Abstract
Of 400 patients with hilar cholangiocarcinoma who were treated at Nagoya University Hospital from 1977 to 2004, 301 (75%) underwent surgical resection. Most patients underwent major hepatectomy with extrahepatic bile duct resection. The overall mortality rate was 7.6% but had decreased to 2.5% in the last 5 years (p=0.007). The overall survival rates at 5, 10 and 15 years were 22%, 13% and 10%, respectively. The survival rates of 233 patients undergoing R0 resection were 27%, 16% and 13%, respectively. R1 or R2 resection, lymph node metastasis and portal vein involvement were significant negative prognostic factors, although survival was better than in patients with unresected tumours. The results show that aggressive surgical treatment of hilar cholangiocarcinoma offers good outcomes with an acceptable mortality rate.
Keywords: Hilar cholangiocarcinoma, prognostic factor, Nagoya experience
Introduction
Although many articles have been published regarding hilar cholangiocarcinoma, the resection rate of this intractable disease is still low in most centres. Aggressive surgery to achieve a curative resection can offer a better survival than conservative therapy 1,2,3. Precise preoperative evaluation of tumour extent is mandatory to plan a curative resection, and hepatectomy with caudate lobe resection is necessary in most cases 4. Concomitant portal vein resection and reconstruction have been applied to increase the resection rate and to prolong patients’ survival 1,2,5,6,7. Still controversial, however, is the issue of combining pancreatoduodenectomy and major liver resection in the treatment of hilar cholangiocarcinoma 8. We have treated patients with hilar cholangiocarcinoma following an aggressive strategy and herein describe the results of our 27 years of experience at the Nagoya University Hospital.
Patients and surgical indications
From 1977 to 2004, 400 patients with hilar cholangiocarcinoma were treated at the Nagoya University Hospital and were included in this study. There were 259 males and 141 females, with an average age of 62 years (range 24–84 years).
Exclusion criteria for surgical resection are lung metastasis, peritoneal dissemination, perineural invasion around the common hepatic artery and liver metastasis in the future liver remnant. When the tumour extends along the intrahepatic bile ducts proximally to both the umbilical fissure and the confluence of the right posterior superior and inferior segmental ducts, surgery is not attempted. In all other cases, aggressive surgery is planned.
Most patients presented with obstructive jaundice and underwent percutaneous transhepatic biliary drainage (PTBD). PTBD encompassed all occluded biliary branches of all hepatic segments except the caudate lobe, to establish precise diagnosis of proximal tumour involvement and to relieve jaundice. Recently, PTBD has only been applied to the bile ducts of hepatic segments which will be preserved. It may be performed in second intention in isolated bile ducts in cases of segmental cholangitis, insufficient biliary drainage and/or insufficient cholangiography precluding precise diagnosis of tumour extent. Major hepatectomy is performed only after serum total bilirubin level has decreased to below 2 mg/dl.
Categorical variables were compared by the Fisher's exact test. Long-term survival was calculated using the Kaplan–Meier method and compared using the log-rank test. A p value <0.05 was considered statistically significant.
Results
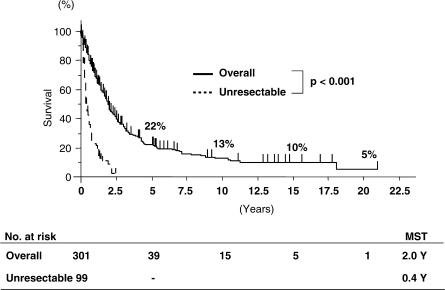
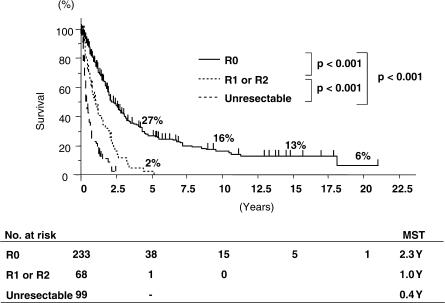
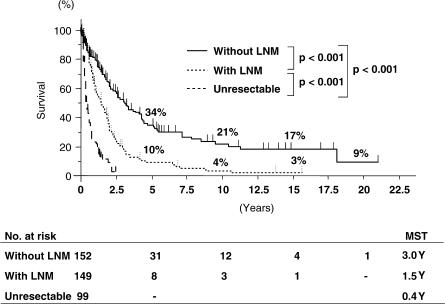
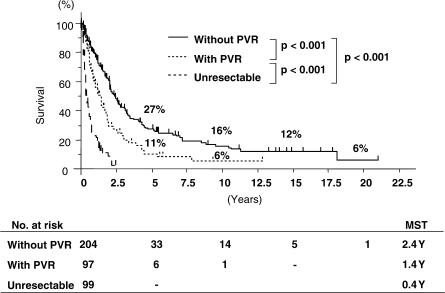
Of the 400 patients, 301 (75%) underwent surgical resection. Operative procedures are shown in Table I. Most patients underwent major hepatectomy with extrahepatic bile duct resection. Surgical techniques and perioperative management have obviously improved throughout the years. Therefore, the mortality rate in the last 5 years is significantly better than that during the preceding 20 years (3/117 vs 20/184, p = 0.007) (Table II). The overall survival rates at 5, 10 and 15 years for the 301 patients undergoing surgical resection were 22%, 13% and 10%, respectively (Figure 1). Overall median survival was 2.0 years. Survival rates of 233 patients undergoing R0 resection were 27%, 16% and 13%, respectively, with a median survival of 2.3 years (Figure 2). Patients without lymph node metastasis survived significantly longer than those with lymph node metastasis (Figure 3). However, 8 of the 149 patients with positive lymph node metastasis survived >5 years. Patients who underwent combined portal vein resection survived for a significantly shorter time than those who did not need portal vein resection (Figure 4). Six of the 97 patients undergoing combined portal vein resection and reconstruction survived >5 years. Survival times of patients with lymph node metastasis and/or portal vein resection were significantly better than those of unresected patients.
Table I. Surgical procedures performed in 301 consecutive patients resected for hilar cholangiocarcinoma (1977–2004).
| Procedures | No. of patients | WithPV | WithPD |
|---|---|---|---|
| BDR only | 16 | 3 | 4 |
| Hepatectomy + BDR | 285 | 94 | 28 |
| S1,4,5,6,7,8 | 22 | 11 | 2 |
| S1,5,6,7,8±S4a | 89 | 34 | 17 |
| S1,2,3,4,5,8 | 34 | 12 | 2 |
| S1,2,3,4 | 108 | 33 | 1 |
| S1,4,5,8 | 11 | 3 | 1 |
| Others | 21 | 1 | 5 |
| Total | 301 | 97 (32%) | 32 (11%) |
PV, portal vein resection; PD, pancreatoduodenectomy; BDR, bile duct resection; S4a, inferior part of the left medial hepatic segment. Note that one patient in category “S1,2,3,4” did not undergo caudate lobe resection because all of the biliary branches from the caudate lobe could be preserved.
Table II. Incidence of liver failure and mortality among 301 patients resected for hilar cholangiocarcinoma.
| Time period | No. of resected patients | Liver failure | Mortality |
|---|---|---|---|
| 1977–1989 | 60 | 9 (15%) | 8 (13%) |
| 1990–1994 | 55 | 16 (29%) | 4 (7%) |
| 1995–1999 | 69 | 15 (22%) | 8 (12%) |
| 2000–2004 | 117 | 7 (6%) | 3 (2.5%) |
| Total | 301 | 47 (16%) | 23 (7.6%) |
Figure 1. .
Overall survival curves of patients who underwent resection for hilar cholangiocarcinoma (1977–2004). MST, median survival time.
Figure 2. .
Survival curves according to completeness of resection. R0, microscopically radical resection; R1, macroscopically radical resection; R2, macroscopically non-radical resection.
Figure 3. .
Survival curves according to the presence of lymph node metastasis (LNM).
Figure 4. .
Survival curves according to portal vein resection. PVR, portal vein resection and reconstruction.
Discussion
We have performed aggressive surgery for patients with hilar cholangiocarcinoma and obtained good outcomes. There are 38 5-year survivors in the R0 resection group resulting in a 27% 5-year survival rate. Of 80 patients without cancer invasion of the portal vein nor regional lymph node metastasis, 23 survived >5 years after R0 resection and the 5-year survival rate was 50% 9. Lymph node micro-metastasis has no impact on survival in patients with otherwise negative lymph nodes, whereas extended lymph node dissection may provide a survival benefit in patients with hilar cholangiocarcinoma 10,11.
Although lymph node metastasis and macroscopic portal vein involvement were independent negative prognostic factors in multivariate analysis of our recent series 12, the 5-year survival rate obtained in patients with portal vein resection or lymph node metastasis still was about 10%. Even in patients with both cancer invasion of the portal vein and regional lymph node metastasis, or with para-aortic lymph node metastasis, curative resection resulted in significantly longer survival than that found in unresected patients 9,10.
As surgical techniques and perioperative management have improved, resection can be performed more safely than before even though our surgical strategy has become more aggressive. During the past 5 years, our mortality rate was <5%. It still remains controversial whether radiotherapy and/or chemotherapy with/without tumour resection can prolong survival of patients with hilar cholangiocarcinoma 13,14.
In conclusion, aggressive surgery for hilar cholangiocarcinoma can offer good outcomes with an acceptable mortality rate. These findings may encourage surgeons to follow an aggressive strategy in surgical treatment for hilar cholangiocarcinoma.
References
- 1.Tashiro S, Tsuji T, Kanemitsu K, Kamimoto Y, Hiraoka T, Miyauchi Y. Prolongation of survival for carcinoma at the hepatic duct confluence. Surgery. 1993;113:270–8. [PubMed] [Google Scholar]
- 2.Launois B, Terblanche J, Lakehal M, Catheline JM, Bardax-oglou E, Landen S, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230:266–75. doi: 10.1097/00000658-199908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–74. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–44. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 5.Nimura Y, Hayakawa N, Kamiya J, Maeda S, Kondo S, Yasui A, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78:727–31. doi: 10.1002/bjs.1800780629. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Nakamura S. Surgery of the portal vein in resection of cancer of the hepatic hilus. Surgery. 1986;99:344–9. [PubMed] [Google Scholar]
- 7.Munoz L, Roayaie S, Maman D, Fishbein T, Sheiner P, Emre S, et al. Hilar cholangiocarcinoma involving the portal vein bifurcation: long-term results after resection. J Hepatobiliary Pancreat Surg. 2002;9:237–41. doi: 10.1007/s005340200025. [DOI] [PubMed] [Google Scholar]
- 8.Nimura Y, Hayakawa N, Kamiya J, Maeda S, Kondo S, Yasui A, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology. 1991;38:170–5. [PubMed] [Google Scholar]
- 9.Nishio H, Nagino M, Oda K, Ebata T, Arai T, Nimura Y. TNM classification for hilar cholangiocarcinoma: comparison between 5th and 6th editions of the AJCC/UICC staging system. Langenbecks Arch Chir. 2005;390:319–27. doi: 10.1007/s00423-005-0561-8. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–92. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tojima Y, Nagino M, Ebata T, Uesaka K, Kamiya J, Nimura Y. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg. 2003;237:201–7. doi: 10.1097/01.SLA.0000048446.18118.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma. Ann Surg. 2003;238:720–7. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–75. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhards MF, van Gulik TM, Gonzale Gonzalez D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–9. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]