Abstract
The relationship between the P450 component elicited by affective stimuli and: a personal history of alcohol dependence, antisocial personality disorder/conduct disorder (ASPD/CD) or affective anxiety disorders (ANYAXAF) was examined in Mexican Americans, a group with high rates of heavy drinking. Data from two hundred and twenty two young adults between the ages of 18 and 30 were used in the analyses. ERPs were collected using a task that required discrimination between faces with neutral, sad and happy facial expressions. DSM-IIIR diagnoses were obtained using a structured interview and personality traits were indexed using the Maudsley personality inventory. Men had significantly diminished P450 responses, when compared to women which were further reduced in men with ASPD/CD; whereas, a significant increase in P450 amplitudes was seen in those participants with ANYAXAF. P450 amplitudes were also significantly increased in men with high extraversion scores and in women with high neuroticism scores. No significant associations were seen between the P450 amplitude and the diagnosis of alcohol dependence. These data suggest that interpretations of P450 responses in Mexican Americans need to take into account the interactions between gender, the affective valence of the eliciting stimuli, as well as psychiatric status.
Keywords: Mexican-Americans, Alcohol Dependence, ASPD, Emotion, P450, Affective Stimuli, Anxiety, Affective, Extraversion, Neuroticism
1. Introduction
Hispanic American males, like Native Americans, as a group, are more likely to drink frequently and to consume larger quantities of alcohol than Whites or Blacks (Caetano, 1984; Caetano and Kaskutas, 1995; Dawson, 1998; Nielsen, 2000; Stinson et al., 1998). Additionally, the total lifetime prevalence rate of alcoholism (alcohol abuse and dependence) has been found to be higher among Hispanic American men than among White men in the Epidemiologic Catchment Area (ECA) study (Helzer et al., 1991). Hispanic American subgroups bring with them a diversity of racial heritage as well as cultures that vary in psychosocial, religious, and economic bases. The importance of specifying subgroups of Hispanics to avoid inaccurate generalizations has been stressed (Caetano et al., 1998). Mexican Americans represent the largest subgroup of Hispanic Americans, nearly two thirds of the total U.S. Hispanic population, followed by Puerto Ricans, Cubans, Caribbeans, Central and South Americans. A recent report suggested that Mexican Americans show high rates of heavy drinking and alcohol-related problems (Caetano, 2003).
There is evidence to suggest that electrophysiological variables may represent ‘markers’ of vulnerability to or endophenotypes for alcohol dependence (see Begleiter and Porjesz, 1999; Ehlers et al., 1999; Enoch et al., 1995, 1999; Polich et al., 1994). The P300 or P3 component of the event-related potential (ERP) has received considerable attention as a possible neurophysiological marker for alcohol dependence risk. Many studies have demonstrated that the P3 amplitude is reduced in subjects with a family history of alcohol dependence, but who have not yet developed the disorder (Begleiter et al., 1984; Berman et al., 1993; Elmasian et al., 1982; Hill et al., 1988, 1990, 1995, 1999a, 1999b; O’ Connor et al., 1987; Porjesz and Begleiter, 1990, 1998; Whipple et al., 1988). Findings from other studies are less conclusive or do not support this hypothesis (see Bauer, 1997; Bauer and Hesselbrock, 1999a, 1999b; Polich and Bloom, 1988; Rodriguez Holguin et al., 1998). Additionally, reduced P3 amplitudes have been found to be associated with other psychiatric disorders that have significant comorbidity with alcohol dependence, such as conduct disorder, borderline personality disorder and depression (see Bauer, 1997, 2002; Bauer and Hesselbrock, 1999a, 1999b, 2001, 2003; Bauer et al., 1994a, 1994b, 2001; Carlson et al., 1999; Ceballos et al., 2006; Costa et al., 2000; Hill et al., 1999a; Houston et al., 2003, 2004a, 2004b, 2005; Iacono et al., 2002, 2003; O’ Connor et al., 1994).
The P3 component, can in some paradigms, consist of two separate positive subcomponents, P3a and P3b, or early and late P300 (for a review, see Polich and Criado 2006). The earlier P3a component (in the 300 msec range in visual tasks) has a fronto-central distribution and has been associated with the “novelty” of a stimulus, and the redirection of attention monitoring (Barcelo et al., 2000; Courchesne et al., 1975; Hartikainen et al., 2003; Knight 1990, 1997; Spencer et al., 2001). In contrast, the late P3b component (400 msec range in a visual task) has a temporo-parietal distribution and has been associated with attention and may index memory updating (Knight et al 1989; Yamaguchi and Knight, 1992; for a review, see Polich and Criado, 2006). Previous studies have shown that presentation of affective stimuli elicits a P3b-like component that peaks between 300 and 600 msec after stimulus presentation (Barret and Rugg, 1989; Johnston et al., 1986; Sommer et al., 1991). This component, which is generated by a paradigm different from those traditionally used to generate P3a and P3b components, has been labeled a P450 wave (Kestenbaum and Nelson, 1992; Lang et al., 1990). Using a modified version of the facial recognition task developed by Erwin et al. (1992), we have demonstrated, in Southwest California (SWC) adults, a significant relationship between alcohol dependence and a reduction in the amplitude of the P450 component (Criado and Ehlers, in submission). These findings are consistent with previous reports showing that the P3 component is reduced in alcoholic individuals, supporting the view of its potential as an important endophenotype for alcohol dependence (see Begleiter and Porjesz, 1999; Ehlers et al., 1999; Enoch et al., 1995, 1999; Polich et al., 1994). The relationship between the P450 component elicited by affective stimuli and a personal history of alcohol dependence in Mexican Americans is not well understood.
The expression of certain personality traits has been suggested to be one of the most prominent factors implicated to increase the risk for alcoholism and substance abuse (see Acton, 2003; Cloninger et al., 1988; Larkins and Sher, 2006; Pihl and Peterson, 1995; Sher and Trull, 1994; Sher, et al., 1999, 2000). There is also support to the view that biological mechanisms proposed to underlie a number of personality traits may be risk factors for alcoholism (Acton, 2003). Previous studies have also provided evidence to suggest that personality traits may influence the P300 component of the ERP (Cahill and Polich, 1992; Daruna and Karrer, 1984; Ditraglia and Polich, 1991; Orlebeke et al., 1989; Pritchard, 1989; Simons et al., 1982; Stelmack et al., 1993). Two personality traits, extraversion and neuroticism, have been suggested to influence the use and abuse of alcohol (see Larkins and Sher, 2006; Read and O’Connor, 2006; Sher and Trull, 1994). However, the relationship between alcohol dependence, P450 amplitude and personality traits has not been previously studied in Mexican Americans.
In the present study, a facial discrimination task was used to generate the P450 component of the ERP in a population of young adult Mexican Americans. Participants in the study had to be self-identified as Mexican American and both parents had to be of Mexican heritage. This study has several objectives. First, we sought to determine the relationships among P450 amplitudes, a personal history of alcohol dependence and two frequently comorbid disorders: antisocial personality disorder/conduct disorder (ASPD/CD) and affective/anxiety disorders (ANYAXAF). Studying the relationship between alcohol dependence other psychiatric disorders that may be co-morbid with alcohol dependence is important since this Mexican American sample had high co-morbidity of alcohol dependence with anxiety and affective disorders (Gilder et al., 2007), whereas Native Americans living in the same county have been reported to have low co-morbidity of alcohol dependence with anxiety and affective disorders (Gilder et al., 2004). Secondly, we investigated the relationship between P450 and the basic personality traits of extraversion and neuroticism. Because women as a whole have larger P450 amplitudes, the analyses were conducted in the population as a whole and in men and women separately.
2. Materials and methods
2.1. Participants
Participants were recruited using a commercial mailing list that provided the addresses of individuals with Hispanic surnames in 11 zip codes in San Diego County that were identified as having a population that was over 20% Hispanic heritage and were within 25 miles of the research site. The mailed invitation stated that potential participants must be of Mexican American heritage, be between the ages of 18 and 30 years, be residing in the United States legally, and be able to read and write in English. Potential participants were requested to phone research staff for more information. During the phone interview potential participants were screened for the presence of the inclusion criteria as listed on the invitation, and were excluded if they were: pregnant or nursing, currently had a major medical or neurological disorder, or a head injury that might bias the ERP testing. Participants were asked to refrain from alcohol or any other substance use for 24 hours prior to testing. None of the participants exhibited physical or behavioral signs of alcohol withdrawal. On the test day, after a complete description of the study to the participants, written informed consent was obtained using a protocol approved by The Institutional Review Board of The Scripps Research Institute.
2.2. Psychiatric Diagnoses
Each participant completed a face-to-face interview with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), which was used to make substance use and other psychiatric disorder diagnoses according to DSM-III-R criteria. The SSAGA is a fully structured, polydiagnostic psychiatric interview that has undergone both reliability and validity testing (Bucholz et al., 1994; Hesselbrock et al., 1999). Participants were eliminated from the current data analyses if they were taking psychoactive medication that may affect the ERP or had a positive breath-analyzer test on the day of the evaluation. Lifetime history of alcohol dependence, antisocial personality disorder/conduct disorder (ASPD/CD) and affective/anxiety disorders (ANYAXAF= any of the following diagnoses: major depressive disorder, social phobia, agoraphobia, panic disorder or obsessive compulsive disorder), in this population, were defined by DSM-III-R criteria. Basic features of personality were assessed using the Maudsley personality inventory (MPI) (Eysenck and Eysenck, 1975), which yields scores for extraversion (E) and neuroticism (N). For statistical analyses, ERP variables in participants with scores that were greater than one standard deviation from the mean, for each scale of the Maudsley (E and N), were compared to the remainder of the participants in the study. Additionally, Pearson correlations between P450 amplitudes and scores on the Maudsley were also calculated (E and N).
2.3. ERP Collection and Analyses
Seven channels of ERP data (FZ, CZ, PZ, F3, F4, F7, and F8, referenced to linked ear lobes with a forehead ground, international 10–20 system) were obtained using gold-plated electrodes with impedances held below 5 KΩ. Frontal electrodes were emphasized in the montage, as previous data had suggested that P3 decrements in frontal areas distinguished subjects with a risk for alcohol dependence (see Bauer, 1997). An electrode placed left lateral infraorbitally and referenced to the left earlobe was used to monitor both horizontal and vertical eye movements. ERP signals were amplified (time constant 0.3 s, 35 Hz low pass) using a Nihon Kohden EEG machine and were transferred on-line to a PC. The combined gain of the EEG amplifiers and the analog-to-digital multiplexer amplifier was 50K.
The present study used a facial discrimination task (Erwin et al., 1992; Gur et al., 1992; Heimberg et al., 1992) that was adapted for use in an ERP paradigm (Orozco and Ehlers, 1998). The stimuli were digital photographs of happy, neutral and sad faces presented on a computer screen for 1000 ms with an inter-trial interval of 1000–1500 ms. The pre-stimulus interval was 150 ms. Participants were instructed to depress a counter whenever a happy or sad face was displayed (36 trials each) and not to respond to neutral faces (144 trials). There were 36 total faces (12 each of happy, neutral, and sad) presented in random order for a total of 216 trials. The number of male and female faces presented was also equally distributed among neutral, sad and happy stimuli.
The ERP trials were digitized at a rate of 256 Hz (bandwidth 0.5–35 Hz). Individual trials containing excessive eye movement artifact as well as trials where the EEG exceeded ±250 microvolts (<5% of the trials) were eliminated before averaging. Trials in which subjects responded below the 300 ms and above the 1000 ms latency window were excluded. The occurrence of eye movements was noted on individual trials and eliminated prior to averaging. For target stimuli, only trials with correct identification were included in the averaging. The P450 component of the ERP was quantified using a computerized peak detection routine that identifies baseline-to-peak amplitudes (in μV) within specified latency windows (400–600 msec). The baseline was determined by averaging the 100 ms of pre-stimulus activity obtained for each trial. The routine is user-driven and each peak detection must be verified by the user. All peaks were quantified by one investigator (R EEG Tech), and verified by a second investigator, both of whom were blind to participants’ characteristics. Time of recording with respect to the menstrual cycle was not controlled, as previous studies have demonstrated that the ERP variables under study are not sensitive to time during the cycle (see Ehlers et al., 1996).
2.4. Data Analysis
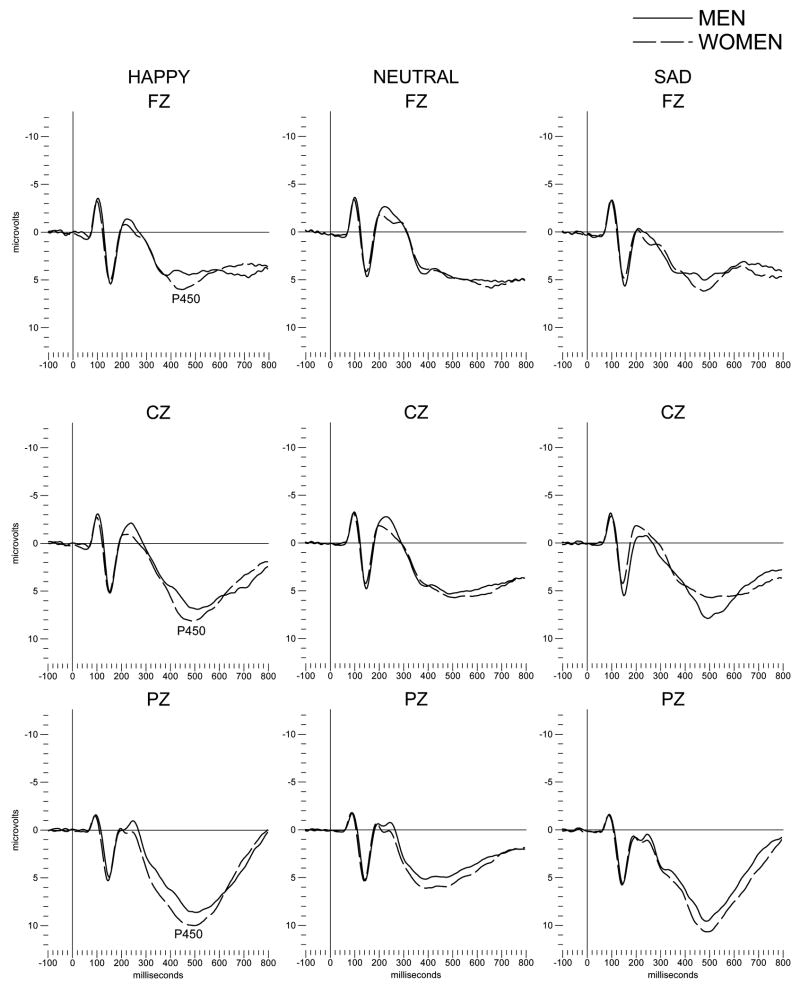
Data analyses focused on several hypotheses that were generated based on previous ERP research in other ethnic populations at varying degrees of risk for the development of alcohol dependence (see Bauer and Hesselbrock, 1999a, 1999b; Ehlers et al., 1998). To analyze the P450 component of the ERP, a principal component analysis (PCA) was performed over the seven electrode locations for P450 amplitudes to the stimuli in the facial recognition task, as described previously (Ehlers et al., 2001) (Fig. 1). For each of the stimuli, varimax rotation yielded two components (eigenvalues > 1, range= 1.0–4.7). The electrode sites loading on the first factor were the frontal leads (FZ, F3, F4, F7, F8), and on the second factor were the two more posterior leads (CZ, PZ) (loadings ranged from .67 to .91).
Figure 1.
Grand averages of event-related potentials (ERPs) elicited by a facial discrimination task in Mexican American young adults. Averages are presented for frontal (Fz) and central (Cz) and parietal (Pz) leads as elicited by each of the three types of facial expressions (happy, neutral, sad). The P450 component is indicated; solid lines are for male participants and dashed lines for females.
The two orthogonal factors each explained between 15–68% of the variance for the ERP task. P450 amplitudes were averaged across the electrode sites within each of the two identified components: 1 = (FZ, F3, F4, F7, F8), 2 = (CZ, PZ), generating a mean for each of the two regions. These regionally averaged scores were generated for each stimulus condition (neutral, sad, happy), generating mean amplitudes for each of the two component regions, for each stimulus category for each individual. The regionally averaged P450 amplitudes generated by the facial discrimination task were used as dependent variables. P450 amplitudes identified in the PCA (frontal leads, centro-parietal leads) generated to the stimuli obtained from the facial discrimination task (happy, neutral, sad) were evaluated using multivariate ANOVAs for the entire group and for men and women separately. Statistical significance was set at the 0.05 probability level. Pearson correlations were used to compare P450 amplitudes and scores on the Maudsley Personality Inventory.
3. Results
3.1. Descriptive Data and gender differences in P450
Demographic data on the 222 participants with valid ERP data are presented in Table 1. When P450 responses were compared in the entire population between men and women significant differences emerged as seen in figure 1. Women were found to have significantly higher amplitude P450 responses to Happy (frontal: F= 6.1; df=1,216; p<0.01, centro-parietal: F=5.7; df=1,221; p<0.02) and Sad (frontal: F= 4.2; df=1,216; p<0.04, centro-parietal: F=4.1; df=1,219; p<0.04) but not neutral facial expressions. Women also had significantly longer latencies from presentation of the stimuli to identification by depressing the correct button (Happy, men: 635 ± 7 msec, women: 656 ± 6 msec F= 5.8 df=1,221; p<0.016, and Sad, men: 659 ± 7 msec, women: 681 ± 6 msec F= 5.5 df=1,221; p<0.02).
Table 1.
Demographic characteristics of Mexican American participants comparing alcohol dependence groups, ASPD/CD diagnosis groups, any anxiety or affective disorder groups, extraversion groups and neuroticism groups (n = 222)
| Demographic variable | Total sample (n = 222) | Alcohol dependence (n = 53) | ASPD/CD diagnosis (n = 25) | Any anxiety or affective disorder (n = 64) | Extraversion score ≥18 (n = 18) | Neuroticism score ≥16 (n = 22) |
|---|---|---|---|---|---|---|
| Age (years) | 23.3 ± 0.3 | 24.1 ± 0.6 | 24.6 ± 0.9 | 22.3 ± 0.5 * | 21.3 ± 0.9 * | 21.9 ± 0.9 |
| Gender (n) | ||||||
| Male | 92 | 27 | 13 | 23 | 9 | 6 |
| Female | 130 | 26 | 12 | 41 | 9 | 16 |
| Years of education | 13.5 ± 0.1 | 14.0 ± 0.2 * | 13.1 ± 0.4 | 13.1 ± 0.2 * | 13.0 ± 0.4 | 13.1 ± 0.3 |
| Employed | ||||||
| No | 58 | 11 | 9 | 21 | 4 | 10 * |
| Yes | 164 | 42 | 16 | 43 | 14 | 12 |
| Extraversion Score | 13.0 ± 0.2 | 13.7 ± 0.5 | 13.8 ± 0.6 | 12.7 ± 0.4 | N/A | N/A |
| Neuroticism Score | 8.4 ± 0.3 | 10.6 ± 0.7 * | 9.6 ± 1.2 | 11.2 ± 0.7 * | N/A | N/A |
Values are Means ± SEM unless indicated
p < 0.05 compared to larger control sample
3.2. Associations of P450 responses in Mexican American young adults with Alcohol Dependence, ASPD/CD and ANYAXAF
P450 amplitudes were evaluated in the frontal and centro-parietal leads in the entire population (n=222), and in men and women separately, as a function of alcohol dependence, ASPD/CD and ANYAXAF. Multivariate ANOVA revealed that those participants with a lifetime DSM-III diagnosis of alcohol dependence did not differ from those without alcohol dependence on any P450 variables (data not shown). No significant differences in the latencies from presentation of the stimuli to identification by depressing the correct button was found based on a lifetime diagnosis of alcohol dependence, ASPD/CD and/or ANYAXAF.
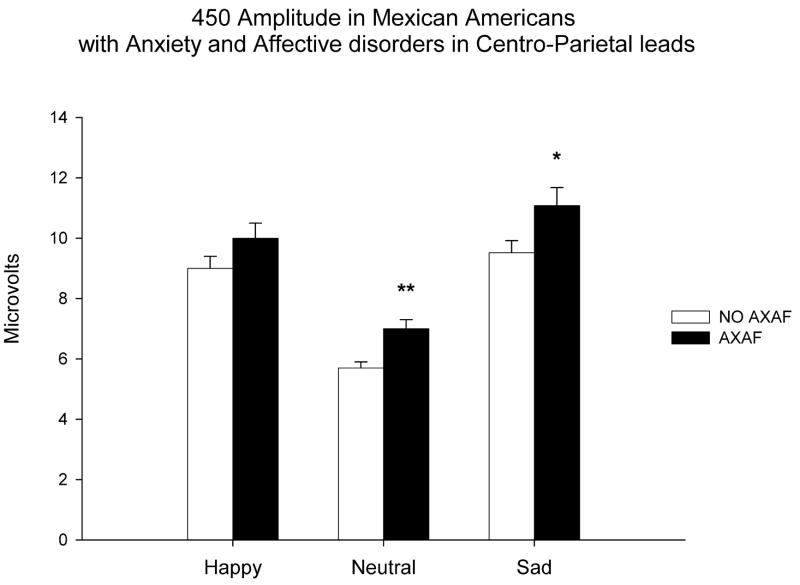
A significant association was found between P450 amplitudes and a lifetime diagnoses of ANYAXAF. As seen in figure 2, a significant increase in P450 amplitudes to sad (F=4.67; df=1,218; p<0.03) and to neutral (F=8.47; df=1,220; p<0.004) faces in centro-parietal areas was found in those participants with ANYAXAF. Associations between a personal history of ANYAXAF and P450 amplitudes were additionally tested in men and women separately. Women with a personal history of ANYAXAF were also found to have significantly elevated P450 response to neutral faces (F=5.23; df=1,128; p<0.02) when compared to women without those disorders.
Figure 2.
P450 Amplitude (microvolts) to happy, neutral, and sad facial expressions in those participants with a select anxiety or affective disorder (AXAF) in the black bars, and in those without a select anxiety or affective disorder (no AXAF) in the open bars. Values are given as means±SEM. Asterisk indicates a significant difference between groups. (*p<0.05, ** p<0.01)
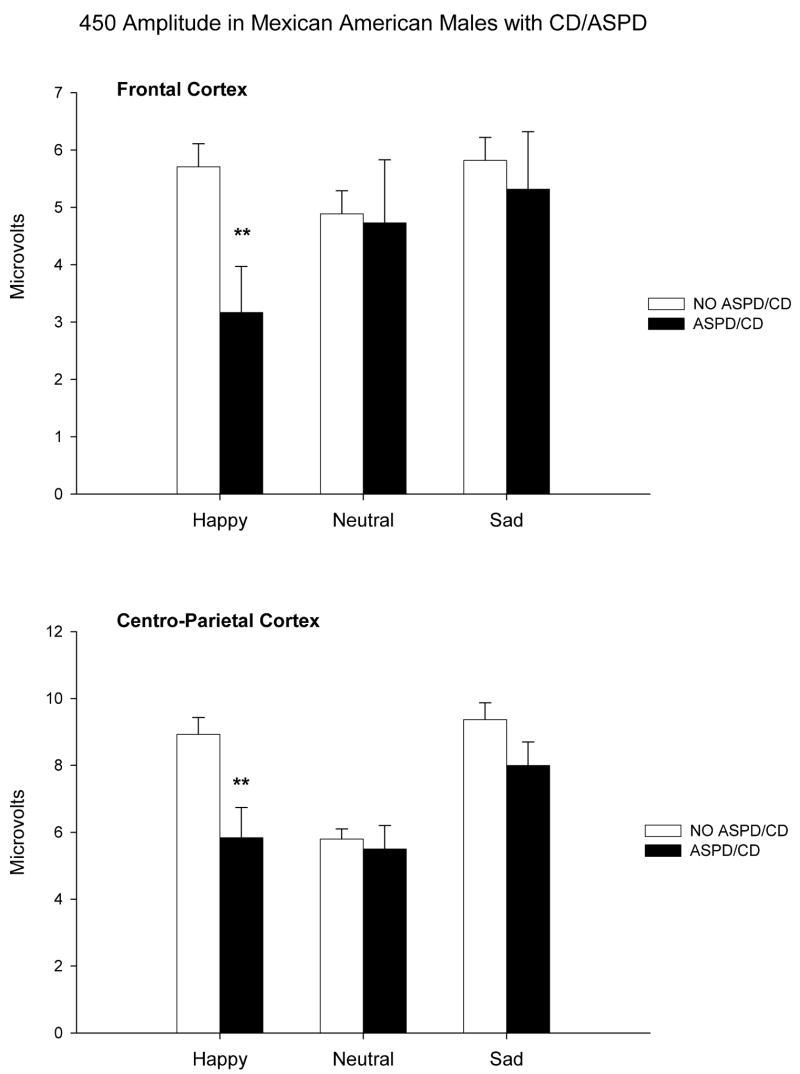
Multivariate ANOVA revealed no significant associations between ASPD/CD and P450 amplitudes when the entire population was evaluated in the analyses. However, as seen in figure 3, associations between a personal history of ASPD/CD and P450 amplitudes were additionally tested in men and women separately and significant reductions in P450 amplitudes to happy faces in frontal (F=6.54; df=1,90; p<0.01) and centro-parietal (F=6.72; df=1,90; p<0.01) areas were found in men with ASPD/CD as compared to men without the disorder. No significant associations between CD/ASPD and the P450 component were found in women.
Figure 3.
P450 Amplitude (microvolts) to happy, neutral, and sad facial expressions in male participants with conduct disorder and/or antisocial personality disorder (ASPD/CD) in the black bars and in those without conduct disorder and/or antisocial personality disorder (no ASPD/CD) in the open bars. Upper graph is data from Frontal Cortical Leads. Lower graph is from centro-parietal leads. Data are presented as means±SEM. Asterisks indicate significant difference between the groups. **P<0.01)
3.3. Associations of P450 responses in Mexican American young adults with Extraversion and Neuroticism
To evaluate further the relationship between P450 amplitudes and basic personality traits, the Maudsley personality inventory (MPI) was used to measure two dimensions of personality: extraversion and neuroticism. ANOVA revealed that those participants with extraversion scores that were over one standard deviation from the mean had significant elevated P450 responses to sad faces in frontal leads (F=8.2; df=1,213; p<0.005) when compared to the remainder of the population. When associations between extraversion and the P450 component were tested in men and women separately, men with extraversion scores greater than one standard deviation from the mean were found to have significantly higher component amplitudes in response to happy (centro-parietal; F = 7.8; df = 1,88; p<0.006) and sad faces (frontal; F = 8.2; df = 1,88; p<0.005) when compared to men with lower scores. No significant associations between extraversion and the P450 component were found in women (see table 2).
Table 2.
P450 amplitudes (μV) in response to affective stimuli in Mexican Americans with extraversion or neuroticism traits
| Extraversion Score ≥ 18 | |||
|---|---|---|---|
| Men (n = 9) | Women (n = 9) | Total (n = 18) | |
| Frontal lead | |||
| Facial expression | |||
| Happy | 7.11 ± 1.52 | 7.67 ± 1.24 | 7.39 ± 0.95 |
| Sad | 8.92 ± 1.69** | 9.07 ± 2.55 | 8.99 ± 1.48** |
| Neutral | 5.73 ± 1.46 | 6.01 ± 1.11 | 5.87 ± 0.89 |
| Centro-Parietal lead | |||
| Facial expression | |||
| Happy | 11.99 ± 1.58*** | 9.40 ± 1.05 | 10.69 ± 0.97 |
| Sad | 12.15 ± 2.10* | 11.35 ± 1.44 | 11.75 ± 1.24 |
| Neutral | 6.65 ± 0.91 | 6.55 ± 1.01 | 6.60 ± 0.66 |
| Mean Extraversion Score (for entire sample, n = 222) | 13.5 ± 0.4 | 12.6 ± 0.3 | 13.0 ± 0.2 |
| Neuroticism Score ≥ 16 | |||
| Men (n = 6) | Women (n = 16) | Total (n = 22) | |
| Frontal lead | |||
| Facial expression | |||
| Happy | 7.04 ± 1.96 | 7.42 ± 0.82 | 7.31 ± 0.79 |
| Sad | 7.77 ± 2.15 | 8.68 ± 1.05 | 8.43 ± 0.94* |
| Neutral | 5.10 ± 1.05 | 6.05 ± 0.82 | 5.79 ± 0.66 |
| Centro-Parietal lead | |||
| Facial expression | |||
| Happy | 10.73 ± 0.91 | 13.15 ± 0.89** | 12.49 ± 0.72*** |
| Sad | 10.65 ± 1.67 | 14.25 ± 1.21** | 13.27 ± 1.03** |
| Neutral | 6.64 ± 0.94 | 8.51 ± 0.65** | 8.00 ± 0.56** |
| Mean Neuroticism Score (for entire sample, n = 222) | 4.9 ± 0.5 | 5.3 ± 0.5 | 8.4 ± 0.3 |
Values are Means ± SEM
p < 0.05,
p < 0.01,
p < 0.001 comparing groups shown to Mexican American participants without extraversion or neuroticism traits
A significant relationship was also found between P450 amplitudes and neuroticism scores in both frontal and centro-parietal leads in response to happy, neutral and sad faces as seen in Table 2. In the entire population sample, participants with scores above one standard deviation from the mean on the neuroticism scale of the MPI were found to have increases in P450 amplitudes in response to happy (centro-parietal; F= 13.1; df = 1,218; p<0.0001), neutral (centro-parietal; F= 11.0; df = 1,218; p<0.001) and sad (frontal; F = 6.2; df = 1,213; p<0.01; centro-parietal; F = 12.5; df = 1,216; p<0.001) facial expressions. Significant correlations between scores on the neuroticism scale and P450 amplitude were also found using Pearsons test (Happy: frontal leads r=0.145, p<0.034; centro-parietal leads r=0.172, p<0.011, Neutral: centro-posterior leads r=0.164, p<0.015, Sad: frontal leads r=0.194, p<0.004, centro-posterior leads r=0.187, p<0.005). No significant correlations were found between extraversions scores and the P450 amplitude.
When men and women were run separately significant increases in P450 amplitudes continued to be seen in women with neuroticism scores over one standard deviation from the mean in response to happy (centro-parietal areas: F= 9.8; df = 1,126; p<0.002), neutral (centro-parietal areas: F= 9.4; df = 1,126; p<0.003) and to sad faces (centro-parietal: F= 10.5; df = 1,124; p<0.002), as compared to women with lower scores. No significant associations between neuroticism and the P450 component were found in men. Because neuroticism and ANAXAF may be indexing similar or different constructs in their relationship to P450 amplitude, an ANOVA was conducted in women for ANAXAF using their neuroticism score as a covariate in the analysis. Significant associations between ANYAXAF and P450 responses to: happy faces in the centro-posterior leads (F=4.12; df=2,127; p<0.018), neutral faces in the centro-posterior leads (F=3.12; df=2,127; p<0.048), and sad faces in the centro-posterior leads (F=3.80; df=2,127; p<0.025) were still found in that analysis, suggesting that the relationship between ANYAXAF and P450 amplitude is not entirely accounted for by neuroticism. No significant differences in the latencies from presentation of the stimuli to identification by depressing the correct button were found based on extraversion or neuroticism scores.
4. Discussion
This investigation extends our previous studies in other ethnic groups (see Ehlers et al., 2001, 2003; Orozco and Ehlers, 1998), by characterizing, in Mexican American young adults, associations between the amplitude of the P450 component of the ERP and alcohol dependence and related comorbid disorders. The present study found a series of relationships between P450 amplitude, gender, the affective valence of the eliciting stimuli, and the psychiatric status of the participant group. Women displayed higher P450 amplitude responses to the target stimuli (happy and sad faces) than men; however, there were no differences between men and women in response to neutral faces. These data could be interpreted as either a greater electrophysiological response to emotional stimuli in women or a less intense response to emotional stimuli in men. These data confirm what has been reported previously for simple oddball tasks (see Hoffman and Polich, 1999) where women were found overall to have higher amplitude P350 and P450 ERP components than men.
Data from a number of electrophysiological and behavioral genetics studies have converged on the idea that substance dependence and antisocial behavioral disorders comprise a spectrum that may have common risk factors (Begleiter and Porjesz, 1999; Button et al., 2005; Du et al., 2006; Iacono et al., 2003; Patrick et al., 2006; Waldman and Slutske, 2000). There is also ample evidence supporting an association between low P3 amplitude and these externalizing disorders (Bauer, 1997; Bauer and Hesselbrock, 1999a, 1999b, 2003; Bauer et al., 1994a; Costa et al., 2000; Iacono et al., 2002, 2003; Kamarajan et al., 2005, 2006). The present study also found that a personal history of ASPD/CD was associated with significant reductions in P450 amplitudes; specifically to happy faces in frontal and centro-parietal areas and only in men, not in women. Previous studies that have reported P3 amplitude reductions in populations with externalizing disorders have primarily focused on paradigms that did not use affective stimuli. Additionally, most studies evaluated participants that were entirely or predominantly male (e.g. Bauer and Hesselbrock, 1999b; Costa et al., 2000; Iacono et al., 2003), as well as under age 30. Our studies suggest that, in this select population of Mexican American young adults, P450 reductions are associated with a personal history of ASPD/CD, only in males, and only in response to happy facial expressions.
Reductions in P450 amplitudes, as a function of alcohol dependence, have been reported in a population of Native Americans using the same task that was employed in the present study (Criado and Ehlers, in submission). In contrast to what has been reported previously in the P450 literature, no P450 amplitude decrements were found as a function of personal history of alcoholism in this population of Mexican American young adults. One reason that this population may not have significant associations of P450 amplitudes and alcohol dependence may be that it was a community sample of young adults with relatively milder alcohol dependence than what is seen in Native Americans residing in the same county (Ehlers et al., 2004), or what has been reported for the COGA study (Schuckit et al., 2002).
Another potential difference in the present population of Mexican American young adults, that may have influenced the expression of P450 responses, is the pattern of comorbidity of alcohol dependence with other psychiatric disorders. A high prevalence of anxiety and affective disorders has been found in this population (Gilder et al., 2007) when compared to Native Americans living in the same geographical region (Gilder et al., 2004). Having a lifetime diagnosis of any of a select group of anxiety and affective disorders (ANYAXAF) was found to be associated with increases in P450 amplitudes to sad and neutral faces in centro-parietal areas in this Mexican American population. These studies confirm previous findings in a Native American population residing in a similar geographic location where an increase in both P350 and P450 amplitudes were found in individuals with a personal history of affective disorder (Criado and Ehlers, in submission). These findings, however, do not confirm the study of Hill et al. (1999a) who found reduced P300 amplitude in female alcoholics with a comorbid lifetime diagnosis of depression. However, two other studies have also found larger P3 amplitudes in participants with affective disorder. In a study of female adolescents with a history of a major depressive episode, P300 amplitude was less dependent on task suggesting “cognitive inflexibility” (Houston et al., 2004a). Additionally, Bruder et al. (2002) found a late P3 (P400) elicited by a phonetic task was larger in patients having comorbidity of anxiety and depressive disorders. Taken together these studies underscore the importance of taking comorbidity into account in studies of cognitive ERPs and drug use disorders.
The present study found that scores on the MPI for the extraversion and introversion scales that were above one standard deviation from the mean were also associated with P450 amplitude. Men with high extraversion scores showed significantly higher P450 amplitudes in response to happy and sad faces. These findings are consistent with a study by Hansenne (1999) who found that P300 amplitude was positively correlated with novelty seeking on the Temperament and Character Inventory (TCI), a trait often associated with extraversion. Previous studies have shown that extraverts may exhibit lower P300 amplitude than introverts in tasks where many trials are presented (Daruna et al., 1985; O’Connor, 1983; Polich and Martin, 1992). However, in tasks where fewer target trials are recorded, extraverts show larger P300 amplitudes than introverts (Cahill and Polich, 1992; Ditraglia and Polich, 1991). Studies by Ditraglia and Polich (1991) also suggest that P300 amplitudes show habituation to target stimuli in extraverted, but not introverted participants. These results might be due to the tendency of extraverts to react more strongly during the initial trials (Eysenck, 1967; Marton, 1972), or as in the present study, to affectively salient target stimuli.
The present study also found increases in P450 amplitudes in response to happy and sad faces in the entire sample and in women, with high neuroticism scores. Anxiety has been shown to be an essential trait of the neurotic personality dimension (Otten et al., 1995). In one study of a community sample, it was found that auditory P300 amplitudes were high in individuals with anxiety disorders (Enoch et al., 2001), although not all studies have confirmed these findings (Bauer et al., 2001). The present findings suggest that trait neuroticism may increase electrophysiological responses to affective stimuli, particularly in women.
In summary, these data suggest that P450 response to affective stimuli in Mexican American young adults differ depending on gender and diagnosis. Mexican American men with ASPD/CD were found to have diminished P450 responses to happy facial expressions. Mexican American women with anxiety and affective disorders and/or high neuroticism scores were found to have enhanced P450 response to neutral and sad facial expressions. These data suggest that interpretations of P450 responses need to take into account both ethnicity and the interactions between gender, the affective valence of the eliciting stimuli, as well as psychiatric status.
The results of this study should be interpreted in the context of several limitations. First, the findings may not generalize to all Mexican Americans, or all Hispanic young adult Americans. Over half of the participants in the present were women and thus findings many not generalize to previous studies that have focused on samples of entirely male participants. Second, the study was limited to young adults between the ages of 18 and 30 years, and the sample size limits interpretation of gender differences. Further studies employing a longitudinal design will be required to test the relationship of P450 amplitude, gender, alcohol dependence and comorbid disorders. Despite these limitations, this report represents an important step in an ongoing investigation to determine risk and protective factors associated with development of substance use disorders in Mexican Americans.
Acknowledgments
Supported in part by National Institute on Alcoholism and Alcohol Abuse grant AA06420 and AA10201, the National Center for Minority Health/Health Disparities, and by the Stein Endowment fund. JRC is recipient of the Dallas and Mary Clark Fellowship in Human Neurophysiology at the Brain Research and Treatment Center, Scripps Clinic, La Jolla, CA. The computer programs were written by Dr. James Havstad. The authors thank David Gilder, Phil Lau, Susan Lopez, Evelyn Phillips and Gina Stouffer for assistance in data collection and analyses, and Shirley Sanchez for help in editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton GS. Measurement of impulsivity in a hierarchical model of personality traits: implications for substance use. Subst Use Misuse. 2003;38:67–83. doi: 10.1081/ja-120016566. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Barrett SE, Rugg MD. Event-related potentials and the semantic matching of faces. Neuropsychologia. 1989;27:913–22. doi: 10.1016/0028-3932(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol Depend. 1997;44:1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Differential effects of alcohol, cocaine, and opioid abuse on event-related potentials recorded during a response competition task. Drug Alcohol Depend. 2002;66:137–45. doi: 10.1016/s0376-8716(01)00190-9. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Costa L, Hesselbrock VM. Effects of alcoholism, anxiety and depression on P300 in women: a pilot study. J Stud Alcohol. 2001;62:571–9. doi: 10.15288/jsa.2001.62.571. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999a;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999b;46:263–72. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents “at-risk”: evidence of frontal cortex dysfunction in conduct disorder. Biol Psychiatry. 2001;50:600–8. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on P300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:106–15. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM, O’Connor S, Roberts L. P300 differences between nonalcoholic young men at average and above average risk for alcoholism: effects of distraction and task modality. Prog Neuropsychopharmacol Biol Psychiatry. 1994a;18:263–77. doi: 10.1016/0278-5846(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcohol Clin Exp Res. 1994b;18:1300–5. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–35. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–6. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clin Electroencephalogr. 2002;33:119–24. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behav Genet. 2005;35:115–20. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Caetano R. A note on arrest statistics for alcohol-related offenses. Drinking Pract Surv. 1984;19:12–7. [Google Scholar]
- Caetano R. Alcohol-related health disparities and treatment-related epidemiological findings among whites, blacks, and Hispanics in the United States. Alcohol Clin Exp Res. 2003;27:1337–9. doi: 10.1097/01.ALC.0000080342.05229.86. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark CL, Tam T. Alcohol consumption among racial/ethnic minorities: theory and research. Alcohol Health Res World. 1998;22:233–41. [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Kaskutas LA. Changes in drinking patterns among whites, blacks and Hispanics, 1984–1992. J Stud Alcohol. 1995;56:558–65. doi: 10.15288/jsa.1995.56.558. [DOI] [PubMed] [Google Scholar]
- Cahill JM, Polich J. P300, probability, and introverted/extroverted personality types. Biol Psychol. 1992;33:23–35. doi: 10.1016/0301-0511(92)90003-d. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–90. [PubMed] [Google Scholar]
- Ceballos NA, Houston RJ, Hesselbrock VM, Bauer LO. Brain maturation in conduct disorder versus borderline personality disorder. Neuropsychobiology. 2006;53:94–100. doi: 10.1159/000092217. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1988;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biol Psychiatry. 2000;47:1064–71. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Daruna JH, Karrer R. Event-related potential correlates of intelligence and personality. Ann N Y Acad Sci. 1984;425:565–9. doi: 10.1111/j.1749-6632.1984.tb23578.x. [DOI] [PubMed] [Google Scholar]
- Daruna JH, Karrer R, Rosen AJ. Introversion, attention and the late positive component of event-related potentials. Biol Psychol. 1985;20:249–59. doi: 10.1016/0301-0511(85)90001-8. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Beyond black, white and Hispanic: race, ethnic origin and drinking patterns in the United States. J Subst Abuse. 1998;10:321–39. doi: 10.1016/s0899-3289(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Ditraglia GM, Polich J. P300 and introverted/extraverted personality types. Psychophysiology. 1991;28:177–84. doi: 10.1111/j.1469-8986.1991.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Du J, Li J, Wang Y, Jiang Q, Livesley WJ, Jang KL, Wang K, Wang W. Event-related potentials in adolescents with combined ADHD and CD disorder: a single stimulus paradigm. Brain Cogn. 2006;60:70–5. doi: 10.1016/j.bandc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Cloutier D, Phillips E. Electroencephalographic responses to alcohol challenge in Native American Mission Indians. Biol Psychiatry. 1999;45:776–87. doi: 10.1016/s0006-3223(98)00113-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Sobel DF, Phillips E. Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology. 1998;18:282–92. doi: 10.1016/S0893-133X(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Parry BL. Electrophysiological findings during the menstrual cycle in women with and without late luteal phase dysphoric disorder: relationship to risk for alcoholism? Biol Psychiatry. 1996;39:720–32. doi: 10.1016/0006-3223(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Sweeny A, Slawecki CJ. Event-related potential responses to alcohol-related stimuli in African-American young adults: relation to family history of alcoholism and drug usage. Alcohol Alcohol. 2003;38:332–8. doi: 10.1093/alcalc/agg080. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Spence JP, Wall TL, Gilder DA, Carr LG. Association of ALDH1 promoter polymorphisms with alcohol-related phenotypes in Southwest California Indians. Alcohol Clin Exp Res. 2004;28:1481–6. doi: 10.1097/01.alc.0000141821.06062.20. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Elmasian R, Neville H, Woods D, Schuckit M, Bloom F. Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proc Natl Acad Sci U S A. 1982;79:7900–3. doi: 10.1073/pnas.79.24.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Rohrbaugh JW, Davis EZ, Harris CR, Ellingson RJ, Andreason P, Moore V, Varner JL, Brown GL, Eckardt MJ. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am J Med Genet. 1995;60:400–8. doi: 10.1002/ajmg.1320600510. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin Exp Res. 1999;23:1312–9. [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Rohrbaugh JW, Goldman D. Alcohol use disorders and anxiety disorders: relation to the P300 event-related potential. Alcohol Clin Exp Res. 2001;25:1293–300. [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992;42:231–40. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Publication no. 689. Springfield, IL: Thomas; 1967. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck personality questionnaire (junior and adult) London: Hodder and Stoughton; 1975. [Google Scholar]
- Gilder DA, Lau P, Gross A, Ehlers CL. Co-morbidity of alcohol dependence with other psychiatric disorders in young adult Mexican Americans. J Addict Dis. 2007 doi: 10.1300/J069v26n04_05. In Press. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Wall TL, Ehlers CL. Comorbidity of select anxiety and affective disorders with alcohol dependence in Southwest California Indians. Alcohol Clin Exp Res. 2004;28:1805–13. doi: 10.1097/01.alc.0000148116.27875.b0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hansenne M. P300 and personality: an investigation with the Cloninger’s model. Biol Psychol. 1999;50:143–55. doi: 10.1016/s0301-0511(99)00008-3. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Knight R. Lateral and orbital prefrontal cortex contributions to attention. In: Polich J, editor. Detection of change: Event-related potential and fMRI findings. The Netherlands: Kluwer Academic Press; 2003. pp. 99–116. [Google Scholar]
- Heimberg C, Gur RE, Erwin RJ, Shtasel DL, Gur RC. Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psychiatry Res. 1992;42:253–65. doi: 10.1016/0165-1781(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Burnam A, McEvoy LT. Alcohol abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric disorders in America: the epidemiologic catchment area study. New York: The Free Press; 1991. pp. 81–115. [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–70. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol Psychiatry. 1999a;46:982–9. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biol Psychiatry. 1995;38:622–32. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999b;46:970–81. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J, Baughman T. Event-related potentials as markers for alcoholism risk in high density families. Alcohol Clin Exp Res. 1988;12:545–54. doi: 10.1111/j.1530-0277.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31:163–74. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Houston RJ, Bauer LO, Hesselbrock VM. Depression and familial risk for substance dependence: a P300 study of young women. Psychiatry Res. 2003;124:49–62. doi: 10.1016/s0925-4927(03)00074-x. [DOI] [PubMed] [Google Scholar]
- Houston RJ, Bauer LO, Hesselbrock VM. P300 evidence of cognitive inflexibility in female adolescents at risk for recurrent depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004a;28:529–36. doi: 10.1016/j.pnpbp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Houston RJ, Bauer LO, Hesselbrock VM. Effects of borderline personality disorder features and a family history of alcohol or drug dependence on P300 in adolescents. Int J Psychophysiol. 2004b;53:57–70. doi: 10.1016/j.ijpsycho.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Houston RJ, Ceballos NA, Hesselbrock VM, Bauer LO. Borderline personality disorder features in adolescent girls: P300 evidence of altered brain maturation. Clin Neurophysiol. 2005;116:1424–32. doi: 10.1016/j.clinph.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–7. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–78. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Johnston VS, Miller DR, Burleson MH. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23:684–94. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005;116:1049–61. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–34. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestenbaum R, Nelson CA. Neural and behavioral correlates of emotion recognition in children and adults. J Exp Child Psychol. 1992;54:1–18. doi: 10.1016/0022-0965(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Knight R. Neural mechanisms of event-related potentials from human lesion studies. In: Rohbraugh J, Parasuraman R, Johnson R, editors. Event-Related brain potentials: Basic issues and applications. New York: Oxford University Press; 1990. pp. 3–18. [Google Scholar]
- Knight R. Distributed cortical network for attention. J Cogn Neurosci. 1997;66:21–34. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Res. 1989;502:109–16. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Lang SF, Nelson CA, Collins PF. Event-related potentials to emotional and neutral stimuli. J Clin Exp Neuropsychol. 1990;12:946–58. doi: 10.1080/01688639008401033. [DOI] [PubMed] [Google Scholar]
- Larkins JM, Sher KJ. Family history of alcoholism and the stability of personality in young adulthood. Psychol Addict Behav. 2006;20:471–7. doi: 10.1037/0893-164X.20.4.471. [DOI] [PubMed] [Google Scholar]
- Marton M. The theory of individual differences in New-Behaviorism and in the typology of higher nervous activity. In: Nebylitsyn VD, Gray JA, editors. Biological bases of individual behavior. New York: Academic Press; 1972. pp. 221–35. [Google Scholar]
- Nielsen AL. Examining drinking patterns and problems among Hispanic groups: results from a national survey. J Stud Alcohol. 2000;61:301–10. doi: 10.15288/jsa.2000.61.301. [DOI] [PubMed] [Google Scholar]
- O’Connor K. Individual differences in components of slow cortical potentials: implications for models of information processing. Pers Individ Differ. 1983;4:403–10. [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1307–21. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A, DePalma N. P3 amplitudes in two distinct tasks are decreased in young men with a history of paternal alcoholism. Alcohol. 1987;4:323–30. doi: 10.1016/0741-8329(87)90030-9. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Kok A, Zeillemaker CW. Disinhibition and the processing of auditory stimulus intensity: An ERP-study. Pers Individ Differ. 1989;10:445–51. [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44:281–9. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Gaillard AW, Wientjes CJ. The relation between event-related brain potential, heart rate, and blood pressure responses in an S1-S2 paradigm. Biol Psychol. 1995;39:81–102. doi: 10.1016/0301-0511(94)00969-5. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB. Alcoholism: the role of different motivational systems. J Psychiatry Neurosci. 1995;20:372–96. [PMC free article] [PubMed] [Google Scholar]
- Polich J, Bloom FE. Event-related brain potentials in individuals at high and low risk for developing alcoholism: failure to replicate. Alcohol Clin Exp Res. 1988;12:368–73. doi: 10.1111/j.1530-0277.1988.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Martin S. P300, cognitive capability, and personality: a correlational study of university undergraduates. Pers Individ Differ. 1992;13:533–43. [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7:465–9. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Genetic basis of event-related potentials and their relationship to alcoholism and alcohol use. J Clin Neurophysiol. 1998;15:44–57. doi: 10.1097/00004691-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. P300 and EPQ/STPI personality traits. Pers Individ Differ. 1989;10:15–24. [Google Scholar]
- Read JP, O’Connor RM. High- and low-dose expectancies as mediators of personality dimensions and alcohol involvement. J Stud Alcohol. 2006;67:204–14. doi: 10.15288/jsa.2006.67.204. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Corral M, Cadaveira F. Visual and auditory event-related potentials in young children of alcoholics from high- and low-density families. Alcohol Clin Exp Res. 1998;22:87–96. doi: 10.1111/j.1530-0277.1998.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Reich T, Bucholz KK, Bierut LJ. Similarities in the clinical characteristics related to alcohol dependence in two populations. Am J Addict. 2002;11:1–9. doi: 10.1080/10550490252801594. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–29. [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ, Bartholow BD, Vieth A. Personality and alcoholism: issues, methods and etiological processes. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. New York: Guilford Press; 1999. pp. 54–105. [Google Scholar]
- Simons RF, MacMillan FW, III, Ireland FB. Anticipatory pleasure deficit in subjects reporting physical anhedonia: slow cortical evidence. Biol Psychol. 1982;14:298–310. doi: 10.1016/0301-0511(82)90010-2. [DOI] [PubMed] [Google Scholar]
- Sommer W, Schweinberger SR, Matt J. Human brain potential correlates of face encoding into memory. Electroencephalogr Clin Neurophysiol. 1991;79:457–63. doi: 10.1016/0013-4694(91)90165-z. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–58. [PubMed] [Google Scholar]
- Stelmack RM, Houlihan M, McGarry-Roberts PA. Personality, reaction time, and event-related potentials. J Pers Soc Psychol. 1993;65:399–409. [Google Scholar]
- Stinson FS, Yi H, Grant BF, Chou P, Dawson DA, Pickering R. Drinking in the United States: Main Findings from the 1992 National Longitudinal Alcohol Epidemiologic Survey (NLAES) 1998 [Google Scholar]
- Waldman ID, Slutske WS. Antisocial behavior and alcoholism: a behavioral genetic perspective on comorbidity. Clin Psychol Rev. 2000;20:255–87. doi: 10.1016/s0272-7358(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Whipple SC, Parker ES, Noble EP. An atypical neurocognitive profile in alcoholic fathers and their sons. J Stud Alcohol. 1988;49:240–4. doi: 10.15288/jsa.1988.49.240. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Effects of temporal-parietal lesions on the somatosensory P3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol. 1992;84:139–48. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]