytokines associated with innate immunity. It controls and regulates the expression of numerous genes, and in doing so leads to cutaneous responses in psoriasis (Victor et al., 2003; Nickoloff and Nestle, 2004; Schottelius et al., 2004). It has been postulated that TNF-α produced locally in psoriatic lesions creates a TNF-α positive feedback loop that amplifies and sustains the inflammatory process within plaques (Banno et al., 2004). In fact, recently developed anti-inflammatory therapies based on blocking TNF-α signalling have been shown to be effective in the treatment of psoriasis and could become a highly promising option for this pathology (Asadullah et al., 2002; Gottlieb, 2005).
A crucial link between high levels of TNF-α and NF-κB activation has been found in psoriatic patients, suggesting an important role of this nuclear factor in the pathogenesis of the disease (Johansen et al., 2005; Lizzul et al., 2005). The Rel/NF-κB proteins belong to a family of related transcription factors, which regulates the expression of a large number of genes, including proinflammatory cytokines (for example, IL-1, IL-2, IL-6, TNF-α, etc.), and has a well-characterized role in immune and inflammatory responses (Tak and Firestein, 2001). The intracellular events from the TNF-α receptor to NF-κB activation in epidermal keratinocytes have been extensively studied (Umezawa and Chaicharoenpong, 2002; Banno et al., 2005). In addition, the involvement of intracellular redox-systems and the arachidonic acid cascade in the TNF-signal transduction pathway leading to the activation of NF-κB in skin have also been described (Thommesen et al., 1998; Kohler et al., 2001; Briganti and Picardo, 2003; Mrowietz and Asadullah, 2005). Thus, on the basis of anti-oxidative properties of TA and its inhibitory behaviour against TNF-α release and PLA2 activity, we determined the possible effect of this compound on NF-κB activation, showing that TA inhibited TNF-α-induced DNA binding of nuclear NF-κB in human HaCaT keratinocytes. Thus, by interfering with NF-κB activation, TA could act not only as an inhibitor of TNF-α-induced cellular functions, but also as an inhibitor of TNF-α production, because its transcription is, at least partly, dependent on the NF-κB pathway.
It is interesting to note that although activation of the NF-κB transcription factor system has been implicated in the induction of COX-2 gene expression in many cell types (Yamamoto and Gaynor, 2001), TA did not inhibit the expression of this enzyme in stimulated human monocytes and similar results were obtained in RAW 264.7 macrophages (data not shown). It is relevant to note that inhibition of NF-κB activation had no repressive effect on transcriptional activation at the COX-2 promoter level in LPS-stimulated macrophages, suggesting the existence of a NF-κB-independent pathway that regulates COX-2 gene activation (Wadleigh et al., 2000; Lo, 2003).
For the in vivo studies, we first determined the effect of TA in the 4h-zymosan-injected mouse air pouch, an acute model of inflammation, which allows measurement of a range of proinflammatory metabolites, including eicosanoids and cytokines, in pouch exudates (Posadas et al., 2000). In this model, the avarol derivative exhibited an inhibitory behaviour that correlated well with its in vitro effects, reducing in a dose-dependent manner and by both local (intra-pouch) and systemic (oral) routes, the levels of PGE2, LTB4 and TNF-α. It is interesting to note that TA also inhibited IL-1 β release in pouch exudates, suggesting the ability of this compound to reduce other inflammatory cytokines regulated by NF-κB activation.
The pathogenic mechanism of psoriasis is unclear, and good animal models do not yet exist. However, there are some similarities between human psoriatic lesions and mouse skin following a topical application of TPA (Reynolds et al., 1997; Moriyama et al., 2004; Sato et al., 2004; Pittelkow, 2005). TPA induces biochemical and histopathological changes in mouse skin, such as cell infiltration within the dermis and epidermis, oedema, epidermal hyperplasia, production of proinflammatory cytokines and chemokines and activation of the NF-κB pathway (Han et al., 2001; Afaq et al., 2005). Thus, we investigated the effects of TA on the TPA-induced hyperplasia in murine skin. Our results demonstrated that topical treatment with TA strongly reduced cutaneous oedema and MPO activity, determined in skin homogenates as an index of cell infiltration (Sato et al., 2004). This feature correlated well with the histopathological study, which showed a clear reduction of epidermal hyperplasia, associated ridges and pegs and cell infiltration in skin of mice treated with TA. As expected, the inhibitory effect of TA on PGE2 and LTB4 levels in skin homogenates correlated with the ability of this compound to reduce the release of both eicosanoids, by different routes of administration.
The involvement of TNF-α in the TPA-induced hyperplasia was also investigated by immunohistochemical staining of skin. TPA-induced TNF-α was mainly localized in or close to the epidermis, which is in agreement with other suggested reports (Oberyszyn et al., 1998; Murakawa et al., 2006). Results obtained in animals treated with TA demonstrated the ability of this compound to decrease TNF-α in mouse skin. It is interesting to note that TA also reduced IL-1β and IL-2 levels in skin homogenates. In this regard, psoriasis is characterized by a type 1 cytokine pattern, IL-2, IL-1 and TNF-α being predominantly expressed (Krueger, 2002). All these cytokines are induced by TPA in skin (Pittelkow, 2005) and are largely released by T cells activated in psoriasis. As we have mentioned above, both adaptative and innate immunity share common effector molecules, particularly cytokines and chemokines, and recruitment of lymphocytes in skin also plays a relevant role in initiating and amplifying the inflammatory process in psoriatic lesions (Cribier, 2006; Gaspari, 2006). Thus, the ability of TA to decrease the production of TNF-α, IL-1β and IL-2 in mouse skin may be relevant to the control of inflammatory processes observed in psoriatic lesions.
Finally, we demonstrated that TA was capable of suppressing TPA-stimulated NF-κB activation in mouse skin. It is noteworthy that this nuclear factor controls the expression of genes encoding IL-2, IL-1 and TNF-α. In addition, TNF-α can likewise induce IL-1β and IL-2 in psoriatic skin (Bos et al., 2005; Kim et al., 2006). From the present data, the inhibition of NF-κB activation and downstream NF-κB-dependent pro-inflammatory pathways could be the hallmarks of the mode of action of TA. A complex regulatory scheme controls Rel/NF-κB transcriptional activity in skin. One of the most well characterized mechanism is the control of NF-κB subcellular localization by IκB family proteins. Phosphorylation of the inhibitory proteins, the initial regulated step of IκB degradation and thus of NF-κB activation, is mediated by cytosolic high molecular weight complexes, collectively termed the IκB kinase complex (Yamamoto and Gaynor, 2001; Lizzul et al., 2005). Beside this classical activation, alternative pathways have also been described (Bell et al., 2002; Gloire et al., 2006). Therefore, further studies will be required to clarify whether TA specifically blocks activation of epidermal NF-κB and the upstream intracellular signal-transduction pathways that are influenced by TA.
In summary, our results suggest that TA could be a promising antipsoriatic agent because it inhibits, in vitro and in vivo, several biomarkers related to the inflammatory response of psoriatic skin. Its mechanism of action is related to the inhibition of NF-κB activation and can be mediated by the downregulation of intracellular signal-transduction pathways influenced by ROS, TNF-α and arachidonic acid metabolism.
Acknowledgments
M Amigó was the recipient of a Research Fellowship from FPU program of Spanish Ministerio de Educación y Ciencia. This work was supported in part by Grant GV04B74 from Generalitat Valenciana and FIS-P1051659. The authors are grateful to Aitana Braza-Boïls (Department of Pharmacology, University of Valencia), and Carmine Iodice (Istituto di Chimica Biomolecolare CNR, Napoli, Italy), for the technical assistance to obtain the tissue sections and the avarol derivative, respectively.
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- DTT
dithiothreitol
- EMSA
electrophoretic mobility-shift assay
- FCS
fetal calf serum
- HTAB
hexadecyltrimethylammonium bromide
- IL
interleukin
- LO
lipoxygenase
- LPS
lipopolysaccharide
- LTB4
leukotriene B4
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MPO
myeloperoxidase
- NF-κB
nuclear factor-κB
- PGE2
prostaglandin E2
- PMSF
phenylmethyl sulphonyl fluoride
- ROS
reactive oxygen species
- sPLA2
secretory phospholipase A2
- TA
avarol-3′-thiosalicylate
- TNF-α
tumour necrosis factor-α
- TPA
12-O-tetradecanoylphorbol-13-acetate
Conflict of interest
The authors state no conflict of interest.
References
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- Amigó M, Terencio MC, Mitova M, Iodice C, Payá M, De Rosa S. Potential antipsoriatic avarol derivatives as antioxidants and inhibitors of PGE(2) generation and proliferation in the HaCaT cell line. J Nat Prod. 2004;67:1459–1463. doi: 10.1021/np049873n. [DOI] [PubMed] [Google Scholar]
- Andersen S, Sjursen W, Laegreid A, Volden G, Johansen B. Elevated expression of human nonpancreatic phospholipase A2 in psoriatic tissue. Inflammation. 1994;18:1–12. doi: 10.1007/BF01534593. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Solhaug A, Johansen B. Functional coupling between secretory and cytosolic phospholipase A2 modulates tumor necrosis factor-alpha and interleukin-1beta-induced NF-kappa B activation. J Biol Chem. 2001;276:30527–30536. doi: 10.1074/jbc.M008481200. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Darragh J. Signaling downstream of p38 in psoriasis. J Invest Dermatol. 2006;126:1689–1691. doi: 10.1038/sj.jid.5700280. [DOI] [PubMed] [Google Scholar]
- Asadullah K, Volk HD, Sterry W. Novel immunotherapies for psoriasis. Trends Immunol. 2002;23:47–53. doi: 10.1016/s1471-4906(01)02119-6. [DOI] [PubMed] [Google Scholar]
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- Banno T, Gazel A, Blumenberg M. Pathway-specific profiling identifies the NF-kappa B-dependent tumor necrosis factor alpha-regulated genes in epidermal keratinocytes. J Biol Chem. 2005;280:18973–18980. doi: 10.1074/jbc.M411758200. [DOI] [PubMed] [Google Scholar]
- Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand H. Involvement of NF-κB signalling in skin physiology and disease. Cell Signalling. 2002;15:1–7. doi: 10.1016/s0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- Betts WH.Detecting oxy radicals by chemiluminescence Handbook of Methods for Oxygen Radical Research 1985CRC Press: Boca Ratón; 197–201.In: Greenwald R (eds). [Google Scholar]
- Borenfreund E, Babick H, Martin-Alguacil N. Comparison of two in vitro cytotoxicity assays. The natural red (NR) and tetrazolium (MTT) test. Toxic In Vitro. 1988;2:1–6. doi: 10.1016/0887-2333(88)90030-6. [DOI] [PubMed] [Google Scholar]
- Bos JD, De Rie MA, Teunissen MB, Piskin G. Psoriasis: dysregulation of innate immunity. Br J Dermatol. 2005;152:1098–1107. doi: 10.1111/j.1365-2133.2005.06645.x. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- Bustos G, Ferrándiz ML, Sanz MJ, Payá M, Alcaraz MJ. A study of the novel anti-inflammatory agent florifenine topical anti-inflammatory activity and influence on arachidonic acid metabolism and neutrophil functions. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:298–304. doi: 10.1007/BF00233250. [DOI] [PubMed] [Google Scholar]
- Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G. The NF-kappaB signaling pathway in human genetic diseases. Cell Mol Life Sci. 2005;62:1682–1691. doi: 10.1007/s00018-005-5031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribier B. Psoriasis under the microscope. J Eur Acad Dermatol Venereol. 2006;20:3–9. [Google Scholar]
- De Rosa S.Mediterranean marine organisms as source of new potential drugs Natural Products in the New Millennium: Prospects and Industrial Application 2002Kluwer Academic Press: Dordrecht; 441–461.In: Rauter AP et al. (eds). [Google Scholar]
- De Vry CG, Valdez M, Lazarov M, Muhr E, Buelow R, Fong T, et al. Topical application of a novel immunomodulatory peptide, RDP58, reduces skin inflammation in the phorbol ester-induced dermatitis model. J Invest Dermatol. 2005;125:473–481. doi: 10.1111/j.0022-202X.2005.23831.x. [DOI] [PubMed] [Google Scholar]
- Ferrándiz ML, Sanz MJ, Bustos G, Payá M, Alcaraz MJ, De Rosa S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur J Pharmacol. 1994;253:75–82. doi: 10.1016/0014-2999(94)90759-5. [DOI] [PubMed] [Google Scholar]
- Franson R, Patriarca P, Elsbach P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res. 1974;15:380–388. [PubMed] [Google Scholar]
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–S80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;30:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53:S3–S16. doi: 10.1016/j.jaad.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Haas U, Podda M, Behne M, Gurrieri S, Alonso A, Furstenberg G, et al. Characterization and differentiation-dependent regulation of secreted phospholipases A in human keratinocytes and in healthy and psoriatic human skin. J Invest Dermatol. 2005;124:204–211. doi: 10.1111/j.0022-202X.2004.23513.x. [DOI] [PubMed] [Google Scholar]
- Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001;108:527–536. doi: 10.1172/JCI12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Keum YS, Seo HJ, Chun KS, Lee SS, Surh YJ. Capsaicin suppresses phorbol ester-induced activation of NF-kappaB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. 2001;164:119–126. doi: 10.1016/s0304-3835(01)00378-0. [DOI] [PubMed] [Google Scholar]
- Ikai K. Psoriasis and the arachidonic acid cascade. J Dermatol Sci. 1999;21:135–146. doi: 10.1016/s0923-1811(99)00042-0. [DOI] [PubMed] [Google Scholar]
- Johansen C, Flindt E, Kragballe K, Henningsen J, Westergaard M, Kristiansen K, et al. Inverse regulation of the nuclear factor-kappaB binding to the p53 and interleukin-8 kappaB response elements in lesional psoriatic skin. J Invest Dermatol. 2005;124:1284–1292. doi: 10.1111/j.0022-202X.2005.23749.x. [DOI] [PubMed] [Google Scholar]
- Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Kohler HB, Huchzermeyer B, Martin M, De Bruin A, Meier B, Nolte I. TNF-alpha dependent NF-kappa B activation in cultured canine keratinocytes is partly mediated by reactive oxygen species. Vet Dermatol. 2001;12:129–137. doi: 10.1046/j.1365-3164.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–23. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- Lan CC, Yu HS, Wu CS, Kuo HY, Chai CY, Chen GS. FK506 inhibits tumour necrosis factor-alpha secretion in human keratinocytes via regulation of nuclear factor-kappaB. Br J Dermatol. 2005;153:725–732. doi: 10.1111/j.1365-2133.2005.06779.x. [DOI] [PubMed] [Google Scholar]
- Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, et al. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005;124:1275–1283. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- Lo CJ. MAPK regulation of prostaglandin E2 production by lipopolysaccharide-stimulated macrophages is not dependent on nuclear factor kappaB. J Surg Res. 2003;113:189–194. doi: 10.1016/s0022-4804(03)00186-0. [DOI] [PubMed] [Google Scholar]
- Moriyama H, Tsukida T, Inoue Y, Yokota K, Yoshino K, Kondo H, et al. Azasugar-based MMP/ADAM inhibitors as antipsoriatic agents. J Med Chem. 2004;47:1930–1938. doi: 10.1021/jm0304313. [DOI] [PubMed] [Google Scholar]
- Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol. 1988;40:787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Moustafa M, Szabo M, Ghanem GE, Morandini R, Kemp EH, MacNeil S, et al. Inhibition of tumor necrosis factor-alpha stimulated NFkappaB/p65 in human keratinocytes by alpha-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol. 2002;119:1244–1253. doi: 10.1046/j.1523-1747.2002.19602.x. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends Mol Med. 2005;11:43–48. doi: 10.1016/j.molmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Secretory phospholipase A2. Biol Pharm Bull. 2004;27:1158–1164. doi: 10.1248/bpb.27.1158. [DOI] [PubMed] [Google Scholar]
- Murakawa M, Yamaoka K, Tanaka Y, Fukuda Y. Involvement of tumor necrosis factor (TNF)-alpha in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem Pharmacol. 2006;71:1331–1336. doi: 10.1016/j.bcp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–1675. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DA. Mechanisms of action of topical therapies and the rationale for combination therapy. J Am Acad Dermatol. 2005;53:S17–S25. doi: 10.1016/j.jaad.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Oberyszyn TM, Tober KL, Ross MS, Robertson FM. Inhibitory effects of pentoxifylline on ultraviolet B light-induced cutaneous inflammation. Mol Carcinog. 1998;22:16–25. [PubMed] [Google Scholar]
- Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4:517–519. doi: 10.2174/1568010054526386. [DOI] [PubMed] [Google Scholar]
- Payá M, Halliwell B, Hoult JR. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem Pharmacol. 1992;44:205–214. doi: 10.1016/0006-2952(92)90002-z. [DOI] [PubMed] [Google Scholar]
- Pennanen N, Lapinjoki S, Palander A, Urtti A, Monkkonen J. Macrophage-like RAW 264 cell line and time-resolved fluoroimmunoassay (TRFIA) as tools in screening drug effects on cytokine secretion. Int J Immunopharmacol. 1995;17:475–480. doi: 10.1016/0192-0561(95)00030-6. [DOI] [PubMed] [Google Scholar]
- Pittelkow MR. Psoriasis: more than skin deep. Nat Med. 2005;11:17–18. doi: 10.1038/nm0105-17. [DOI] [PubMed] [Google Scholar]
- Posadas I, Terencio MC, Guillen I, Ferrándiz ML, Coloma J, Payá M, et al. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:98–106. doi: 10.1007/s002109900150. [DOI] [PubMed] [Google Scholar]
- Quivy V, Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Reynolds NJ, McCombie SW, Shankar BB, Bishop WR, Fisher GJ. SCH 47112, a novel staurosporine derivative, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and epidermal hyperplasia in hairless mouse skin. Arch Dermatol Res. 1997;289:540–546. doi: 10.1007/s004030050236. [DOI] [PubMed] [Google Scholar]
- Rioja I, Terencio MC, Ubeda A, Molina P, Tarraga A, Gonzalez-Tejero A, et al. A pyrroloquinazoline derivative with anti-inflammatory and analgesic activity by dual inhibition of cyclo-oxygenase-2 and 5-lipoxygenase. Eur J Pharmacol. 2002;434:177–185. doi: 10.1016/s0014-2999(01)01539-4. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. The inflammatory response in mild and in severe psoriasis. Br J Dermatol. 2004;150:917–928. doi: 10.1111/j.1365-2133.2004.05984.x. [DOI] [PubMed] [Google Scholar]
- Rys-Sikora KE, Konger RL, Schoggins JW, Malaviya R, Pentland AP. Coordinate expression of secretory phospholipase A(2) and cyclooxygenase-2 in activated human keratinocytes. Am J Physiol Cell Physiol. 2000;278:C822–C833. doi: 10.1152/ajpcell.2000.278.4.C822. [DOI] [PubMed] [Google Scholar]
- Rys-Sikora KE, Pentland AP, Konger RL. Pertussis toxin-sensitive secretory phospholipase A2 expression and motility in activated primary human keratinocytes. J Invest Dermatol. 2003;120:86–95. doi: 10.1046/j.1523-1747.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Nakayama Y, Yamashita C, Uno H. Anti-inflammatory effects of tacalcitol (1,24(R)(OH)2D3, TV-02) in the skin of TPA-treated hairless mice. J Dermatol. 2004;31:200–217. doi: 10.1111/j.1346-8138.2004.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Sawa M, Tsukamoto T, Kiyoi T, Kurokawa K, Nakajima F, Nakada Y, et al. New strategy for antedrug application: development of metalloproteinase inhibitors as antipsoriatic drugs. J Med Chem. 2002;45:930–936. doi: 10.1021/jm010349c. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK., III Biology of tumor necrosis factor-alpha- implications for psoriasis. Exp Dermatol. 2004;13:193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Sipkema D, Osinga R, Schatton W, Mendola D, Tramper J, Wijffels RH. Large-scale production of pharmaceuticals by marine sponges: sea, cell, or synthesis. Biotechnol Bioeng. 2005;90:201–222. doi: 10.1002/bit.20404. [DOI] [PubMed] [Google Scholar]
- Sjursen W, Brekke OL, Johansen B. Secretory and cytosolic phospholipase A(2)regulate the long-term cytokine-induced eicosanoid production in human keratinocytes. Cytokine. 2000;12:1189–1194. doi: 10.1006/cyto.1999.0727. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson JE, Randall RW, Reynolds CH, Jackson WP, Bhattacherjee P, Salmon JA, et al. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br J Pharmacol. 1988;94:528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommesen L, Sjursen W, Gasvik K, Hanssen W, Brekke OL, Skattebol L, et al. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- Umezawa K, Chaicharoenpong C. Molecular design and biological activities of NF-kappaB inhibitors. Mol Cells. 2002;14:163–167. [PubMed] [Google Scholar]
- Victor FC, Gottlieb AB, Menter A. Changing paradigms in dermatology: tumor necrosis factor alpha (TNF-alpha) blockade in psoriasis and psoriatic arthritis. Clin Dermatol. 2003;21:392–397. doi: 10.1016/j.clindermatol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Inaloz HS, Baysal V, Delibas N. The role of oxidants and antioxidants in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:34–36. doi: 10.1046/j.1468-3083.2003.00641.x. [DOI] [PubMed] [Google Scholar]