Abstract
Background. Hilar cholangiocarcinoma is a rare tumour which is best managed by an aggressive surgical approach. The role of adjuvant or neoadjuvant radiation therapy, chemotherapy or chemoradiation remains controversial, as no prospective randomized studies have been performed. Methods. This review summarizes the recent literature regarding the role of radiation, chemotherapy and chemoradiation in hilar cholangiocarcinoma. The results of a biliary cancer questionnaire regarding current treatment strategies are also reported. Results. A number of retrospective studies have shown that patients treated with adjuvant radiation therapy have prolonged survival compared with untreated patients. However, most of these reports did not control for tumour stage or performance status. A carefully controlled trial from the Johns Hopkins Hospital did not demonstrate any benefit for adjuvant radiation therapy. A number of phase II trials of chemotherapy have demonstrated modest response rates (20–40%). The best responses have been reported with 5-fluorouracil (5-FU) in combination with interferon-alpha or with leucovorin and mitomycin C. Recent non-randomized reports of chemoradiation with 5-FU with or without gemcitabine as the radiosensitizer suggest, but do not prove, improved survival. Adjuvant chemoradiation is currently being employed at specialized centres most often in the Americas (71%) and the Asia/Pacific region (55%) and to a lesser degree in Europe (29%). Discussion. The only chance for long-term survival in patients with hilar cholangiocarcinoma is complete resection with negative margins. Neither radiation therapy nor chemotherapy alone has been proven to prolong survival in completely or partially resected patients or in unresected patients. Recent uncontrolled data suggest that chemoradiation may improve survival in resected and locally unresectable patients. However, prospective, randomized multicentre trials need to be performed to confirm efficacy.
Keywords: Chemoradiation, chemotherapy, cholangiocarcinoma, radiation therapy
Introduction
Cholangiocarcinoma is an uncommon tumour that may occur anywhere along the intrahepatic or extrahepatic biliary tree. In the United States approximately 4000–5000 cholangiocarcinomas are diagnosed per year. The hepatic duct bifurcation is the most frequently involved site, and approximately 60–80% of cholangiocarcinomas are found in this perihilar region 1. The role of radiation therapy, chemotherapy and chemoradiation as adjuvants to surgical resection in patients with perihilar cholangiocarcinoma remains controversial. Aggressive surgical resection obtaining a negative microscopic margin offers the only chance for long-term survival. Unfortunately, many patients will only be candidates for palliative bypass or operative or non-operative intubation aimed to provide biliary drainage and prevent cholangitis and hepatic failure. Radiation therapy, chemotherapy and/or chemoradiation also can be used in these patients with non-resectable disease in an attempt to palliate symptoms and extend survival.
Five-year survivals as high as 30–40% have been reported for perihilar cholangiocarcinomas in the subset of patients that can be resected with negative microscopic margins 1,2,3. Unfortunately, despite recent advances in radiological imaging and improved staging of perihilar cholangiocarcinoma, less than half of the tumours are resectable at the time of exploration. In patients undergoing potentially curative resection for hilar cholangiocarcinomas loco-regional recurrence is found approximately 60% of the time while distant metastases are seen with or without local recurrence in almost 40% 4. Therefore, many authorities recommend the addition of adjuvant chemoradiation for patients with resected perihilar cholangiocarcinoma.
Radiation therapy
Initially, bile duct tumours were thought to be radioresistant, but several studies have shown that radiotherapy can palliate symptoms and may contribute to improvement in survival. Ionizing radiation likely acts by the production of free radicals that cause damage to DNA resulting in double-stranded DNA breaks. These DNA lesions are unable to be repaired or are repaired inadequately in tumour tissue; and as a result, the malignant cells are unable to divide. Therapeutic gain is achieved by the difference in repair capacity and fidelity of repair of DNA lesions in tumour as opposed to normal tissue. Radiotherapy is delivered as a fractionated daily dose allowing normal tissue to repair DNA damage between treatments. Tumours repair this damage less well. Factors which limit the usefulness of radiotherapy in bile duct tumours include the inherent sensitivity of tumours to radiation, hypoxic radioresistant regions within tumour, the repopulation of surviving tumour cells, and the initial number of viable tumour cells in the resected field.
Radiation therapy has been evaluated in patients with cholangiocarcinoma using a variety of methods including external beam radiotherapy, intraoperative radiotherapy, internal radiotherapy, radioimmuno-therapy, and charged particle radiation. External beam radiotherapy has been the most commonly used modality and is typically administered to a total dose of 45–60 Gy. Internal radiotherapy is normally delivered through either percutaneous or endoscopically placed biliary stents using iridium-192 as the radiation source. Total radiation doses may vary from 20 to 60 Gy up to 1 cm from the source. Radioimmunotherapy has also been performed with iodine-131 anti-CEA (carcinoembryonic antigen).
Multiple retrospective analyses have suggested that radiation therapy augments survival in patients with perihilar cholangiocarcinoma. In a recent study from Japan, Todoroki and colleagues 5 examined 63 patients who underwent resection of a perihilar cholangiocarcinoma. Twenty-one patients underwent resection alone and 42 patients received adjuvant radiation therapy. Intraoperative radiation therapy (IORT) alone was given to 12 patients (mean dose 21.0±0.6 Gy), 8 patients were treated with postoperative radiation therapy (PORT) (mean dose 43.6±1.4 Gy) and 22 patients received both IORT and PORT. The loco-regional control rate was significantly better in the adjuvant therapy group compared with the resection alone group, 80% vs 31%, respectively. The actuarial 5-year survival also was significantly better in the resection + IORT + PORT group (39.2%) compared with the resection alone group (13.5%).
A recent study from the Academic Medical Center in Amsterdam 6 examined 91 patients with resected hilar cholangiocarcinoma. Twenty patients had no adjuvant radiation therapy, 30 patients had external beam radiotherapy (46.0±11.3 Gy) and 41 patients had combination external beam therapy (42.3±4.9 Gy) and intraluminal brachytherapy with iridium seeds (10.4±1.7 Gy). Overall median survival was significantly longer in patients treated with adjuvant radiotherapy than in those who underwent resection without additional radiotherapy (24 months vs 8 months). The combination of external radiation and brachytherapy did not result in longer survival than external irradiation alone (21 months and 30 months, respectively).
In many of these retrospective reports, patients receiving radiotherapy tended to have more favourable, often resectable tumours, and were relatively fit. These patients who have undergone radiotherapy, and are expected to have good outcomes, have been compared with patients with unresectable tumours, metastatic disease, or poor performance status who did not receive radiotherapy. Thus, the fact that patients receiving radiotherapy in these analyses have survived longer is not surprising.
To more objectively assess the benefit, if any, of adjuvant radiotherapy, we have previously reported the results of a prospective trial of adjuvant radiation therapy for patients with operable perihilar cholangiocarcinoma at the Johns Hopkins Hospital 7. All patients were surgically staged and found to have cholangiocarcinoma localized to the perihilar biliary tree, with no evidence of intraperitoneal or distant metastases. All patients had histological confirmation of malignancy. Patients were included with either resected, partialIy resected, or unresected tumour, but were stratified on the basis of extent of resection. A Karnofsky performance status of at least 60 at the time of hospital discharge was required for inclusion. In addition, patients had to be fit to begin radiation therapy within 8 weeks after surgery.
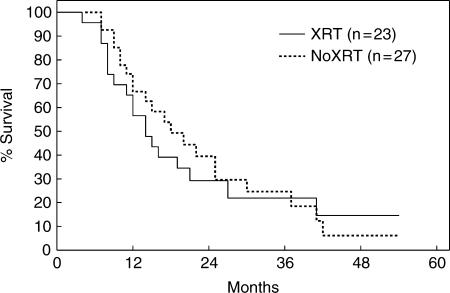
During the 5-year study period, 50 patients were evaluated, whereas 34 were excluded. Radiation ranged from 45 to 63 Gy and consisted of external beam plus iridium-I92 seeds for resected patients and external beam plus cone down port for palliated patients. None of the patients received adjuvant chemotherapy. Patients undergoing curative resection survived significantly longer than patients undergoing operative palliation. Among 31 resected patients, radiation had no effect on mean (24 vs 24 months), median, or actuarial survival. Similarly, among 19 palliated patients radiation had no effect on mean (10 vs 13 months), median, or actuarial survival. Thus, for all 50 patients, adjuvant radiation therapy had no effect on overall survival (Figure 1). Moreover, multivariate analysis identified resection as the only positive predictive factor for prolonged survival.
Figure 1. .
Actuarial survival for 50 patients with perihilar cholangiocarcinoma with (n=23) and without (n=27) adjuvant radiation therapy. From Pitt et al.7.
Chemotherapy
Most trials evaluating the efficacy of chemotherapeutic agents in cholangiocarcinoma represent small single institution phase II trials. These trials often combine both gallbladder cancers and intrahepatic and distal cholangiocarcinomas with perihilar cholangiocarcinomas. Several small studies of single agent systemic chemotherapy regimens for unresectable cholangiocarcinoma using drugs such as 5-fluorouracil (5-FU), methansulfon-m-anisidide, cisplatin, rifampicin, mitomycin C, paclitaxel and gemcitabine have been reported. In general, these trials have shown little efficacy, with partial response rates ranging from 0% to 9% and median survivals between 2 and 12 months 8.
The most extensively investigated chemotherapeutic agent for cholangiocarcinoma has been 5-FU. A prospective randomized trial by the Eastern Cooperative Oncology Group (ECOG) 9 comparing oral 5-FU to oral 5-FU + streptozotocin (Stz) and oral 5-FU + methyl-CCNU (MeCCNU) in 34 patients with unresectable cholangiocarcinoma demonstrated a partial response rate of only 9%. The addition of either streptozotocin or MeCCNU to oral 5-FU therapy did not improve the response rate and was associated with a decrease in median survival from 26 weeks for 5-FU alone to 12 weeks for 5-FU + Stz and 8 weeks for 5-FU + MeCCNU.
Because of the poor response rates with single agent therapy for cholangiocarcinoma, several authors have used combination chemotherapy in an attempt to achieve better response rates and longer survival (Table I). Cholangiocarcinomas are believed to have a large percentage of hypoxic cells since they appear as hypovascular lesions on abdominal imaging and angiography. Therefore, mitomycin C has been proposed as a potential chemotherapeutic agent for cholangiocarcinoma because high levels of the drug can be achieved in bile and because it has a preferential toxicity in hypoxic cells.
Table I. Systemic chemotherapy for unresectable perihilar cholangiocarcinoma*.
| Chemotherapy | n | Response rate | Median survival |
|---|---|---|---|
| 5-FU | 12 | 9% | 6 months |
| 5-FU, cisplatin, epirubicin | 9 | 22% | 5 months |
| 5-FU, adriamycin, mitomycin C | 14 | 29% | 11.5 months |
| 5-FU, methotrexate, leukovorin, cisplatin | 10 | 30% | 4 months |
| 5-FU, interferon-alpha | 25 | 38% | 12 months |
| 5-FU, leukovorin, mitomycin C | 7 | 57% | 17 months |
5-FU, 5-fluorouracil.
*Adapted from Todoroki 8.
In one study a regimen of intravenous bolus cisplatin (60 mg/m2) and epirubicin (50 mg/m2) was given on day 1, and repeated every 3 weeks followed by 5-FU 200 mg/m2/day given as a continuous 24-hour infusion throughout the treatment course in nine patients with advanced extrahepatic cholangiocarcinoma (Table I). Two patients (22%) had a partial response with duration of response of 10 months, three patients (33%) had stable disease and four patients (44%) had evidence of disease progression on therapy. The median survival was 5 months (range 3–13 months). In another study the efficacy of combination therapy with 5-FU, doxorubicin, and mitomycin C (FAM regimen) was investigated in 14 patients with unresectable cholangiocarcinoma (Table I). A partial response (50% or greater reduction in tumour size by imaging) was obtained in four patients (29%). The median duration of response was 8.5 months, and the median survival was 11.5 months. An additional six (43%) patients had evidence of disease stabilization for a median of 6.7 months.
Patt and colleagues 10 used systemic intravenous 5-FU and subcutaneous recombinant human interferon-alpha-2b (rIFN-alpha-2b) in 25 patients with cholangiocarcinoma. Patients received a continuous infusion of 750 mg/m2/d of 5-FU on days 1–5 and a subcutaneous injection of 5 MU/m2 of rIFN-alpha-2b on days 1, 3 and 5. Treatment cycles were repeated every 14 days for 8 weeks. Nine of 24 (38%) assessable patients had a partial response. The median time to disease progression was 9.5 months, and the median survival time was 12 months. Unfortunately, none of the above regimens has been proven to enhance survival in patients with cholangiocarcinoma.
Chemoradiation
Combinations of chemotherapy with radiation have been attempted for many localized tumours. The benefit of combining chemotherapeutic agents with radiosensitizing properties such as 5-FU, mitomycin C and cisplatin with radiation has been demonstrated with several gastrointestinal tumour types but not for biliary tract cancers. Nevertheless, chemoradiation has been applied to patients with cholangiocarcinoma at several centres. In general, these regimens are well tolerated; however, the number of patients has been small, both resected and unresected patients have been treated, and no control patients have been included.
In a recent retrospective review Kim et al.11 used adjuvant external beam radiotherapy (40 Gy) combined with concomitant bolus 5-FU (500 mg/m2) chemotherapy for the first 3 days of each 2 weeks of radiation in 84 patients with extrahepatic cholangiocarcinoma. Monthly maintenance chemotherapy with 5-FU (500 mg/m2, first 5 days of the month) was then administered for 1 year. The overall 5-year actuarial survival rate was 31%. When patients were stratified by residual tumour the 5-year survival rate was 36% for patients with negative microscopic margins at the time of resection, 35% for patients with positive microscopic margins and 0% for patients with gross residual disease. However, the 5-year actuarial survival was only 14% overall for the subgroup with perihilar tumours.
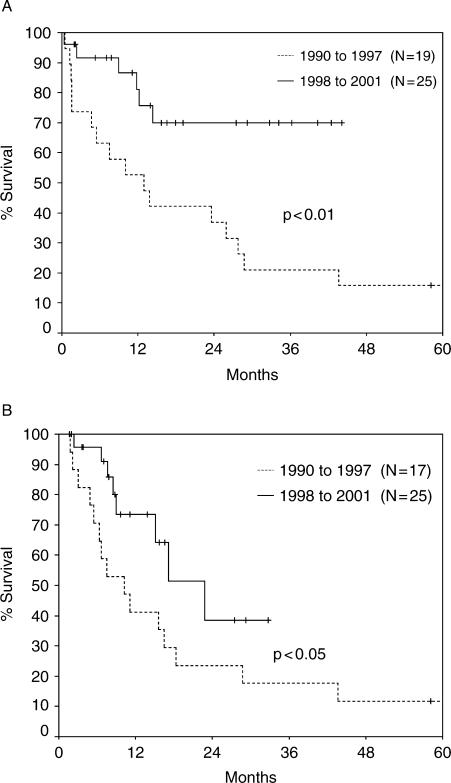
We have recently reported 140 patients with biliary malignancies managed at the Medical College of Wisconsin over a 12-year period. One hundred and eleven (79%) had cholangiocarcinomas, and 72 of these (65%) were perihilar. Over the past 4 years of this analysis, improved staging, active biliary stenting and aggressive surgery led to improved survival (p<0.01, 70% at 44 months) in resected patients (Figure 2A). Chemoradiation with confocal radiation, 5-FU and gemcitabine was employed more frequently in the patients resected since 1998. In addition, this regimen of chemoradiation resulted in better survival (p<0.05) than a regimen with less sophisticated radiation and 5-FU alone which was used in the early 1990s (Figure 2B).
Figure 2. .
(A) Actuarial survival for resected patients by time period. From Nakeeb et al.2. (B) Actuarial survival for patients treated with chemoradiation by time period. From Nakeeb et al.2.
In 2001–2002 a questionnaire was sent to members of the International Hepato-Pancreato-Biliary Association, the American Hepato-Pancreato-Biliary Association and the American College of Surgeons Oncology Group to assess current trends in adjuvant therapy for biliary malignancies 12. Responses were received from 331 authorities at 262 centres in 39 countries worldwide. Presently, adjuvant chemoradiation is used at the majority of centres, with this approach being employed most frequently in the Americas (71%) followed by the Asia/Pacific region (55%) and Europe (29%) (Table II). Interestingly, considerable enthusiasm (88%) for a prospective randomized trial was expressed by the respondents. However, to date, a multi-institutional, multinational trial has not been performed.
Table II. Current use of adjuvant radiation therapy, chemotherapy, and chemoradiation in biliary malignancies*.
| Treatment | Americas | Asia/Pacific | Europe | Total |
|---|---|---|---|---|
| Radiation therapy | 70%† | 40% | 29% | 59% |
| Chemotherapy | 66% | 79% | 68% | 68% |
| Chemoradiation | 71%† | 55% | 29% | 63% |
*Adapted from Pitt et al.12.
†p<0.05 versus other regions.
References
- 1.Nakeeb A, Pitt HA, Coleman J, Hraban RH, Abrams RA, Cameron JL. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–75. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakeeb A, Tran KQ, Black MJ, Ritch PA, Quebbeman EJ, Wilson SD, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–64. doi: 10.1067/msy.2002.127555. [DOI] [PubMed] [Google Scholar]
- 3.Tsao JI, Nimura Y, Kamija J, Hayakowa N, Kondo S, Naqino M, et al. Management of hilar cholangiocarcinoma: comparison of an American and Japanese experience. Ann Surg. 2000;232:166–74. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 5.Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:581–7. doi: 10.1016/s0360-3016(99)00472-1. [DOI] [PubMed] [Google Scholar]
- 6.Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–9. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- 7.Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, et al. Perihilar cholangiocarcinoma: postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788–97. doi: 10.1097/00000658-199506000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todoroki T. Chemotherapy for bile duct carcinoma in the light of adjuvant chemotherapy to surgery. Hepatogastroenterology. 2000;47:644–9. [PubMed] [Google Scholar]
- 9.Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965–9. doi: 10.1002/1097-0142(19840915)54:6<965::aid-cncr2820540603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Patt YZ, Jones DV, Jr, Hoque A, Lozano R, Markowitz A, Raijman I, et al. Phase II trial of intravenous fluorouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311–15. doi: 10.1200/JCO.1996.14.8.2311. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Kim SW, Bang YJ, Heo DS, Ha SW. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54:414–19. doi: 10.1016/s0360-3016(02)02952-8. [DOI] [PubMed] [Google Scholar]
- 12.Pitt HA, Broelsch C, Fong Y, Gouma DJ, Lau JW, Nagorney DM, et al. Adjuvant therapy for biliary malignancies: international trends and possibilities. J Gastrointest Surg. 2003;7:309A. [Google Scholar]