Abstract
Narcolepsy is linked to a widespread loss of neurons containing the neuropeptide hypocretin (HCRT), also named orexin. A transgenic (TG) rat model has been developed to mimic the neuronal loss found in narcoleptic humans. In these rats, HCRT neurons gradually die as a result of the expression of a poly-glutamine repeat under the control of the HCRT promoter. To better characterize the changes in HCRT-1 levels in response to the gradual HCRT neuronal loss CSF HCRT-1 levels were measured in various age groups (2–82 weeks) of wildtype (WT) and transgenic (TG) Sprague-Dawley rats. TG rats showed a sharp decline in CSF HCRT-1 level at week 4 with levels remaining consistently low (26% ± 9%, mean± SD) thereafter compared to WT rats. In TG rats, HCRT-1 levels were dramatically lower in target regions such as the cortex and brainstem (100 fold), indicating decreased HCRT-1 levels at terminals. In TG rats, CSF HCRT-1 levels significantly increased in response to 6 h of prolonged waking, indicating that the remaining HCRT neurons can be stimulated to release more neuropeptide. REM sleep in TG rats (n=5) was consistent with a HCRT deficiency. In TG rats HCRT immunoreactive (HCRT-ir) neurons were present in the lateral hypothalamus (LH), even in old rats (24 months) but some HCRT-ir somata were in various stages of disintegration. The low output of these neurons is consistent with a widespread dysfunction of these neurons, and establishes this model as a tool to investigate the consequences of partial hypocretin deficiency.
Keywords: Hypothalamus, Peptide, Sleep, Hypocretin, Lesion, REM Sleep, Orexin, Ataxin, Narcolepsy
Introduction
Neurons containing the neuropeptide hypocretin (HCRT), also known as orexin, are found only in the lateral hypothalamus from where they innervate virtually the entire brain and spinal cord 7,9,17,20,22, providing especially heavy innervation to neurons regulating arousal. HCRT binds to two receptors whose distribution in the brain has been determined 15. Abnormal HCRT transmission causes narcolepsy in animals and humans. In familial canine narcolepsy, the disease is caused by mutations in the HCRT-2 receptor 14. Further, mice genetically engineered to lack hypocretin also display narcolepsy-like symptoms 5. Finally, human narcoleptics have low to negligible levels of HCRT-1 in the CSF 18 indicating a defect in the release of the peptide, or an actual loss of HCRT neurons. Postmortem tissue analysis has confirmed a loss of HCRT neurons19,24, since dynorphin and neuronal activity-related pentraxin (NARP), which colocalize with HCRT are also absent in human with narcolepsy 4,6.
In humans, CSF measurements of HCRT-1 are now an acceptable and valuable diagnostic tool for narcolepsy 16, 21. However, precise assessment of the relationship between CSF HCRT-1 levels and HCRT neuronal loss can only be made at time of autopsy. Additionally, how CSF HCRT-1 levels change as the disease progresses is unknown. We have previously determined that a 73% loss of HCRT and other LH neurons caused by the neurotoxin, hypocretin-2-saporin, resulted in a significant, albeit more modest (50%) decline in CSF HCRT-1 levels 11. This suggests some level of compensation. Nevertheless, how CSF HCRT-1 levels change with a more selective HCRT neuronal loss is unknown.
This important question can be investigated in a transgenic line of rats created to mimic the loss of HCRT neurons seen in human narcoleptics. In these rats, there is a gradual and selective loss of HCRT neurons as a result of a HCRT promoter-driven accumulation of the cytotoxic gene product poly-Q-ataxin -33. At three weeks of age, HCRT neurons start to die, and rats also begin to show narcoleptic symptoms3. In the present study, CSF HCRT-1 levels were measured at various developmental stages, from 2 weeks to adulthood. A precipitous decline in HCRT-1 levels was found when HCRT neurons are known to degenerate (3–4 weeks). Interestingly, however, we also found hypocretin-ir somata even in old rats, but these were unable to maintain a normal level of hypocretin release. These results establish the ataxin-hypocretin transgenic rats as a valid model for partial hypocretin deficiency.
Materials and methods
Animals
The transgenic rats are derived from the Sprague-Dawley strain. Two independent groups of rats were used in the present study, one maintained at the West Roxbury VA hospital and the other at Stanford University. However, both groups of rats were derived from rats obtained from the original colony in University of Texas Southwestern Medical Center Dallas, Texas 3. Genotype analysis of tail snips was used to confirm that the ataxin-3 transgene was present. Since the previous study3 demonstrated that the HCRT neurons die when the transgene is present, it was not necessary to determine whether the rats were heterozygous or homozygous with respect to the transgene. Male and female transgenic and wild-type (from Charles River Labs, MA) Sprague-Dawley rats were housed singly (or by litter before weaning) in Plexiglas cages with wood-shavings, in a room with controlled temperature (21 ± 0.5 °C) under light-dark cycle (7AM–7PM lights-on; 150 lux).
Collection of CSF and 6h total sleep deprivation
CSF was collected from the cisterna magna under Isofluorane anesthesia 10 with a needle (27G) and immediately frozen on dry ice and maintained at −70°C until analysis.
In one group of rats, CSF was collected at ZT0 (lights-on) or ZT8. These two points were chosen based on previous data8,10,28. These rats were all less than 44 weeks old. After CSF collection, rats were euthanized and brains dissected. The cerebral cortex and brainstem were quickly frozen on dry ice and maintained at −70°C until analysis. The portion of the brain containing the hypothalamus was placed in 4% paraformaldehyde for immunohistochemistry studies.
To investigate the effects of sleep deprivation on CSF HCRT-1 levels, we used 49–82 week-old rats. CSF was collected at ZT8. Two weeks later, these same rats were kept awake for six hours (ZT2–ZT8) and CSF collected again (post-total sleep deprivation, TSD) at ZT8. The post-sleep deprivation data point was compared with CSF collected from the same rat at the same ZT time but without any sleep deprivation (repeated ANOVA with Neuman-Keuls post-hoc test).
Hypocretin Radioimmunoassay
CSF HCRT-1 was measured using commercially available 125I RIA kits (Phoenix Pharmaceuticals, Belmont, CA). Twenty five μl of CSF was mixed with 75μl of RIA buffer and directly applied to the RIA. The detection limits of the assay were 100 pg/mL. All comparative samples were measured in a single assay and the intra-assay variation was <5 %.
Frozen brain tissue of animals sacrificed at ZT3–ZT5 were extracted with 1 mL of 0.5M acetic acid and boiled in water bath for 15 minutes. Samples were cooled on ice and centrifuged at 5000× g for 10 minutes. Protein concentration in the supernatant was measured using the Bradford method (Bio-Rad Laboratories, Hercules, CA). The supernatants were dried overnight at 50°C and reconstituted in RIA buffer for radioimmunoassay. The HCRT contents were corrected against protein concentrations.
EEG Sleep Recording
Five WT (age= 55weeks) and five TG (ages= 60, 90, 118, 124, 124 weeks) rats were implanted under anesthesia (Isofluorane 2%, continuous) with sleep recording electrodes. Four stainless steel screw electrodes were positioned in the skull to sit on the surface of the cortex and were used to record the EEG. Two miniature screws were inserted 2 mm on either side of the sagittal sinus and 3 mm anterior to bregma (frontal cortex). The other two screws were located 3 mm on either side of the sagittal sinus and 6 mm behind bregma (occipital cortex). The EEG was recorded from two contralateral screws (frontal-occipital). To record muscle activity (electromyogram, EMG), two flexible multi-stranded wires were inserted in the nuchal muscles. The six electrodes were inserted into a plastic plug, which was then secured onto the skull using dental cement.
After surgery, the animals were connected to lightweight recording cables and two weeks later the electroencephalogram (EEG) and electromyogram (EMG) were recorded for a 48-hour period. The EEG and EMG signals were recorded on a Grass model 15 polygraph and stored using an A/D board (National Instruments).
CSF was not collected from the five TG rats whose sleep was recorded. The brains of these rats were processed for visualization of HCRT-ir neurons.
Analysis of Sleep Data
Contralateral frontal-occipital EEG screw electrodes were used for EEG acquisition. The data were filtered at 70 Hz (low pass filter) and 0.3 Hz (high pass filter) using a Grass electroencephalograph and continuously sampled at 128 Hz. The 48 h EEG and EMG recordings were scored manually on a computer (Icelus software, M. Opp) in 12 second epochs for awake, slow wave sleep and REM sleep by staff (E. Winston) blind to the age and genotype of the animals. Wakefulness was identified by the presence of desynchronized EEG and high EMG activity. Non-REM sleep consisted of high amplitude slow waves together with a low EMG tone relative to waking. REM sleep was identified by the presence of regular theta activity coupled with low EMG relative to slow wave sleep. The percent of time spent in wakefulness, non-REM and REM was determined for each hour. The number and length of each bout of wake, non-REM and REM sleep was determined, with the minimum length of a bout being 12 sec (minimum scoring epoch). Sleep onset REM sleep (SOREMP) episodes were scored according to our previous study in rats 11. Briefly, an episode of REM sleep was scored as a SOREMP if it occurred after 2 min or more of wakefulness with less than 2 min of an intervening episode of non-REM sleep. Repeated measures ANOVA and t-tests (where appropriate) were used to compare changes in sleep parameters.
Immunohistochemistry
Rat brains were examined for presence of HCRT immunoreactive neurons in the lateral hypothalamus. In most rats, the hypothalamus was freshly dissected and fixed in phosphate-buffered 4% paraformaldehyde (pH 7.0) for 3 days, equilibrated in 30% sucrose, and stored at 4°C. The TG rats whose sleep was recorded were deeply anesthetized with pentobarbital (150 mg/kg i.p.) and perfused transcardially with 0.9% saline (50 ml) followed by 500 ml of phosphate-buffered 4% paraformaldehyde (pH 7.0). The brains were postfixed overnight, equilibrated in 30% sucrose, and stored at 4°C. Five series of coronal sections were cut at 30 μm on a sliding microtome. Each set of coronal brain sections was incubated overnight at room temperature in the primary antibody (rabbit anti-orexin-A (HCRT1) (1:10,000, Peninsula Laboratories, Inc., San Carlos, CA). After washing, the sections were incubated with the secondary antibody for 1 hr (Chemicon; 1:500 dilution) and then reacted with avidin-biotin complex for 1 hr (Vector Laboratories, Burlingame, CA). The DAB method was used to visualize the reaction product. To test for specificity of the antibody, tissue sections from one each randomly selected WT and TG rats were incubated in preadsorbed HCRT-1 antibody and processed as above. The preadsorption of HCRT-1 antibody (1:10, 000) was done with 40 μg of the orexin-A (HCRT-1) peptide (Phoenix Pharmaceuticals Inc., Belmont, CA.), and kept overnight at 4°C before incubating the brain sections.
Results
Age-related changes in CSF and brain tissues
Figure 1 summarizes the age-related changes in HCRT-1 level in CSF and brain tissues in WT and TG rats. Figure 1A shows the CSF HCRT-1 levels in each rat. In WT rats, CSF HCRT-1 was detectable at two weeks, when the samples could be reliably collected. CSF HCRT-1 then increased gradually over the next few weeks to reach a stable level at week five. In contrast, HCRT-1 levels in TG rats continuously decreased from week 2 to week 4, to stabilize at a low level after the fourth week. This decrease was fitted to an exponential decay (r2=0.73 using data from 2–6 week-old rats), resulting in a half-life of decay of 1.4 week. There was a slight overlap between the WT and TG rats at weeks 2 and 3, but after week 4 the average HCRT-1 levels in the TG rats were significantly lower than those observed in WT rats at all ages studied (p<0.001, unpaired two-tailed t-test). The average reduction of CSF HCRT-1 level in the TG rats was 74% ± 9% (mean ± SD), and there was no significant difference between males and females. Table 1 summarizes data for rats older than 5 weeks (age when levels were found to be stable in both TG and WT rats).
Figure 1.
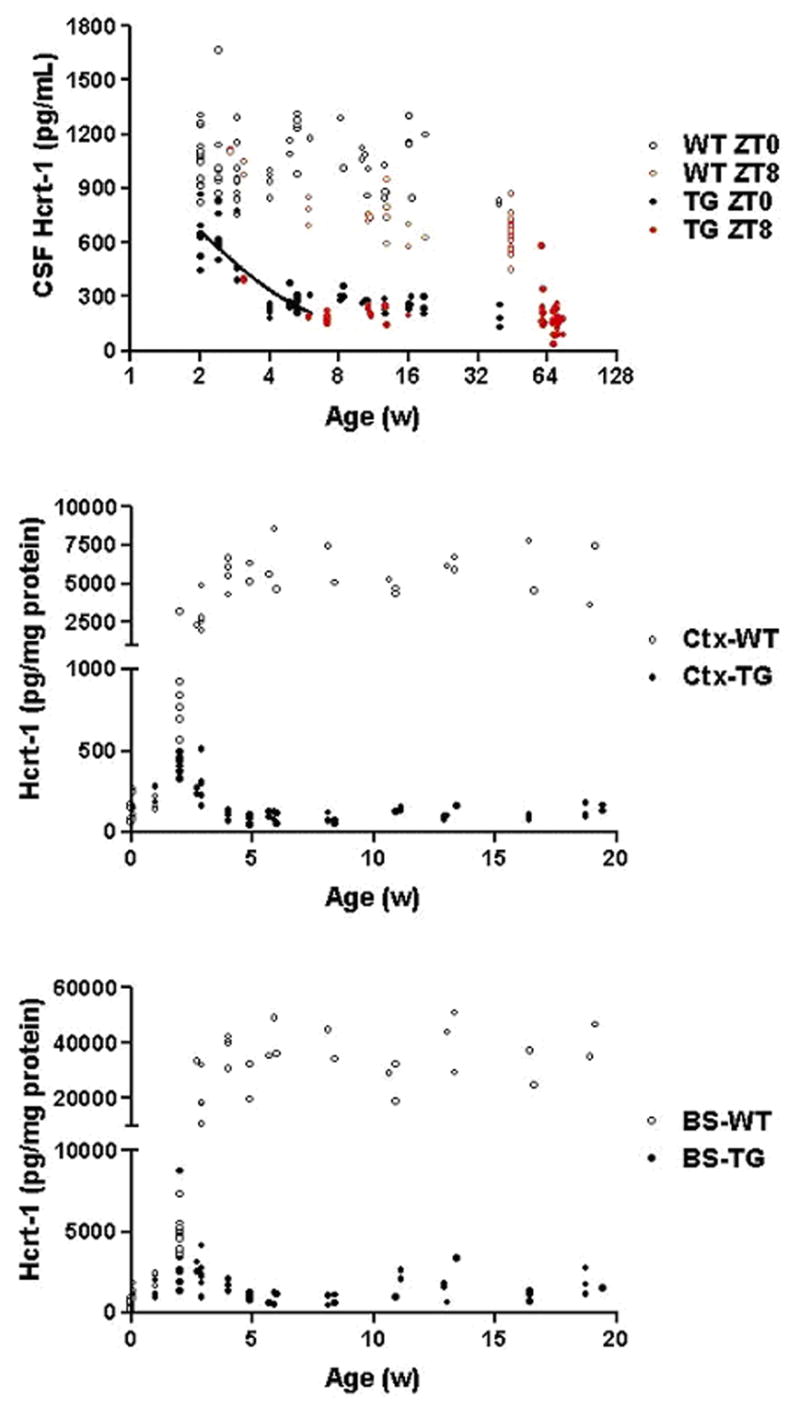
HCRT-1 levels in wildtype (WT) and hypocretin-ataxin transgenic (TG) rats. HCRT-1 was measured in the CSF (top), cortex (middle) and brainstem (bottom) at various ages in WT and TG rats. CSF was extracted at two time points (ZT0 and ZT 8). Brain measurements were all made at ZT 3–5. TG rats have consistently low levels of HCRT-1, especially after the fourth week of age.
Table 1.
CSF HCRT-1 levels (pg/ml ± SEM) in various age groups (age 5–79 weeks) of WT and TG rats
| ZT0
|
ZT8
|
|||
|---|---|---|---|---|
| WT | TG | WT | TG | |
| All | 1079 ± 31 (26) | 260 ± 8 (37)* | 683 ± 20 (33)# | 190 ± 11 (48)*# |
| Male | 1035 ± 37 (10) | 272 ± 9 (16)* | 683 ± 28 (16)# | 176 ± 11 (20)*# |
| Female | 1107 ± 45 (16) | 251 ± 11 (21)* | 683 ± 28 (17)# | 199 ± 18 (28)*# |
Data are expressed as Mean ± SEM
Compare WT and TG at corresponding time, p<0.001 (unpaired two-tailed t-test)
Compare ZT0 and ZT8 within each genotype, p<0.001 (unpaired two-tailed t-test)
Figures 1B and 1C report on HCRT-1 contents in two regions of the brain known to receive HCRT projections. In WT rats, HCRT-1 contents in both the cortex and the brainstem were negligible at birth, and then gradually increased over the next few weeks, to reach stable levels around week five. The amount of HCRT-1 measured in the brainstem after week five was eight-fold higher than those observed in the cortex. In TG rats, HCRT-1 levels in the cortex and brainstem were low compared to WT rats at two weeks of age, surged briefly at weeks 2–3, to decrease until week 5. At this stage, HCRT-1 levels were dramatically lower than in control rats. In contrast to CSF levels where we found a 4 fold decrease, brain content in projection areas were decreased approximately 100 fold in TG rats (Figure 1).
Effects of 6h total sleep deprivation on CSF HCRT-1 levels
To determine whether residual HCRT-1 levels observed after 5 weeks could be increased, 6h of sleep deprivation was performed in older TG rats (age ranging from 64–82 weeks), and was compared to WT rats (49–50 weeks old). As expected, CSF HCRT-1 levels were significantly lower (F1, 114= 309.38; P< 0.001) in TG rats compared to WT in both male (F1, 41= 116.13; P< 0.001) and female (F1, 61= 144.32; P< 0.001) (Figure 2). Surprisingly however, six hours of prolonged waking increased CSF HCRT-1 levels in both WT and TG rats, but the levels in the TG rats were still well below basal WT levels (P< 0.001).
Figure 2.
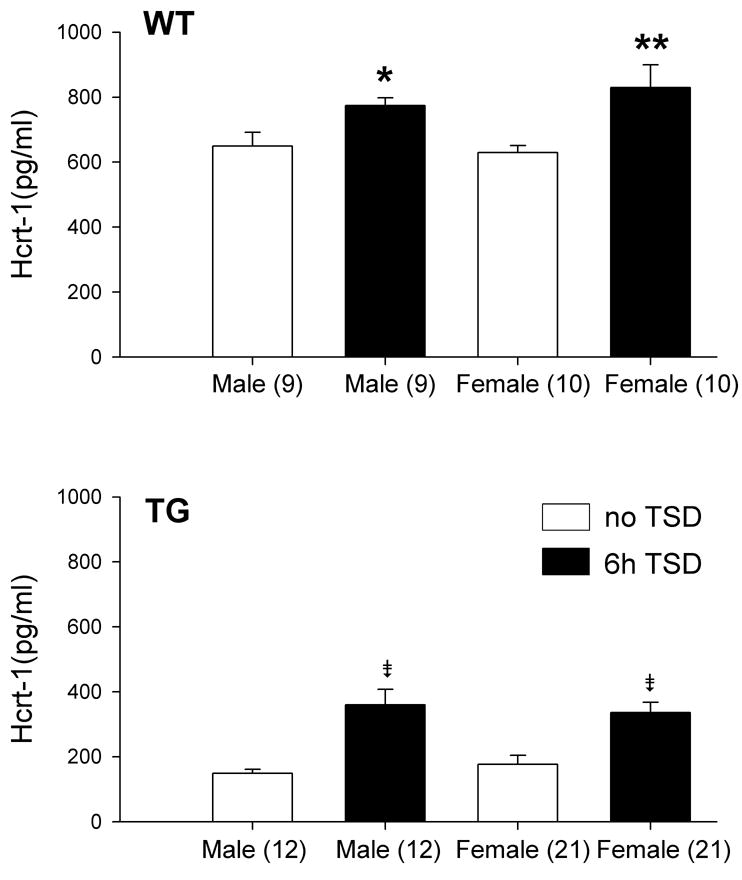
CSF HCRT-1 levels in response to prolonged waking. CSF was collected from male and female WT and TG rats at ZT8 without sleep deprivation (no TSD) and after 6h (ZT2–ZT8) of total sleep deprivation (TSD). Significance levels: * versus no TSD (P< 0.05); ** versus no TSD (P<0.01); ⇟=versus no TSD (P<0.001); # =WT versus TG (P< 0.001). The numbers within brackets represents the sample size of the group.
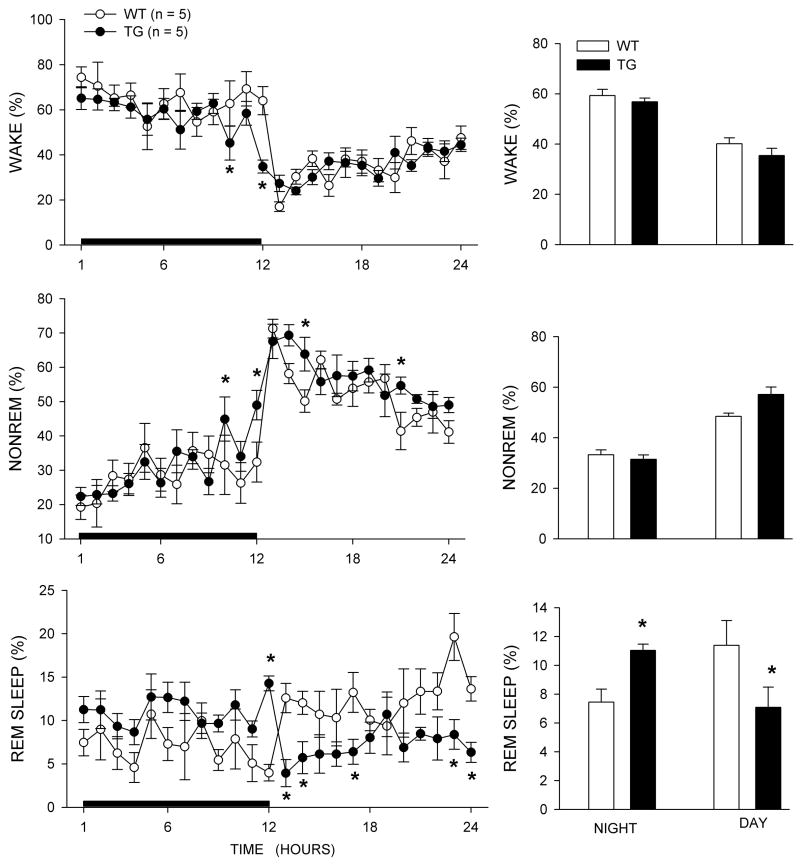
Sleep in TG rats
Figure 3 summarizes the diurnal rhythm of sleep in the two genotypes of rats. The most striking difference between the two genotypes was in the distribution of REM sleep during the day and night. TG rats had more REM sleep at night compared to WT (P<0.05), and less REM sleep during the day compared to WT (P<0.05). For REM sleep, ANOVA identified a significant interaction between genotype and day-night cycle (F1,8= 10.2; P<0.05). The TG rats were significantly less awake and had more non-REM sleep at the end of the night cycle (P<0.05). The daytime decrease in REM sleep in the TG rats was consistent with a decrease in number and length of REM sleep bouts (see Table 2), while the increase in REM sleep at night was consistent with an increase in the length of REM sleep bouts. These changes in REM sleep are consistent with the previous report 3. However, that report noted increased fragmentation of sleep-wake bouts at night which were not found in our study.
Figure 3.
Percent (± SEM) wake, nonREM and REM sleep in old WT and TG rats. The line graphs summarize the average each hour across the 24h period whereas the bar graphs summarize the data during the 12h night and day cycles. The rats were implanted with sleep recording electrodes (isofluorane anesthesia) and two weeks later a 48 h sleep recording was made. CSF was not extracted from the TG rats but the diurnal distribution of REM sleep is indicative of a HCRT deficiency. Asterisks denote significant difference (P<0.05) versus WT.
TABLE 2.
Average (± SEM) number and length (minutes) of wake, non-REM and REM sleep bouts, per hour, in WT (n=5) and TG (n=5) rats.
| DAY | NIGHT | |||
|---|---|---|---|---|
| WT | TG | WT | TG | |
| Number of REM sleep bouts | 5.37 ± 0.29 | 4.05 ± 0.30* | 3.98 ± 0.30 | 4.4 ± 0.27 |
| Length of REM sleep bouts | 1.22 ± 0.05 | 0.74 ± 0.04* | 1.07 ± 0.06 | 1.48 ± 0.09 * |
| Number of non-REM sleep bouts | 14.60 ± 0.40 | 14.91 ± 0.41 | 8.65 ± 0.43 | 8.76 ± 0.38 |
| Length of non-REM sleep bouts | 2.28 ± 0.07 | 2.36 ± 0.09 | 2.07 ± 0.07 | 2.17 ± 0.08 |
| Number of wake bouts | 6.19 ± 0.26 | 7.61 ± 0.24* | 4.96 ± 0.25 | 4.56 ± 0.21 |
| Length of wake bouts | 3.44 ± 0.54 | 2.76 ± 0.21 | 11.66 ± 1.25 | 10.75 ± 1.30 |
| Sleep onset REM sleep bouts | 0 | 1.0 ± 0.316* | 0 | 2.4± 0.51* |
WT vs TG (P< 0.05); two-way ANOVA followed by Neuman Keul’s test
The behavior of these rats was not videotaped and, as such, we were not able to distinguish cataplexy from direct transitions to REM sleep similar to what has been described previously 3,11. However, we inspected the EEG recordings and found that the TG rats had direct transitions to REM sleep (Figure 4) (TG rats day:1.0 + 0.316; P= 0.013 vs. WT, and TG rats night: 2.4+ 0.51; P= 0.002 vs. WT). No direct transitions to REM sleep were detected in WT rats. The previous study reported 3.8 such abnormal REM sleep transitions at night versus 0.3 during the day 3. Thus, the rats in the present study showed fewer abnormal REM transitions during the night compared to the previous report.
Figure 4.
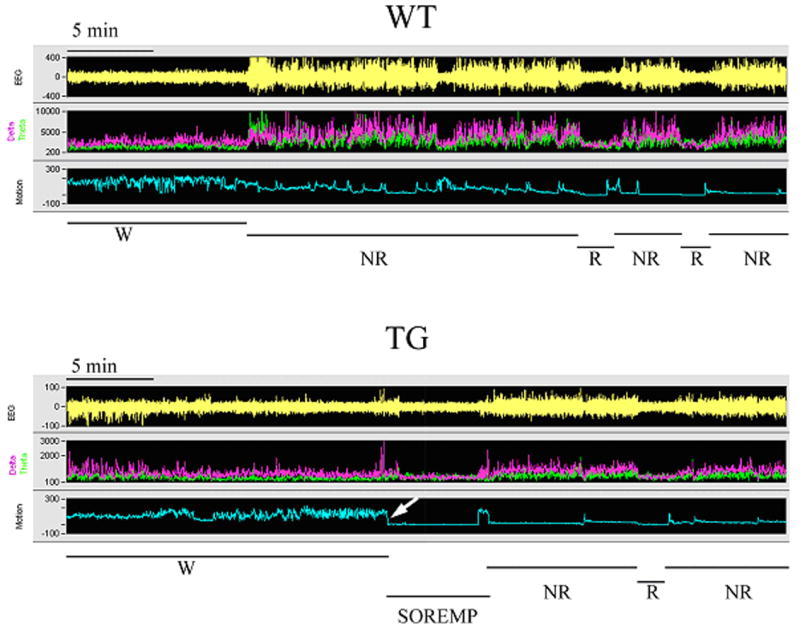
Alternation between wakefulness (W), non-REM sleep (NR) and REM sleep in representative WT and TG rats. The figure represents a 40 minute segment of a sleep-wake recording during the night. Each panel (WT and TG) consists of a recording of the electroencephalogram (EEG), power of the EEG in the delta (0.3–4 hz; pink) and theta bands (4–12 hz; green), and integrated activity of the nuchal muscles (EMG). The sleep-wake state determination, based on the relationship of the EEG, power and EMG activity, is indicated at the bottom of each panel. In the WT rat there are normal transitions from W to non-REM to REM sleep. In TG rats, there are occasional occurrences of sleep-onset REM sleep period (SOREMP). The SOREMP is identified by the abrupt loss of EMG tone (near zero) during a wake bout. Note that the length of the SOREMP episode is quite similar to an average length of a REM sleep bout.
Over the 24h period there was no difference in wake (WT=49.72±2.08; TG=46.12± 1.12; Mean±SEM), non-REM (WT=40.85±1.44; TG=44.28±1.42) or REM sleep (WT=9.42±1.07; TG=9.06± 0.52) between the WT (n=5) and TG (n=5) rats. In the previous report TG rats had more REM sleep over the 24 h period 3.
Hypocretin neurons in TG rats
HCRT immunoreactivity in the hypothalamus was examined at different ages. Figure 5 shows representative images of the WT rats at 1, 3, 4, and 6 weeks old (Figure 5A) and of the TG rats at 1, 3, 5, and 16 weeks old (Figure 5B). Unlike a previous study reporting negative detection of HCRT-1 immunoreactive cells before 2 weeks old 27, we observed HCRT-1-immunoreactive neurons at birth. Further, in older TG rats (60 to 124 weeks), HCRT-ir neurons were clearly present, although staining was generally weaker and there were fewer HCRT axonal and dendritic processes (see Fig 6). At higher magnification, some HCRT-ir somata in TG rats appeared odd shaped, disrupted or fractured, indicating abnormal HCRT neurons (Figure 6).
Figure 5.
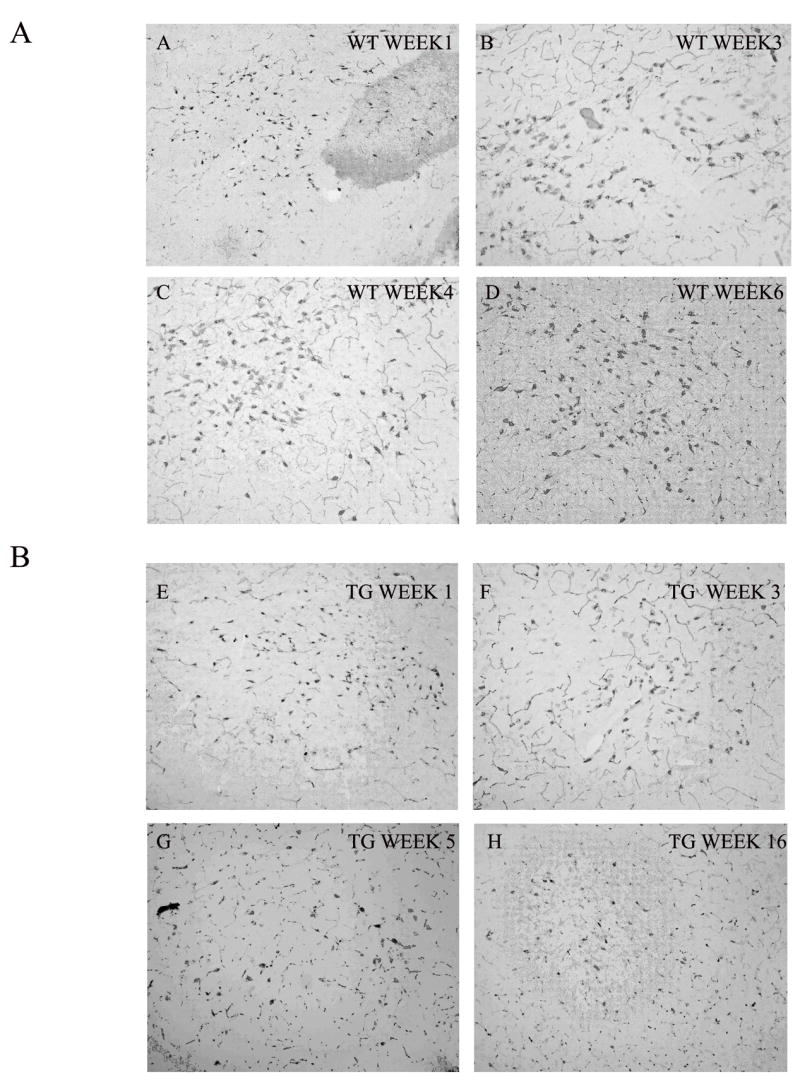
Hypocretin-immunoreactive neurons in the LH of representative WT and TG rats. The top four photos depict LH in WT rats and the lower four photos represent TG rats. HCRT-ir neurons are evident in 3 and 5 week old TG rats. These neurons are still visible in 16 week old rats, albeit they are much lighter.
Figure 6.
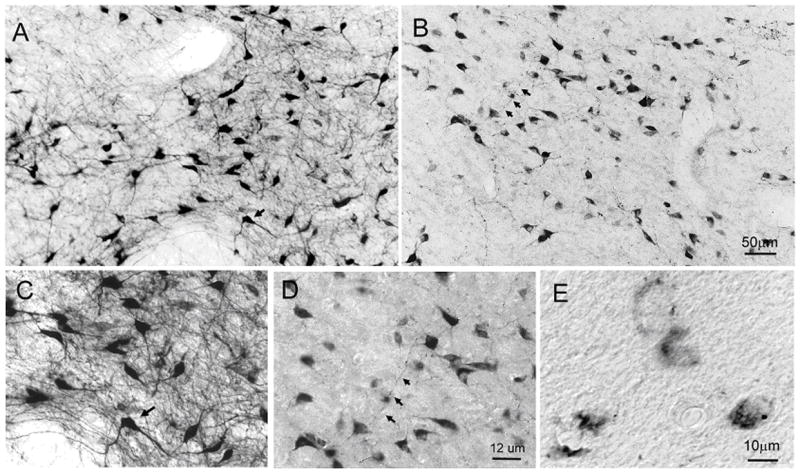
Hypocretin-ir neurons in the LH of representative WT (63 weeks) (photo A and C) and TG (83 wks) (photos B, D and E) rats. In TG rats, HCRT-ir somata were evident in the LH (photo B) but the high density of HCRT processes seen in WT rats (photo A) was lacking. Photomicrographs C and D represent higher magnification (Nomarski optics) of the areas denoted by arrows in photos A and B. Note that in TG rats the somata are visible but they lack the axonal and dendritic processes emanating from it. Upon closer inspection some of the HCRT somata of TG rats were found to be in various stages of disintegration (photo E). Magnification: A=B, C=D.
Discussion
The primary finding of this study is that the TG rats, which represent a rat model of the HCRT neuronal degeneration seen in human narcolepsy, have a 74% decline in CSF levels of HCRT-1. The TG rats showed a significant and precipitous decline in CSF HCRT-1 levels around four weeks of age, consistent with a previous study reporting cell death during this developmental period 3. In the TG rats HCRT neurons were evident even in old (60–124 weeks) rats, but the rich axonal and dendritic arborization which is evident in WT rats was lacking. Beuckmann et al., (2004) reported finding no HCRT neurons in 17 week old TG rats. The TG rats in the present study had an inverted day-night REM sleep pattern similar to that reported previously 3. However, the TG rats had fewer numbers of REM sleep episodes directly from waking, and no sleep fragmentation at night, compared to the previous study.
After the precipitous decline in CSF HCRT-1 levels at around four weeks, the neuropeptide levels quickly stabilized (74% decline) and CSF HCRT-1 remained detectable even at older ages. The decline in HCRT-1 levels was not the result of aging since 21-month old wildtype F344 rats only have a 10% decline compared to young (3 months) F344 rats 8. The relatively stable CSF HCRT-1 levels observed in older TG rats suggest that the viable HCRT neurons were partially, but not completely, able to compensate for the hypocretin dysfunction. The functional capacity of the remaining HCRT neurons may have increased to maintain higher extracellular levels as measured in the CSF.
We found that the HCRT-1 levels in the cortex and brainstem also declined in parallel with the CSF HCRT-1 levels indicating a decrease in HCRT-1 in terminals in all target regions. This suggests that HCRT neuronal dysfunction is uniform across the lateral hypothalamus. Interestingly, we found a surge in HCRT in these target regions at week 2 in TG animals as well as in controls. This suggests that at this developmental age the HCRT neurons may be maturing. The HCRT-1 rise in TG animals was however rapidly aborted, suggesting that ataxin toxicity was most acute once the neurons were fully developed. This may reflect increasing vulnerability to the ataxin transgene at a time when neurons have long processes and increased metabolic demand, or could reflect a stronger effect of ataxin toxicity in terminal areas.
In TG rats, we found that the remaining HCRT neurons could still be stimulated to release more HCRT as CSF-HCRT-1 levels increased in response to 6h of sleep deprivation. This effect was nonetheless insufficient to compensate for the baseline hypocretin deficiency observed in TG rats. This is consistent with a previous study where HCRT neurons were killed using the neurotoxin hypocretin-2 saporin (HCRT-2 SAP). Indeed, in that study, CSF HCRT-1 levels were stable after an initial sharp decline 11 but could still be stimulated by sleep deprivation, without reaching control levels. In the present study, the wake-induced increase in HCRT-1 in the TG rats indicates that in spite of the ataxin gene, HCRT neurons can be stimulated to release more hypocretin. This finding suggests that combined pharmacological or behavioral treatments could further stimulate synthesis and/or release of HCRT in animals with remaining hypocretin neurons. This last observation may be significant, as 5–10% of neurons still remain in human narcoleptic brains 24. In that study, remaining neurons appeared to have normal morphometry, unlike in the present study.
In the present study, immunohistochemistry identified numerous HCRT-containing neurons in the LH of adult and aging TG rats. This finding was unexpected since in the previous report3 HCRT immunoreactive (HCRT-ir) neurons were not present in 17-week old TG rats. In that study, CSF HCRT-1 was not measured. The reason for the presence of HCRT-ir neurons in the present study is not clear and could reflect changes in genetic background, inactivation of the transgene due to further breeding or technical differences in immunostaining. Of these explanations, we believe that the least likely is transgene inactivation. Indeed, although we noted intact HCRT-ir somata, there were neurons that were clearly abnormal and/or stained more weakly for hypocretin. Further, the dramatic decrease in hypocretin content in projection areas argues in favor of a primary degeneration of terminals, with a partial preservation of cell bodies.
Importantly, as in the previous study 3, we found that REM sleep was abnormal in these TG rats. This is consistent with a narcolepsy-like phenotype, and a clear effect of the partial HCRT deficiency observed in this model. In the previous study, sleep was recorded in young (about 4 months old) TG rats whereas here three of the five rats whose EEG was recorded were over two years old, and two were 14 and 21 months old. The old TG rats had a diurnal rhythm of REM sleep which was consistent with HCRT deficiency and not the result of aging, since diurnal distribution of REM sleep in wildtype old rats is blunted, but not inverted 23. We found abnormal REM sleep transitions, albeit only half as many as previously reported 3. We also did not find that the night-time sleep-wake pattern was fragmented compared to the WT rats.
Overall, in our rats, the day versus night distribution of REM sleep is consistent with two previous rat studies 3,11, but we did not find altered non REM sleep and wake bout duration, nor increased transitions between states. Hypocretin null 5 and the hypocretin-ataxin 13 mice show increased transitions between states. The partial hypocretin deficiency in the TG rats in the present study might help stabilize the sleep-wake architecture. We suggest that additional physiological studies in this TG rat model are warranted to further assess interspecies differences and the effect of partial versus complete hypocretin deficiency on various other phenotypes. Indeed, in other species, hypocretin deficiency has been reported to be associated with reduced food intake and metabolism, obesity, cardiovascular abnormalities and altered locomotion following food-restriction30.
A number of animal models of narcolepsy are available. In canines, narcolepsy can result from mutations in the hypocretin receptor-2 14 or from a sporadic loss of hypocretin neurons mimicking human narcolepsy 21. In mice, narcolepsy has been engineered though homologous recombination of the hypocretin gene (hypocretin knockout) 5 or of the hypocretin receptor-2 25. A pharmacological model where HCRT receptor bearing neurons are lesioned using the neurotoxin HCRT2-SAP has also been characterized 11. Finally, mice and rat models with hypocretin cell loss have been engineered by expressing a mutated ataxin gene containing a long poly-glutamine repeat under the control of the HCRT promoter 3,13. These TG rats and mice are the closest rodent models available to human narcolepsy, although our study suggests that the rat model has a more partial hypocretin cell loss than the mice model. Each of these models provides a different approach to investigate the neurobiology of the disease and hypocretin, which may vary across species 30.
CSF HCRT-1 levels have been measured in humans (for review see 30), squirrel monkey 29, canines 26, and rats 8,10,12,28. CSF HCRT-1 cannot be measured reliably in mice as, based on our experience, it is difficult to collect sufficient amounts of CSF for quantitative analysis of the peptide. The TG rats have an advantage over the other models since in rats it is possible to perform a longitudinal assessment of CSF HCRT-1 levels. Such studies in the TG rats will help evaluate whether strategies such as neuronal grafts can elevate CSF HCRT-1 levels and ameliorate symptoms. Two recent studies have reported that 5% of transplanted HCRT neurons survive 1,2. Such grafts could be made in the TG rats and correlated with changes in CSF HCRT-1, and sleep. Further studies using this rat model are needed to substantiate isolated findings made in other species.
Acknowledgments
We thank Suraiya Begum for providing technical assistance, E. Winston for analyzing the sleep data, and Shelley Dixon for expert managerial assistance. Supported by NIH grants NS30140, NS52287, MH55772, MH01600, NS23724, MH073435 and Medical Research Service of the Department of Veterans Affairs. EM and MY are HHMI investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shengwen Zhang, Stanford University, Palo Alto, CA 94304.
Ling Lin, Stanford University, Palo Alto, CA 94304.
Satvinder Kaur, West Roxbury VA Medical Center and Harvard Medical School, 1400 VFW Parkway, West Roxbury, MA, 02132.
Stephen Thankachan, West Roxbury VA Medical Center and Harvard Medical School, 1400 VFW Parkway, West Roxbury, MA, 02132.
Carlos Blanco-Centurion, West Roxbury VA Medical Center and Harvard Medical School, 1400 VFW Parkway, West Roxbury, MA, 02132.
Masashi Yanagisawa, University of Texas Southwestern Medical Center, Dallas, Texas 75390.
Emmanuel Mignot, Stanford University, Palo Alto, CA 94304.
Priyattam J. Shiromani, West Roxbury VA Medical Center and Harvard Medical School, 1400 VFW Parkway, West Roxbury, MA, 02132
Reference List
- 1.Arias-Carrion O, Drucker-Colin R, Murillo-Rodriguez E. Survival rates through time of hypocretin grafted neurons within their projection site. Neurosci Lett. 2006;404:93–97. doi: 10.1016/j.neulet.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Carrion O, Murillo-Rodriguez E, Xu M, Blanco-Centurion C, Drucker-Colin R, Shiromani PJ. Transplantation of hypocretin neurons into the pontine reticular formation: preliminary results. Sleep. 2004;27:1465–1470. doi: 10.1093/sleep/27.8.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–1192. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, Van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. Journal of Comparative Neurology. 1998;402:442–459. [PubMed] [Google Scholar]
- 10.Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S. Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12:993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- 11.Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerashchenko D, Murillo-Rodriguez E, Lin L, Xu M, Hallett L, Nishino S, Mignot E, Shiromani PJ. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Exp Neurol. 2003;184:1010–1016. doi: 10.1016/S0014-4886(03)00388-1. [DOI] [PubMed] [Google Scholar]
- 13.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 15.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 16.Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsch J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 17.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 18.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 19.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 20.Peyron C, Tighe DK, Van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripley B, Overeem S, Fujiki N, Nevsimalova S, Uchino M, Yesavage J, Di Monte D, Dohi K, Melberg A, Lammers GJ, Nishida Y, Roelandse FW, Hungs M, Mignot E, Nishino S. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–2258. doi: 10.1212/wnl.57.12.2253. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 23.Shiromani PJ, Lu J, Wagner D, Thakkar J, Greco MA, Basheer R, Thakkar M. Compensatory sleep response to 12 h wakefulness in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R125–R133. doi: 10.1152/ajpregu.2000.278.1.R125. [DOI] [PubMed] [Google Scholar]
- 24.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 26.Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto Y, Ueta Y, Hara Y, Serino R, Nomura M, Shibuya I, Shirahata A, Yamashita H. Postnatal development of orexin/hypocretin in rats. Brain Res Mol Brain Res. 2000;78:108–119. doi: 10.1016/s0169-328x(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 29.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci. 2006;27:368–374. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]