Abstract
Background and purpose:
L-type calcium channels (Ca (V)1.2) play an important role in cardiac contraction. Roscovitine, a cyclin-dependent kinase inhibitor and promising anticancer drug, has been shown to affect Ca (V)1.2 by inhibiting current amplitude and slowing activation. This research investigates the mechanism by which roscovitine inhibits Ca (V)1.2 channels.
Experimental approach:
Ca (V)1.2 channels were transfected into HEK 293 cells, using the calcium phosphate precipitation method, and currents were measured using the whole-cell patch clamp technique.
Key results:
Roscovitine slows activation at all voltages, which precludes one previously proposed mechanism. In addition, roscovitine enhances voltage-dependent, but not calcium-dependent inactivation. This enhancement resulted from both an acceleration of inactivation and a slowing of the recovery from inactivation. Internally applied roscovitine failed to affect Ca (V)1.2 currents, which supports a kinase-independent mechanism and extracellular binding site. Unlike the dihydropyridines, closed state inactivation was not affected by roscovitine. Inactivation was enhanced in a dose-dependent manner with an IC50=29.5±12 μM, which is close to that for slow activation and inhibition.
Conclusions and implications:
We conclude that roscovitine binds to an extracellular site on Ca (V)1.2 channels to inhibit current by both slowing activation and enhancing inactivation. Purine-based drugs could become a new option for treatment of diseases that benefit from L-channel inhibition such as cardiac arrhythmias and hypertension.
Keywords: voltage-dependent inactivation, slowed activation, enhanced inactivation, whole-cell patch clamp, cardiac action potential, arrhythmia
Introduction
L-type calcium channels (Ca(V)1) play a vital role in regulating a wide range of cellular processes such as cardiac and smooth muscle cell contraction (Bodi et al., 2005), hormone secretion (Bourinet et al., 2004) and gene transcription (Gomez-Ospina et al., 2006). The cardiac L-channel (Ca(V)1.2) generates the Ca2+ influx that shapes the cardiac action potential and triggers Ca2+ release from the sarcoplasmic reticulum (Roden et al., 2002) to drive contraction. Drugs that target Ca(V)1.2 channels are important for the treatment of certain types of arrhythmias as well as hypertension (Roden et al., 2002; Elmslie, 2004).
It has been recently shown that roscovitine, a membrane permanent inhibitor of cyclin-dependent kinases (Meijer et al., 1997), modulates Ca(V)channels in a kinase-independent manner. Roscovitine has been shown to increase neurotransmitter release in central and peripheral neurons (Yan et al., 2002; Cho and Meriney, 2006) through slowed deactivation of Ca(V)2 channels (Yan et al., 2002; Buraei et al., 2005, 2007), which resulted in enhanced calcium influx into nerve terminals. Therefore, roscovitine can be considered an agonist drug for Ca(V)2 channels (Buraei et al., 2007). It has also been demonstrated that roscovitine inhibited voltage-dependent potassium (K(V)) channels by blocking the open channels, and inhibited Ca(V)1.2 channels while slowing activation (Buraei et al., 2007).
Roscovitine is currently undergoing phase II clinical trials as an anticancer treatment (Fischer and Gianella-Borradori, 2003). Thus, it is important to explore the inhibitory effects of roscovitine on Ca(V)1.2 channels so that we can predict the potential impact of this drug on cardiovascular function. We show that therapeutic concentrations of roscovitine inhibit these channels by slowing their activation and enhancing their inactivation. Further, roscovitine slows down the recovery from inactivation of the Ca(V)1.2 current. We conclude that the combination of slowed activation and enhanced inactivation is responsible for the inhibition of Ca(V)1.2 channel activity.
Materials and methods
HEK293 cell transfection
We utilized the calcium phosphate precipitation method to transfect HEK293 cells with cardiac Ca(V)1.2 channels, which provided highly reproducible expression over 24–72 h after transfection. HEK293 cells were maintained in standard Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic solution at 37°C in 5% CO2 incubator. For transfection, the medium was changed to DMEM/F-12 containing 10% FBS. HEK293 cells were transfected by adding 1 ml of precipitated transfecting solution containing 4-(2-hydroxyethyl)-1-piperazine ethane sulphonic acid (HEPES)-buffered saline (HeBS), 50 mM CaCl2 and cDNA plasmids pcDNA3 (Invitrogen, Carlsbad, CA, USA) as follow: 11 μg α1C (rabbit heart, GenBank accession no. X15539), 8.5 μg α2δ, 5.5 μg β1b, 2.15 μg TAG (to increase expression efficiency) and 1 μg green fluorescent protein (to visualize transfected cells) (provided by Dr Blaise Peterson, Penn State College of Medicine, Hershey, PA, USA). Cells were incubated for 8 h after which the medium was replaced by the standard DMEM. The 35-mm dishes served as the recording chamber.
Measurement of ionic currents
Cells were voltage-clamped using the whole-cell configuration of the patch clamp technique. Pipettes were pulled from Schott 8250 glass (Garner Glass, Claremont, CA, USA) on a Sutter P-97 puller (Sutter Instruments Co., Novato, CA, USA). Series resistance ranged from 2 to 15 MΩ, and was compensated at 80% to yield a mean voltage error of 1.2±0.8 mV (±s.d., n=40). Currents were recorded using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA, USA) and digitized with ITC-18 data acquisition interface (InstruTECH Corporation, Port Washington, NY, USA). Experiments were controlled by a Power Macintosh G3 computer (Apple Computer, Cupertino, CA, USA) running S5 data acquisition software written by Dr Stephen Ikeda (NIH, NIAAA, Bethesda, MD, USA). Leak current was subtracted online using a P/4 protocol. All recordings were carried out at room temperature and unless otherwise noted the holding potential was −120 mV. Whole-cell currents were digitized depending on step duration at 50 (25 ms), 10 (200 ms) and 4 kHz (300 and 1000 ms) after analog filtering at 1–10 kHz.
Electrophysiological data analysis
These data were analyzed using IgorPro (WaveMetrics, Lake Oswego, OR, USA) running on a Macintosh computer. Step currents were measured as the average of 1 ms of current at the end of the voltage step. Tail currents were measured as the average of 0.3 ms of current beginning 0.3 ms following hyperpolarization to −60 mV. Activation τ (τAct) was determined by fitting a single exponential function to the step current after a 0.3 ms delay (Buraei et al., 2007). Inactivation τ (τInact) was determined by fitting a single exponential function from peak step current to the end of the step.
Solutions
For the transfection, HeBS contained (in mM) 140 NaCl, 25 HEPES, 1.4 Na2HPO4, with pH 7.10 adjusted using 5 N NaOH. The internal pipette solution contained (in mM) 104 N-methyl-D-glucamine (NMG)-Cl, 14 creatine-PO4, 6 MgCl2, 10 NMG-HEPES, 5 Tris-ATP, 0.3 Tris-GTP and 10 NMG-glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid with osmolarity of 280 mosmol and pH 7.3. The external recording solution contained (in mM) 100 NMG-Cl, 10 NMG-HEPES and 30 BaCl2. For some experiments, 30 mM BaCl2 was replaced with 1 MgCl2 and either 10 BaCl2 or 10 CaCl2. The NMG-Cl concentration was adjusted to maintain osmolarity of 300 mosmol and pH was set to 7.3 using NMG base. Roscovitine was prepared as a 50 mM stock solution in DMSO and stored at −30°C. All external solutions contained the same DMSO concentration so that the roscovitine concentration was the sole variable when changing solutions. Test solutions were applied from a gravity-fed perfusion system with an exchange time of 1–2 s.
Statistical analysis
Group data were calculated and are presented as mean±s.d. throughout the paper. The paired t-test was used for within-cell comparisons.
Chemicals
All experiments utilized R-roscovitine (referred to as roscovitine) from LC Labs (Woburn, MA, USA). DMEM/F12, DMEM, FBS, 100 × antibiotic–antimycotic were from Invitrogen. Other chemicals were obtained from Sigma (St Louis, MO, USA).
Results
Effects of roscovitine on activation of Ca(V)1.2 channels
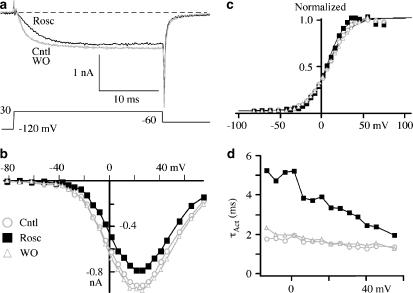
It was shown previously that roscovitine inhibited step current and slowed activation of Ca(V)1.2 channels (Buraei et al., 2007). We have extended this observation to show the effect of 100 μM roscovitine over a range of voltages (Figures 1a and b). The inhibition was similar across the current–voltage relationship (I–V; Figure 1b), so that there was no obvious voltage dependence to the roscovitine-induced inhibition. On average, peak current (+30 mV) was inhibited 26.3±8.2%, while current at +60 mV was inhibited 33.1±14.3% (n=6) by 100 μM roscovitine. The activation–voltage relationship was measured from tail currents (−60 mV) and was well fitted by a single Boltzmann equation (Figure 1c). Roscovitine slightly, but significantly, left-shifted V1/2, which was 12.0±0.5, 9.0±0.5 and 13.6±0.8 mV (P<0.01, n=4) for control, 100 μM roscovitine and recovery, respectively. The voltage dependence of activation became slightly more steep in roscovitine with activation changing e-fold for 17.1±0.4, 15.1±0.4 and 18.2±0.5 mV (P<0.01, n=4) for control, 100 μM roscovitine and recovery, respectively. The inhibitory effect of roscovitine was accompanied by slowed activation at all voltages generating measurable current (Figure 1d). The activation τ (τAct) was more than doubled at hyperpolarized voltages by 100 μM roscovitine, but this increase declined with depolarization (Figure 1d). However, τAct was significantly higher even at the most depolarizing voltage examined (+50 mV, P<0.001, n=9).
Figure 1.
Roscovitine slowed activation of Ca(V)1.2 channels. (a) Ca(V)1.2 currents were activated during 25-ms voltage steps to 30 mV. Roscovitine (Rosc; 100 μM) induced a slowing of activation and inhibition compared to currents before (Cntl) and upon recovery from (WO) roscovitine application. (b) The current–voltage (I–V) relationship shows roscovitine-induced inhibition across all current-generating voltages compared to control and washout. (c) The activation–voltage relationship was measured from tail currents and is shown normalized to maximum current to highlight the small changes induced by roscovitine (symbols are same as in b). The smooth lines are fits using a single Boltzmann function with V1/2=7.9, 6.6 and 9.1 mV, and slope (k)=12.1, 9.9 and 12.9 for control, roscovitine and washout, respectively. (d) Activation τ (τAct) was generated from single exponential fits to current activation (after a 0.3-ms delay) in control, 100 μM roscovitine and washout (symbols are same as in b).
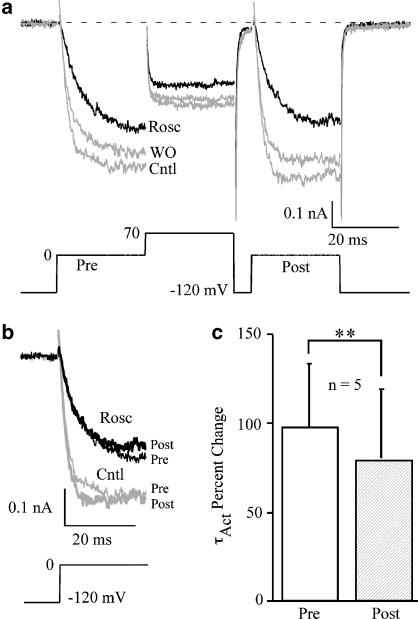
We speculated previously that the roscovitine-induced slowed activation could result from voltage-dependent dissociation of roscovitine from Ca(V)1.2 channel (Buraei et al., 2007), which is analogous to G-protein-mediated inhibition of Ca(V)2.2 channels (Elmslie et al., 1990). The absence of obvious voltage dependence to inhibition measured from the I–V relationship failed to support this idea (Figure 1b). However, a more direct test comes from examining the effect of a strong depolarizing conditioning pulse on inhibition (Elmslie et al., 1990). If roscovitine binding is voltage-dependent, the conditioning pulse will induce dissociation so that current activates normally during a postpulse delivered 10 ms latter. On the contrary, as shown in Figure 2, activation was similarly affected by 100 μM roscovitine before (prepulse) and after (postpulse) a depolarizing pulse to 0 mV. Prepulse activation was slowed from 3.0±0.6 to 5.6±0.4 ms (P<0.001, n=5) and postpulse activation was slowed from 2.6±0.5 to 4.5±0.5 ms (P<0.01, n=5). The percent change of τAct was slightly, but significantly, reduced for the postpulse relative to the prepulse (Figure 2c), but the persistence of significant slowing of postpulse activation suggested that roscovitine did not dissociate from Ca(V)1.2 channels in a voltage-dependent manner.
Figure 2.
Roscovitine did not dissociate from Ca(V)1.2 channels upon channel opening. (a) Ca(V)1.2 currents were evoked by two 25-ms steps to 0 mV (prepulse (Pre) and postpulse (Post)) bracketing a +70-mV conditioning step in control, 100 μM roscovitine and washout. The interval between the conditioning step and postpulse was 10 ms. (b) Superimposed prepulse- and postpulse-evoked currents show slowed activation in the presence of 100 μM roscovitine compared to control. (c) Roscovitine (100 μM) increased activation τ (τAct) for both prepulse and postpulse stimulation, although these results were significantly different (mean±s.d. **P<0.01, n=5).
Roscovitine enhances inactivation of Ca(V)1.2
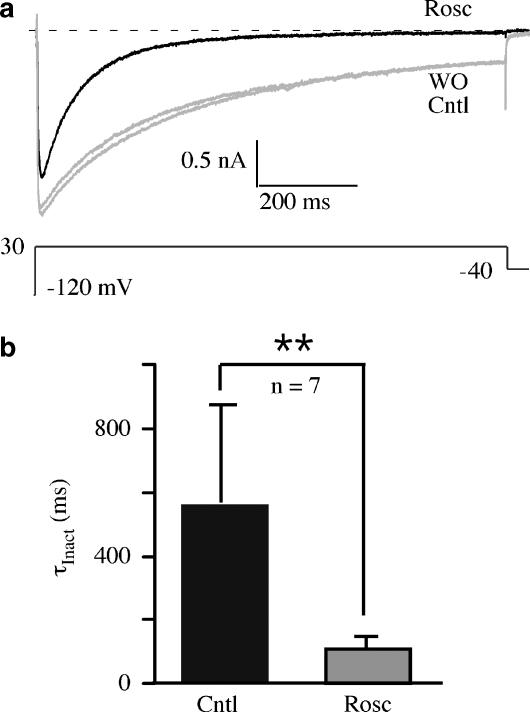
Close inspection of the roscovitine effect on 25 ms voltage steps revealed that inhibition was largest at both the beginning and end of the step (Figure 1a). The late increase of inhibition could be explained by roscovitine-induced enhancement of voltage-dependent inactivation (VDI), since these currents were recorded in solutions containing Ba2+ to eliminate Ca2+-dependent inactivation (CDI). Currents induced by 1-s voltage steps showed both increased magnitude and speed of apparent inactivation in the presence of 100 μM roscovitine (Figure 3). The effect was quantified by calculating the IEnd/IPeak ratio, where IEnd was measured at the end of the 1-s step and IPeak was measured at peak step current. This ratio, which indicates the fraction of current remaining at the end of the step, was reduced from 0.53±0.20 to 0.16±0.09 in control vs roscovitine, respectively (n=7, P<0.001). Single exponential fitting of inactivation showed that the inactivation τ (τInact) was decreased by 100 μM roscovitine (Figure 3b). The roscovitine-induced enhancement of apparent inactivation was fully developed within 10 s of application and complete recovery was observed in ⩽30 s of washout (data not shown). The speed of this effect is similar to that observed for slowed activation and inhibition (Buraei et al., 2007), and suggests the possibility that roscovitine interacts with an extracellular site to enhance Ca(V)1.2 channel inactivation.
Figure 3.
Roscovitine-enhanced inactivation of Ca(V)1.2 channels. (a) Ca(V)1.2 currents evoked by 1-s steps to 30 mV show the enhancement of inactivation by 100 μM roscovitine relative to control and washout. (b) Mean and s.d. of inactivation τ (τInact) obtained from fitting a single exponential function to inactivating currents in control and 100 μM roscovitine. Data are significantly different (**P<0.01, n=7).
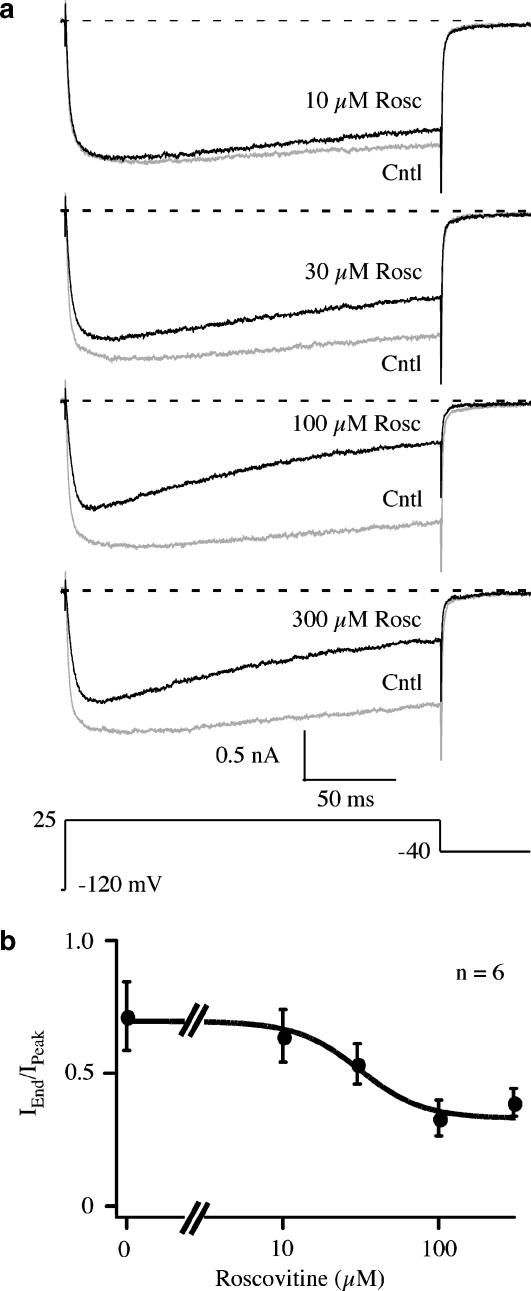
Roscovitine blocks potassium channels by binding to open channels, which gives the appearance of enhanced inactivation (Buraei et al., 2007). Thus, it is possible that enhanced inactivation of Ca(V)1.2 channels resulted from open-state block. This was tested by measuring the effect of 10–300 μM roscovitine on Ca(V)1.2 channel inactivation (Figure 4). The speed of an open-channel effect is directly related to the concentration by the relationship τ−1=kOff+kOn[Rosc], where kOff and kOn are rate constants, and [Rosc] is roscovitine concentration (Buraei et al., 2005). Thus, both the magnitude and speed of apparent inactivation should increase with concentration if roscovitine blocks open Ca(V)1.2 channels. On the other hand, roscovitine could be binding to a site on Ca(V)1.2 channels to increase VDI, which predicts saturation of the effect at high roscovitine concentrations. Inactivation, measured as the IEnd/IPeak ratio, increased with roscovitine concentration, but saturated at concentration ⩾100 μM. IEnd/IPeak ratio was well fitted by the Hill equation yielding an EC50=29.5±12.0 μM and a Hill coefficient=2.3±2.8 (Figure 4b; n=6), which supports a roscovitine enhancement of VDI. For comparison, we also measured the dose–response relationship of the other roscovitine-induced effects and found for slow activation (+10 mV), an EC50 of 20.9±1.2 μM and Hill coefficient of 1.2±0.1 For inhibition (+30 mV) measured at 25 ms, the IC50 was 23.4±4.8 μM, with Hill coefficient of 1.6±0.5. Thus, it is possible that all three effects result from roscovitine binding to a single site with an apparent affinity of 20–30 μM. The differences in Hill coefficient (1.2–2.3) could result from separate binding sites, but statistical analysis showed no significant differences between these values (analysis of variance with Tukey HSD).
Figure 4.
The roscovitine-induced enhancement of inactivation saturated at high drug concentrations. (a) Representative traces from a single cell show the enhancement of inactivation induced by application of 10, 30, 100 and 300 μM roscovitine compared to control. Currents were evoked by 200-ms steps to 25 mV. (b) Inactivation was quantified as the IEnd/IPeak ratio, where IEnd was measured at the end of the 200-ms step and IPeak was measured at the peak current during the step. The smooth line is a fit using the Hill equation with an EC50=29.5 μM and a Hill coefficient of 2.3. Data are presented as mean±s.d. of six cells.
Roscovitine enhances inactivation of Ca(V)1.2 channels by interacting with externally located binding site(s)
Roscovitine is known to inhibit intracellularly located cyclin-dependent kinases (Meijer et al., 1997), and altered calcium channel phosphorylation can affect inactivation (Werz et al., 1993). However, the speed of the roscovitine effect is consistent with an externally facing binding site (Buraei et al., 2005). To test this hypothesis further, 300 μM roscovitine was added to the internal solution and currents were elicited by 200 ms voltage steps (+10 mV) to examine both activation and inactivation. Control cells were dialyzed with internal solutions containing 0.6% DMSO to control for vehicle. We found no significant difference in the IEnd/IPeak ratio between control (0.75±0.14, n=8) and internally applied roscovitine (0.65±0.11, n=8, not significant). In addition, internal roscovitine failed to alter the effect of externally applied roscovitine (100 μM). The fraction of current remaining in external roscovitine was 0.40±0.10 for internal roscovitine vs 0.36±0.07 for DMSO control (n=8; not significant). Activation was also not altered by internally applied roscovitine (τAct=2.42±0.69 vs control τAct=3.0±1.1 ms; n=8, not significant) and external roscovitine significantly slowed activation with either internal roscovitine (τAct=4.6±0.9 ms) or DMSO (τAct=4.2±0.9 ms; n=8). This evidence supports an extracellular binding site for the roscovitine-induced effects on Ca(V)1.2 channels.
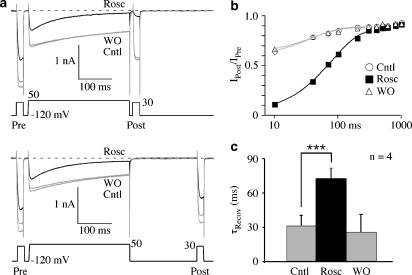
Roscovitine slows recovery from inactivation
We observed that roscovitine speeds inactivation, but recovery from inactivation could also be affected. Recovery from inactivation was examined using a three-pulse protocol, where the first (pre) and third (post) pulses were set to +30 mV (peak current) and the second (inactivating) pulse was +50 mV (duration=300 ms). The interval between the inactivating pulse and postpulse was varied and the IPost/IPre ratio was monitored to follow the recovery from inactivation, which was composed of fast and slow components (Figure 5). The fast component ranged from 80 to 100% of total recovery time course with mean relative amplitude=93±7% in control and 87±2% in roscovitine (n=4, not significant). Roscovitine significantly slowed the fast component of recovery, but had little or no effect on the slow component. The fast recovery τ (τRecov) was increased to more than double the control value in 100 μM roscovitine (Figure 5c). The roscovitine-induced increase of inactivation thus appears to result from both accelerated inactivation and slowed recovery from inactivation.
Figure 5.
Roscovitine slowed recovery from inactivation. (a) This set of Ca(V)1.2 currents shows the recovery from inactivation for 10-ms (top) and 200-ms (bottom) intervals between the inactivating pulse and postpulse. Roscovitine (100 μM) increased inactivation during the 300-ms step to 50 mV and slowed recovery from inactivation at −120 mV relative to control and washout. Pre=prepulse; Post=postpulse. (b) The IPost/IPre ratio is plotted vs recovery time for data from the same cell as in (a). Roscovitine (100 μM) significantly slowed the fast recovery component relative to control and washout. The smooth curves are fits using a single exponential function. (c) The mean and s.d. of recovery τ (τRecov) are shown for control, 100 μM roscovitine and washout. Data are significantly different (*** P<0.001, n=4).
The slowed recovery from inactivation suggests that roscovitine-induced inhibition could be frequency dependent. However, increasing stimulation frequency from 0.1 to 2 Hz (25 ms steps) did not alter the percent inhibition (∼22% for each condition) (see Supplementary Figure 1). This was expected since the slowed recovery from inactivation (τRecov∼72 ms) would not impact inhibition until the interval between stimuli was ⩽100 ms. Thus, use-dependent inhibition is not observed over the frequency range used to observe use-dependent block of Ca(V)1.2 current by phenylalkylamines and benzothiazepines (Hering et al., 1996; Johnson et al., 1996; Motoike et al., 1999; Bodi et al., 2002).
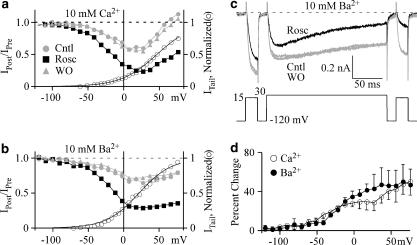
Roscovitine does not affect calcium-dependent inactivation
Our previous results used Ba2+ as the charge carrier to isolate VDI. To determine if CDI was also affected (Peterson et al., 1999, 2000), we compared the effect of 100 μM roscovitine on inactivation in either 10 mM Ca2+ or Ba2+. A three-pulse protocol, similar to that described above, was used to examine the voltage dependence of inactivation. The 200 ms inactivating pulse was varied from −120 to +80 mV and inactivation was measured from the IPost/IPre ratio. In control, inactivation in Ca2+ was minimal at hyperpolarized voltages, peaked at +20 mV and declined with further depolarization (Figure 6a), which mirrored Ca2+ influx as expected for CDI. Inactivation in Ba2+ increased monotonically with voltage as expected for an open-state inactivation mechanism typical for VDI (Figures 6b and c). Thus 100 μM roscovitine enhanced inactivation of Ca(V)1.2 channels in the presence of both external Ca2+ and Ba2+, but this could be explained by enhanced VDI that functions in Ca2+ as well as Ba2+ (Giannattasio et al., 1991). To determine if CDI was affected, we measured the percent effect of roscovitine with voltage (Figure 6d). If CDI was affected, we would expect to observed a peak in this relationship corresponding to peak CDI (+20 mV) in Ca2+, but not Ba2+. Contrary to this prediction, the percent enhancement of inactivation was not significantly different between Ca2+ and Ba2+ at any voltage, which demonstrates that roscovitine does not affect CDI. While VDI was enhanced, roscovitine did not alter voltage dependence as quantified by a single Boltzmann equation fitted to the data from −120 to +30 mV (30 mM Ba2+ external solution), which yielded V1/2 =16.0±5.1 and 16.0±5.2 mV and slope=−14.9±2.8 and −17.1±3.0 (n=6, not significant) for control and 100 μM roscovitine, respectively.
Figure 6.
Roscovitine enhanced voltage-dependent (VDI) but not calcium-dependent inactivation (CDI). (a) The IPost/IPre ratio (left axis) was measured as in Figure 5 and is plotted vs inactivation voltage to show inactivation in 10 mM Ca2+. Data are shown for control, 100 μM roscovitine and washout. The activation–voltage relationship in control (right axis, open circle) was measured as in Figure 1 and is superimposed here for comparison with the voltage dependence of inactivation. Data were collected in the presence of 10 mM Ca solution. (b) The voltage dependence of inactivation in 10 mM Ba2+ was measured as in (a). The same cell was first recorded in 10 mM Ca2+ (a), which was then replaced with 10 mM Ba2+ external solution. (c) Ca(V)1.2 currents evoked by the triple-pulse inactivation protocol used to generate the data of (a) and (b). The 200-ms inactivation pulse to +30 mV is flanked by two 25-ms steps to 15 mV (prepulse and postpulse). Currents were recorded in 10 mM Ba2+ external solution in control, 100 μM roscovitine and washout. (d) 100 μM roscovitine induced a monotonic increase of inactivation with voltage in both 10 mM Ca2+ (n=7) and Ba2+ (n=5). The roscovitine-induced percent change in the IPost/IPre ratio was calculated by averaging control and washout values. There was no significant difference in the roscovitine-induced percent change of inactivation between Ca2+ and Ba2+ at any voltage.
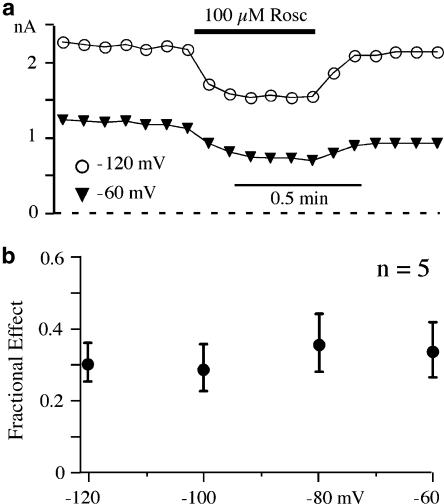
Roscovitine does not affect closed state inactivation
The correlation between the voltage dependence of activation and inactivation (Figure 6b) supports a roscovitine-induced enhancement of open-state inactivation (VDI). We also investigated the effect of roscovitine on closed-state inactivation, which is involved in dihydropyridine-induced inhibition (Bean, 1984; Sanguinetti and Kass, 1984). However, Ca(V)1.2 current inhibition induced by 100 μM roscovitine was not affected by altering the holding potential from −120 to −60 mV (Figures 7a and b). Holding potential was maintained at least 1 min before applying 100 μM roscovitine. The percent inhibition was 30.7±5.4% at −120 mV and 34.2±7.8% at holding potentials of −60 mV (n=5, not significant), despite a 50% reduction in available channels at holding potential of −60 vs −120 mV.
Figure 7.
Closed-state inactivation was not affected by roscovitine. (a) Time course of inhibition of currents evoked by 25-ms steps to 30 mV from holding potential of −120 vs −60 mV. (b) Average fractional of inhibition by 100 μM roscovitine of currents generated by steps to 30 mV from holding potentials ranging from −120 to −60 mV (20 mV increments). Data are presented as mean±s.d. of five cells.
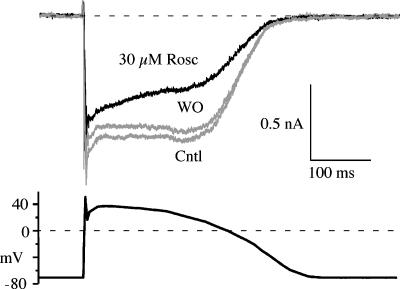
Roscovitine inhibited physiologically activated Ca(V)1.2 current
The slowing of activation and speeding of inactivation (τ=100 ms) suggest that roscovitine will inhibit Ca(V)1.2 current activated by the cardiac action potential. A cardiac action potential waveform was generated to match that measured from human ventricular myocytes (Li et al., 1999). Roscovitine inhibited current activated by the cardiac action potential in a time-dependent manner with inhibition increasing with duration as expected from enhanced inactivation (Figure 8). The charge influx carried by Ba2+, calculated by integrating inward current during the cardiac action potential, was inhibited by roscovitine in a concentration-dependent manner with inhibitions of 16.1±4.5, 30.0±6.8, 53.2±8.7 and 49.3±8.9% for 10, 30, 100 and 300 μM roscovitine, respectively. Hence, clinically relevant doses of roscovitine (10–50 μM; McClue et al., 2002; Hahntow et al., 2004; Raynaud et al., 2005) will significantly inhibit Ca(V)1.2 current.
Figure 8.
Roscovitine inhibited Ca(V)1.2 current activated by a cardiac action potential waveform. Currents are shown for control, washout and in 30 μM roscovitine.
Discussion
Roscovitine is a purine-based compound with a broad spectrum of effects. Initially used as a selective inhibitor of cyclin-dependent kinases (Meijer and Raymond, 2003), roscovitine has developed into a promising anticancer therapy (Hahntow et al., 2004; Raynaud et al., 2005; Benson et al., 2007). However, roscovitine has more recently been recognized as a drug that can affect voltage-dependent ion channels (Yan et al., 2002; Buraei et al., 2005, 2007; Cho and Meriney, 2006). It has been demonstrated previously that roscovitine inhibits and slows activation of Ca(V)1.2 current. We can now add the enhancement of VDI to roscovitine's effect on Ca(V)1.2 channels. This enhancement resulted from both speeding of inactivation and slowing of recovery from inactivation. Internally applied roscovitine failed to reproduce enhanced inactivation or slowed activation, which suggests an extracellularly exposed binding site. In addition, the similar EC50 (20–30 μM) for the roscovitine-induced effects was consistent with a single binding site mediating these effects, but verification awaits further investigation.
Roscovitine-induced slow activation of Ca(V)1.2 channels
Our results support and extend findings that roscovitine slows activation by binding to the closed state (Buraei et al., 2007). We had speculated previously that slowed activation could result from the voltage-dependent dissociation of roscovitine from Ca(V)1.2 channels upon channel opening (Buraei et al., 2007), which is analogous to G-protein-mediated inhibition of N-type (Ca(V)2.2) calcium channels (Elmslie et al., 1990). Our results demonstrate that this possibility is unlikely because activation was slowed at all voltages and current was similarly inhibited across voltages (Figure 1). In addition, activation was slowed in currents following a strong depolarizing pulse designed to activate fully Ca(V)1.2 channels. If roscovitine binding was voltage-dependent, we would expect the strong depolarizing pulse to induce complete dissociation, which should have resulted in normal activation of postpulse-evoked current (Elmslie et al., 1990). The absence of such an effect further supports the notion that roscovitine binding is voltage-independent. Thus, slowed activation is an effect induced by roscovitine occupying a site on Ca(V)1.2 channels.
The change in τAct with voltage was dramatically increased by roscovitine. The τAct was weakly voltage-dependent in control conditions (e-fold change for 125±11 mV, n=9) as expected if the voltage-independent closed–open transition was rate limiting for channel activation (Marks and Jones, 1992). In 100 μM roscovitine, τAct changed e-fold for 54±2 mV (n=9). The increased voltage dependence is likely to result from roscovitine-induced slowing of voltage-dependent close–close transitions, which become rate-limiting to channel opening. It is unlikely that open–close transitions were greatly affected since we found no effect of roscovitine on deactivation of Ca(V)1.2 channels, which suggests that mean open time was not affected. Slowed activation is responsible for the inhibition of Ca(V)1.2 channels at the beginning of the voltage step, but did not appear to have a large impact at early times during the cardiac action potential. The minimal early inhibition of the cardiac action potential-induced Ca(V)1.2 currents likely results from the very depolarized phase 0 and phase 1 portions of the cardiac action potential, which reaches voltages where τAct was less affected by roscovitine.
Roscovitine-enhanced inactivation
We investigated two possible mechanisms for the roscovitine-induced increase of apparent inactivation, open-channel block and enhanced VDI. K(V) channels show open-channel block by roscovitine, which appears as enhanced inactivation. Assuming a first-order reaction process, the speed of block should increase with concentration without saturation (Buraei et al., 2005). Contrary to this prediction, τInact saturates at roscovitine concentrations ⩾100 μM, which suggests that roscovitine binds to Ca(V)1.2 channels to enhance inactivation.
The majority of our studies on inactivation used Ba2+ to isolate VDI (Shi and Soldatov, 2002; Dafi et al., 2004). The magnitude and speed of VDI measured in our study corresponded well with VDI measured from cardiac L-channels (Ferreira et al., 2003). The correlation of roscovitine-enhanced inactivation with the voltage-dependent activation combined with the absence of an effect on holding potential-induced inactivation suggests that roscovitine enhances open-state inactivation (Faber et al., 2007). This enhancement appears to result from an increase in the open to inactivated rate constant and from a decrease in the effective recovery from inactivation rate constant, which combine to increase the number of inactivated channels at steady state. On the other hand, CDI was not affected since the voltage dependence of enhanced inactivation did not differ between Ca2+ and Ba2+ (Figure 6d).
Comparison with other Ca(V)1.2 antagonists
Phenylalkylamines (PAAs), such as gallopamil and verapamil, and benzothiazepines (BNZs), such as diltiazem, enhance VDI (Motoike et al., 1999; Sokolov et al., 2001), but the mechanism of roscovitine's action appears to be unique. For example, the block by PAA and BNZ is use-dependent as it builds with each pulse, but roscovitine block is rapid and nearly complete in the 10-s interval between sweeps. Use dependence results from these blockers preferentially binding to the inactivated and open states combined with slow dissociation so that the block develops with each depolarization that opens Ca(V)1.2 channels (Hockerman et al., 1997). However, roscovitine appears to bind to closed Ca(V)1.2 channels since activation is slowed.
PAAs are thought to bind within the inner vestibule of the Ca(V)1.2 channels, which helps explain use-dependent block and slow dissociation of these drugs from the channel (Hockerman et al., 1997). In contrast, BNZs are thought to bind to an extracellular site on Ca(V)1.2 channels (Hering et al., 1993; Seydl et al., 1993). In spite of this, the binding sites for PAA and BNZ appear to be either partially overlapping or allosterically linked, since mutation studies have identified amino acids that affect both (Hockerman et al., 1997; Striessnig et al., 1998). The absence of an effect of intracellularly applied roscovitine shows that the PAA site is not involved, while the apparent binding of roscovitine to closed channels (slowed activation) suggests that neither the PAA or BNZ sites are involved. On the basis of the similar EC50 for roscovitine-induced slow activation and enhanced inactivation, we tentatively conclude that roscovitine binds to a unique externally facing site to affect Ca(V)1.2 channel gating.
Potential cardiac effects of roscovitine
PAA and BNZ have proven useful as treatments for certain arrhythmias that result from Ca(V)1.2 channel-induced early afterdepolarization (Marban et al., 1986; Wu et al., 1999; Roden et al., 2002). However, the PAA verapamil has been shown to block K(V) channels (Zhang et al., 1999), which will increase cardiac action potential duration and can exacerbate, instead of ameliorate, the arrhythmias. The current thinking is that inhibition of Ca(V)1.2 channels effectively counteracts the pro-arrhythmic effect of K(V) channel block (Bril et al., 1996; Sanguinetti and Tristani-Firouzi, 2006). Roscovitine has also been found to block K(V) channels (Buraei et al., 2007), including the human ether-a-go-go-related gene (HERG) potassium channel that is crucial for cardiac action potential repolarization (SB Ganapathi and KS Elmslie, submitted). However, cardiac arrhythmias have not been reported as a side effect from either phase I or phase II drug trials (Fischer and Gianella-Borradori, 2003; Benson et al., 2007), even though HERG channels were blocked by therapeutic roscovitine concentrations (10–50 μM) (McClue et al., 2002; Hahntow et al., 2004; Raynaud et al., 2005). Like verapamil, it seems likely that roscovitine-induced inhibition of Ca(V)1.2 prevents the negative cardiovascular effects of K(V) channels block. In addition, the incomplete inhibition of Ca(V)1.2 currents by roscovitine could provide a ‘safety factor', as even saturating concentrations would allow considerable residual Ca(V)1.2 channel activity.
In spite of its promiscuity, roscovitine appears to interact with previously uncharacterized sites to uniquely alter the gating of each of class of affected ion channel. Particularly notable is the agonist effect of roscovitine on Ca(V)2 (Buraei et al., 2005, 2007) and the slowed activation and faster inactivation Ca(V)1.2 channels (detailed here). Further investigation of these sites is likely to define unique characteristics that can be exploited for the development of drugs specific for each site. As the intensive study of the ATP-binding site on protein kinases has led to the development of specific inhibitors, the study of roscovitine and its analogues could lead to isolation of a novel class of purine-based drugs that is specific for each class of affected ion channel.
External data objects
Abbreviations
- BNZ
benzothiazepine
- Ca(V)1.2
cardiac L-type voltage-dependent calcium channel
- Ca(V)2.2
N-type voltage-dependent calcium channel
- CDI
calcium-dependent inactivation
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulphoxide
- FBS
fetal bovine serum
- HeBS
HEPES-buffered saline
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethane sulphonic acid
- HERG
human ether-a-go-go-related gene
- K(V)
voltage-dependent potassium channel
- NMG
N-methyl-D-glucamine
- PAA
phenylalkylamine
- VDI
voltage-dependent inactivation
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci USA. 1984;81:6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi I, Koch SE, Yamaguchi H, Szigeti GP, Schwartz A, Varadi G. The role of region IVS5 of the human cardiac calcium channel in establishing inactivated channel conformation. Use-dependent block by benzothiazepines. J Biol Chem. 2002;277:20651–20659. doi: 10.1074/jbc.M200752200. [DOI] [PubMed] [Google Scholar]
- Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Mangoni ME, Nargeot J. Dissecting the functional role of different isoforms of the L-type Ca2+ channel. J Clin Invest. 2004;113:1382–1384. doi: 10.1172/JCI21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril A, Gout B, Bonhomme M, Landais L, Faivre JF, Linee P, et al. Combined potassium and calcium channel blocking activities as a basis for antiarrhythmic efficacy with low proarrhythmic risk: experimental profile of BRL-32872. J Pharmacol Exp Ther. 1996;276:637–646. [PubMed] [Google Scholar]
- Buraei Z, Anghelescu M, Elmslie KS. Slowed N-type calcium channel (CaV2.2) deactivation by the cyclin-dependent kinase inhibitor roscovitine. Biophys J. 2005;89:1681–1691. doi: 10.1529/biophysj.104.052837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraei Z, Schofield G, Elmslie KS. Roscovitine differentially affects CaV2 and Kv channels by binding to the open state. Neuropharmacology. 2007;52:883–894. doi: 10.1016/j.neuropharm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cho S, Meriney SD. The effects of presynaptic calcium channel modulation by roscovitine on transmitter release at the adult frog neuromuscular junction. Eur J Neurosci. 2006;23:3200–3208. doi: 10.1111/j.1460-9568.2006.04849.x. [DOI] [PubMed] [Google Scholar]
- Dafi O, Berrou L, Dodier Y, Raybaud A, Sauve R, Parent L. Negatively charged residues in the N-terminal of the AID helix confer slow voltage dependent inactivation gating to CaV1.2. Biophys J. 2004;87:3181–3192. doi: 10.1529/biophysj.104.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmslie KS. Calcium channel blockers in the treatment of disease. J Neurosci Res. 2004;75:733–741. doi: 10.1002/jnr.10872. [DOI] [PubMed] [Google Scholar]
- Elmslie KS, Zhou W, Jones SW. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca2+ channel and its role in myocyte electrophysiology: a theoretical investigation. Biophys J. 2007;92:1522–1543. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G, Rios E, Reyes N. Two components of voltage-dependent inactivation in Cav1.2 channels revealed by its gating currents. Biophys J. 2003;84:3662–3678. doi: 10.1016/S0006-3495(03)75096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PM, Gianella-Borradori A. CDK inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2003;12:955–970. doi: 10.1517/13543784.12.6.955. [DOI] [PubMed] [Google Scholar]
- Giannattasio B, Jones SW, Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Calcium-dependent and voltage-dependent inactivation. J Gen Physiol. 1991;98:987–1003. doi: 10.1085/jgp.98.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahntow IN, Schneller F, Oelsner M, Weick K, Ringshausen I, Fend F, et al. Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia. 2004;18:747–755. doi: 10.1038/sj.leu.2403295. [DOI] [PubMed] [Google Scholar]
- Hering S, Aczel S, Grabner M, Doring F, Berjukow S, Mitterdorfer J, et al. Transfer of high sensitivity for benzothiazepines from L-type to class A (BI) calcium channels. J Biol Chem. 1996;271:24471–24475. doi: 10.1074/jbc.271.40.24471. [DOI] [PubMed] [Google Scholar]
- Hering S, Savchenko A, Strubing C, Lakitsch M, Striessnig J. Extracellular localization of the benzothiazepine binding domain of L-type Ca2+ channels. Mol Pharmacol. 1993;43:820–826. [PubMed] [Google Scholar]
- Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. Molecular determinants of drug binding and action on L-type calcium channels. Annu Rev Pharmacol Toxicol. 1997;37:361–396. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Hockerman GH, Scheuer T, Catterall WA. Distinct effects of mutations in transmembrane segment IVS6 on block of L-type calcium channels by structurally similar phenylalkylamines. Mol Pharmacol. 1996;50:1388–1400. [PubMed] [Google Scholar]
- Li G-R, Yang B, Feng J, Bosch RF, Carrier M, Nattel S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am J Physiol Heart Circ Physiol. 1999;276:H98–H106. doi: 10.1152/ajpheart.1999.276.1.H98. [DOI] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks TN, Jones SW. Calcium currents in the A7r5 smooth muscle-derived cell line. An allosteric model for calcium channel activation and dihydropyridine agonist action. J Gen Physiol. 1992;99:367–390. doi: 10.1085/jgp.99.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClue SJ, Blake D, Clarke R, Cowan A, Cummings L, Fischer PM, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–468. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong J, Blow J, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res. 2003;36:417–425. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- Motoike HK, Bodi I, Nakayama H, Schwartz A, Varadi G. A Region in IVS5 of the human cardiac L-type calcium channel is required for the use-dependent block by phenylalkylamines and benzothiazepines. J Biol Chem. 1999;274:9409–9420. doi: 10.1074/jbc.274.14.9409. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, Lee JS, Mulle JG, Wang Y, de Leon M, Yue DT. Critical determinants of Ca(2+)-dependent inactivation within an EF-hand motif of L-type Ca(2+) channels. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud FI, Whittaker SR, Fischer PM, McClue S, Walton MI, Barrie SE, et al. In vitro and in vivo pharmacokinetic–pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res. 2005;11:4875–4887. doi: 10.1158/1078-0432.CCR-04-2264. [DOI] [PubMed] [Google Scholar]
- Roden DM, Balser JR, George AL, Jr, Anderson ME. Cardiac ion channels. Annu Rev Physiol. 2002;64:431–475. doi: 10.1146/annurev.physiol.64.083101.145105. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- Seydl K, Kimball D, Schindler H, Romanin C. The benzazepine/benzothiazepine binding domain of the cardiac L-type Ca2+ channel is accessible only from the extracellular side. Pflugers Arch. 1993;424:552–554. doi: 10.1007/BF00374922. [DOI] [PubMed] [Google Scholar]
- Shi C, Soldatov NM. Molecular determinants of voltage-dependent slow inactivation of the Ca2+ channel. J Biol Chem. 2002;277:6813–6821. doi: 10.1074/jbc.M110524200. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Timin E, Hering S. On the role of Ca2+- and voltage-dependent inactivation in Cav1.2 sensitivity for the phenylalkylamine (-)gallopamil. Circ Res. 2001;89:700–708. doi: 10.1161/hh2001.098983. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- Werz MA, Elmslie KS, Jones SW. Phosphorylation enhances inactivation of N-type calcium channel current in bullfrog sympathetic neurons. Pflugers Arch. 1993;424:538–545. doi: 10.1007/BF00374919. [DOI] [PubMed] [Google Scholar]
- Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol. 1999;276 6 Part 2:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P. Roscovitine: a novel regulator of P/Q-type calcium channels and transmitter release in central neurons. J Physiol (Lond) 2002;540:761–770. doi: 10.1113/jphysiol.2001.013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Gong Q, Makielski JC, January CT. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.