Abstract
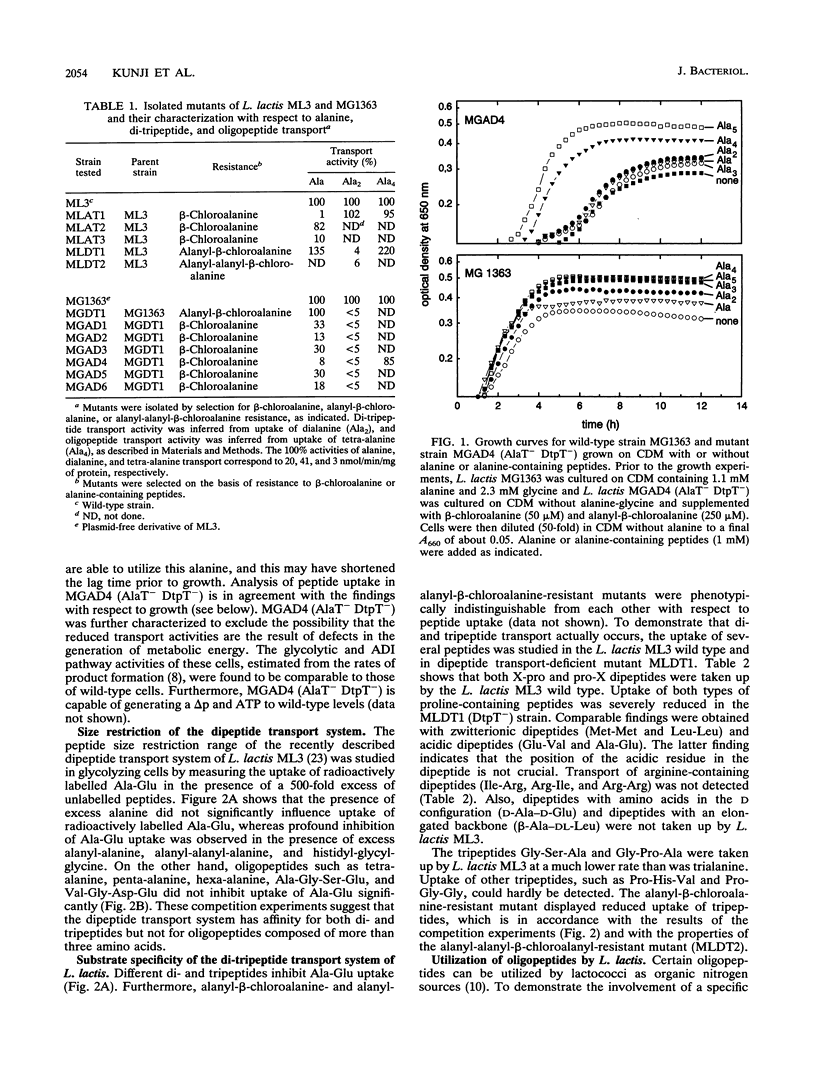
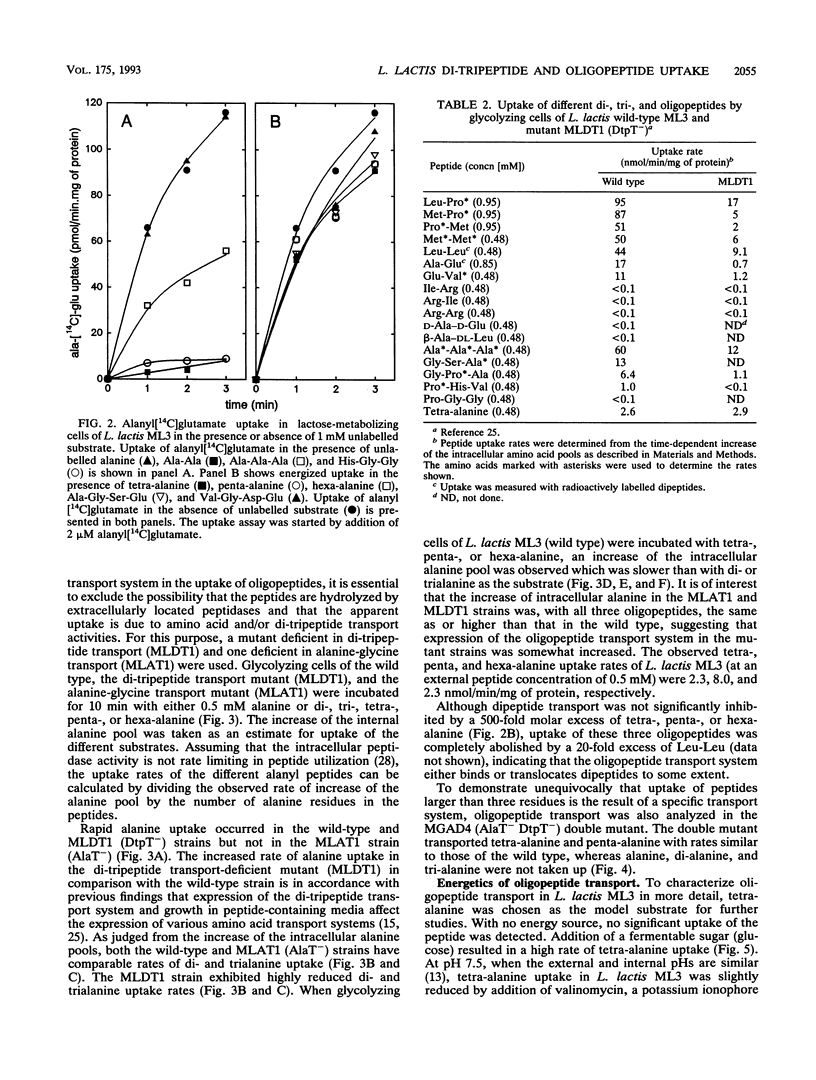
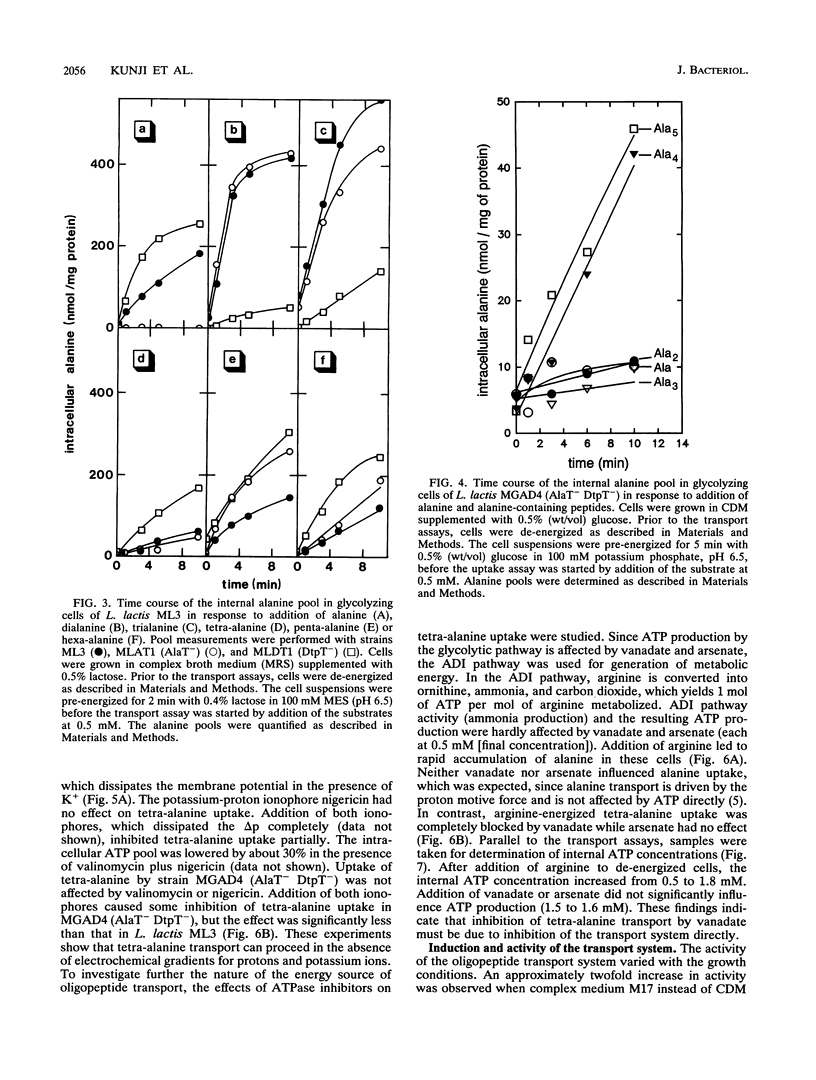
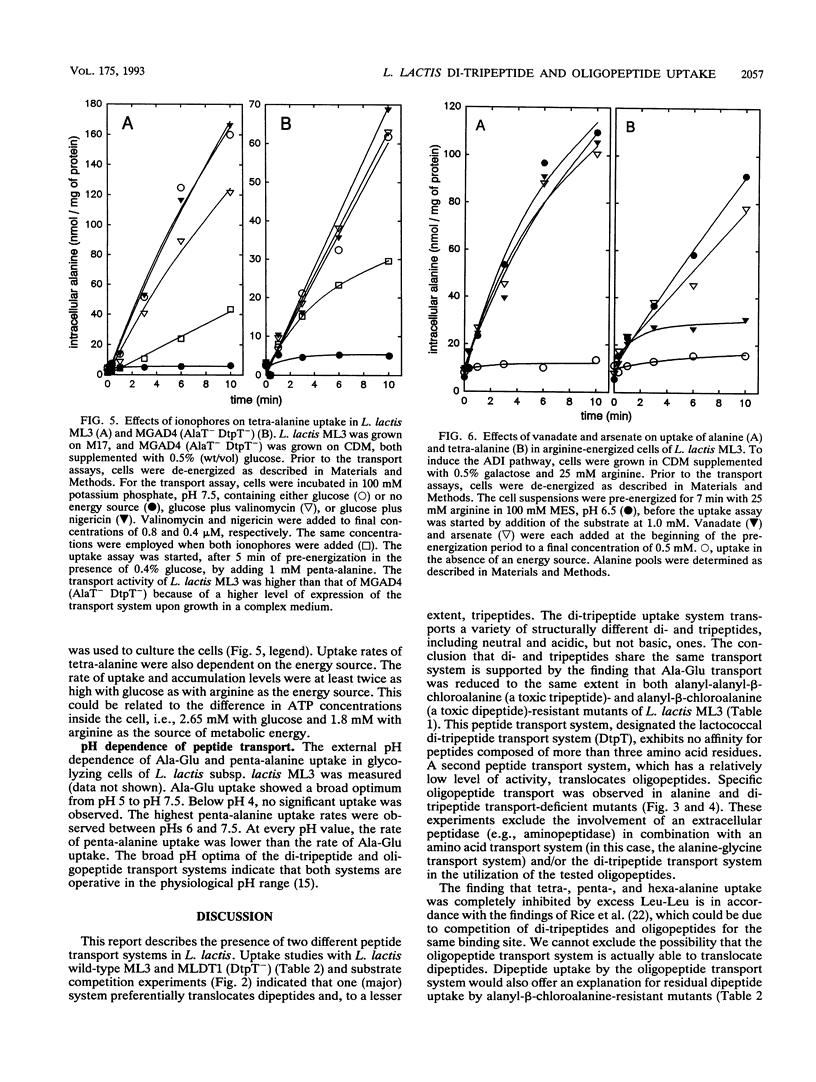
Lactococcus lactis ML3 possesses two different peptide transport systems of which the substrate size restriction and specificity have been determined. The first system is the earlier-described proton motive force-dependent di-tripeptide carrier (E. J. Smid, A. J. M. Driessen, and W. N. Konings, J. Bacteriol. 171:292-298, 1989). The second system is a metabolic energy-dependent oligopeptide transport system which transports peptides of four to at least six amino acid residues. The involvement of a specific oligopeptide transport system in the utilization of tetra-alanine and penta-alanine was established in a mutant of L. lactis MG1363 that was selected on the basis of resistance to toxic analogs of alanine and alanine-containing di- and tripeptides. This mutant is unable to transport alanine, dialanine, and trialanine but still shows uptake of tetra-alanine and penta-alanine. The oligopeptide transport system has a lower activity than the di-tripeptide transport system. Uptake of oligopeptides occurs in the absence of a proton motive force and is specifically inhibited by vanadate. The oligopeptide transport system is most likely driven by ATP or a related energy-rich, phosphorylated intermediate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheldelin V. H., Hoag E. H., Sarett H. P. The Pantothenic Acid Requirements of Lactic Acid Bacteria. J Bacteriol. 1945 Jan;49(1):41–45. doi: 10.1128/jb.49.1.41-45.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law B. A. Peptide utilization by group N streptococci. J Gen Microbiol. 1978 Mar;105(1):113–118. doi: 10.1099/00221287-105-1-113. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of solute transport in streptococci by external and internal pH values. Microbiol Rev. 1987 Dec;51(4):498–508. doi: 10.1128/mr.51.4.498-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Konings W. N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol. 1988 Feb;170(2):700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Nijssen R. M., Konings W. N. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J Bacteriol. 1987 Dec;169(12):5373–5378. doi: 10.1128/jb.169.12.5373-5378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribadeau Dumas B., Brignon G., Grosclaude F., Mercier J. C. Structure primaire de la caséine beta bovine. Séquence complète. Eur J Biochem. 1972 Feb;25(3):505–514. doi: 10.1111/j.1432-1033.1972.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Smid E. J., Driessen A. J., Konings W. N. Mechanism and energetics of dipeptide transport in membrane vesicles of Lactococcus lactis. J Bacteriol. 1989 Jan;171(1):292–298. doi: 10.1128/jb.171.1.292-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Konings W. N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1990 Sep;172(9):5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Plapp R., Konings W. N. Peptide uptake is essential for growth of Lactococcus lactis on the milk protein casein. J Bacteriol. 1989 Nov;171(11):6135–6140. doi: 10.1128/jb.171.11.6135-6140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Poolman B., Konings W. N. Casein utilization by lactococci. Appl Environ Microbiol. 1991 Sep;57(9):2447–2452. doi: 10.1128/aem.57.9.2447-2452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapuhi Y., Schmidt D. E., Lindner W., Karger B. L. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem. 1981 Jul 15;115(1):123–129. doi: 10.1016/0003-2697(81)90534-0. [DOI] [PubMed] [Google Scholar]
- Wiedmeier V. T., Porterfield S. P., Hendrich C. E. Quantitation of Dns-amino acids from body tissues and fluids using high-performance liquid chromatography. J Chromatogr. 1982 Sep 10;231(2):410–417. doi: 10.1016/s0378-4347(00)81865-4. [DOI] [PubMed] [Google Scholar]
- van Boven A., Konings W. N. Energetics of Leucyl-Leucine Hydrolysis in Streptococcus cremoris Wg(2). Appl Environ Microbiol. 1986 Jan;51(1):95–100. doi: 10.1128/aem.51.1.95-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


