Abstract
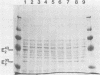
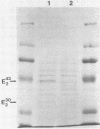
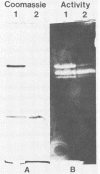
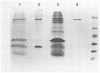
Thermomonospora fusca YX grown in the presence of cellulose produces a number of β-1-4-endoglucanases, some of which bind to microcrystalline cellulose. By using a multicopy plasmid, pIJ702, a gene coding for one of these enzymes (E2) was cloned into Streptomyces lividans and then mobilized into both Escherichia coli and Streptomyces albus. The gene was localized to a 1.6-kilobase PvuII-ClaI segment of the originally cloned 3.0-kilobase SstI fragment of Thermomonospora DNA. The culture supernatants of Streptomyces transformants contain a major endoglucanase that cross-reacts with antibody against Thermomonospora cellulase E2 and has the same molecular weight (43,000) as T. fusca E2. This protein binds quickly and tightly to Avicel, from which it can be eluted with guanidine hydrochloride but not with water. It also binds to filter paper but at a slower rate than to Avicel. Several large proteolytic degradation products of this enzyme generated in vivo lose the ability to bind to Avicel and have higher activity on carboxymethyl cellulose than the native enzyme. Other smaller products bind to Avicel but lack activity. A weak cellobiose-binding site not observed in the native enzyme was present in one of the degradation products. In E. coli, the cloned gene produced a cellulase that also binds tightly to Avicel but appeared to be slightly larger than T. fusca E2. The activity of intact E2 from all organisms can be inactivated by Hg2+ ions. Dithiothreitol protected against Hg2+ inactivation and reactivated both unbound and Avicel-bound Hg2+-inhibited E2, but at different rates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Purification and partial characterization of two acidic proteases from the white-rot fungus Sporotrichum pulverulentum. Eur J Biochem. 1982 Jun;124(3):635–642. doi: 10.1111/j.1432-1033.1982.tb06641.x. [DOI] [PubMed] [Google Scholar]
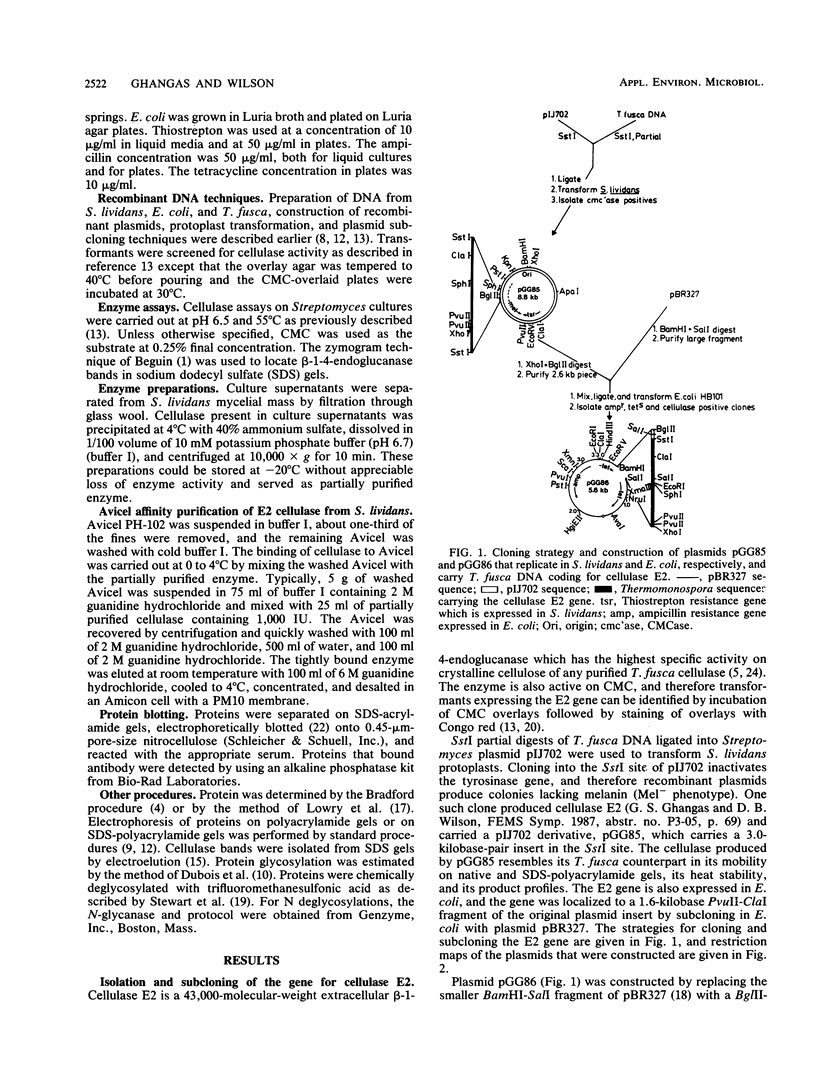
- Ghangas G. S., Wilson D. B. Expression of a Thermomonospora fusca Cellulase Gene in Streptomyces lividans and Bacillus subtilis. Appl Environ Microbiol. 1987 Jul;53(7):1470–1475. doi: 10.1128/aem.53.7.1470-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
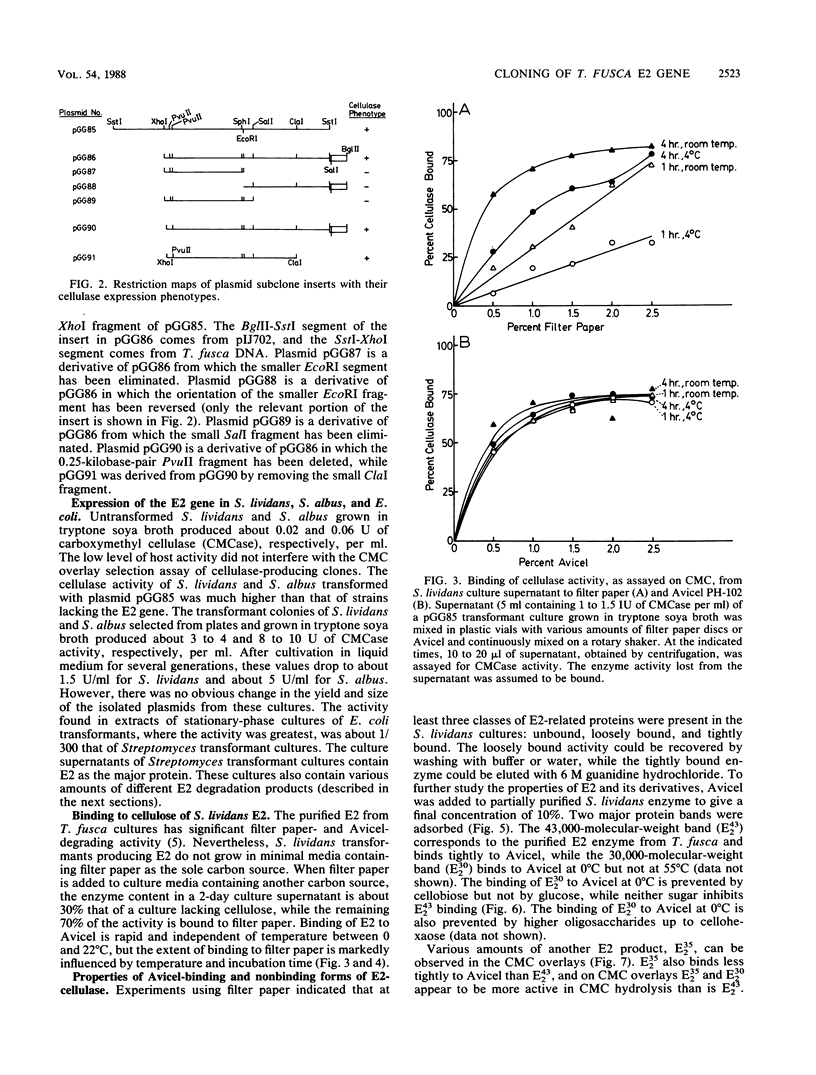
- Ghangas G. S., Wilson D. B. Isolation and characterization of the Salmonella typhimurium LT2 xylose regulon. J Bacteriol. 1984 Jan;157(1):158–164. doi: 10.1128/jb.157.1.158-164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hägerdal B. G., Ferchak J. D., Pye E. K. Cellulolytic Enzyme System of Thermoactinomyces sp. Grown on Microcrystalline Cellulose. Appl Environ Microbiol. 1978 Oct;36(4):606–612. doi: 10.1128/aem.36.4.606-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Chaplin M. F., Kenny A. J. Deglycosylation by trifluoromethanesulphonic acid of endopeptidase-24.11 purified from pig kidney and intestine. Biochem J. 1984 Aug 1;221(3):919–922. doi: 10.1042/bj2210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
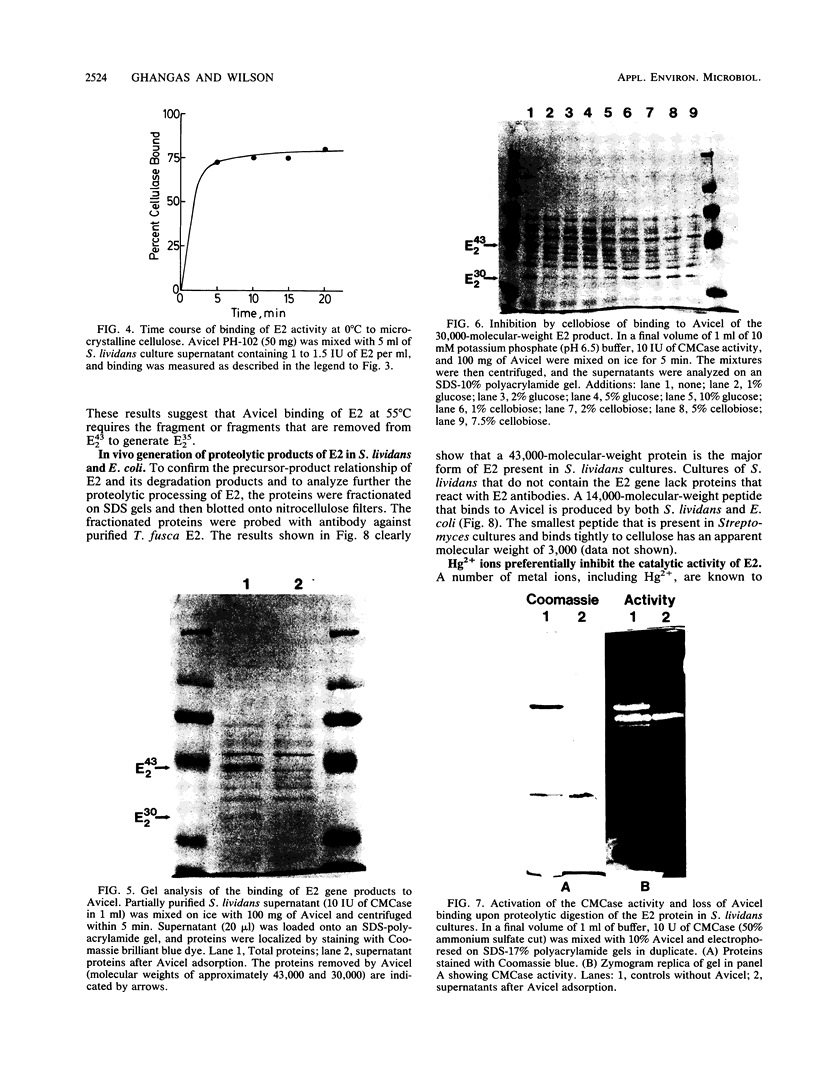
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P., Van Tilbeurgh H., Pettersson G., Van Damme J., Vandekerckhove J., Knowles J., Teeri T., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988 Jan 4;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]