Abstract
Background: The numbers of margin-negative resections and survival times have greatly improved because of a more aggressive surgical approach to resectable hilar cholanciocarcinoma (Klatskin tumour). It was shown initially by Japanese authors that complete resection of the caudate lobe together with partial hepatectomy leads to more margin-negative resections. However, this concept has not been unanimously taken up by Western authors. The aim of this study was to examine the role of complete caudate lobe resection in our series of resected hilar cholangiocarcinomas. Methods: Between January 1993 and January 2003, 54 patients underwent resection for Klatskin tumours. These patients were divided into two groups, according to the two 5-year periods in which they had been operated. In the first period, patients did not routinely undergo complete excision of the caudate lobe, whereas in the second period, partial liver resection was combined with complete excision of the caudate lobe in 15 patients. These two patient groups were evaluated with respect to postoperative morbidity and mortality, microscopic tumour margins and survival time. Results: Postoperative complications occurred in 59% of patients in total, while overall mortality was 11%. No difference was found in postoperative morbidity or mortality between the two periods. A significantly higher number of margin-negative resections was found in the second 5-year period, together with improved survival. Conclusion: Concomitant complete excision of segment 1 for patients with hilar cholangiocarcinoma did not lead to increased morbidity or mortality. Therefore the addition of complete excision of segment 1 is a safe procedure contributing to a higher rate of R0 resections and improved survival.
Keywords: Hilar cholangiocarcinoma, hepatectomy, liver, caudate lobe, survival
Introduction
Due to the central anatomical position of Klatskin tumours within the liver hilum, it has been difficult to perform curative resections for this type of tumour. During the last 15 years, a shift has taken place towards a more aggressive approach in the treatment of hilar cholangiocarcinoma 1,2,3,4. Many authors have emphasized the importance of negative surgical margins to improve survival after resection 5,6,7. Mostly in Bismuth–Corlette type II, III and IV tumours, this can only be achieved by combining hilar resection with partial liver resection 8. Because of frequent anterior and posterior ductal infiltration, partial hepatectomy is advocated in combination with resection of segment 4 and complete excision of the caudate lobe. Japanese surgeons in particular, convincingly demonstrated a survival benefit of a more aggressive approach including extended liver resection and concomitant complete excision of segment 1 2,3,4,9. Despite these results, complete excision of segment 1 has not been fully implemented in the surgical treatment of hilar cholangiocarcinoma by Western surgeons.
Also in our institution, as of the early 1990s, an increasing number of patients with type III or IV hilar cholangiocarcinoma underwent hilar resection in combination with partial liver resection, occasionally including part of segment 1. Starting in 1998, more partial liver resections were performed for hilar cholangiocarcinoma and these were routinely combined with complete excision of the caudate lobe. The aim of this study was to assess the outcome of patients who underwent hilar resection and partial hepatectomy with or without concomitant complete caudate lobe resection with regard to postoperative morbidity and mortality, microscopic tumour clearance and patient survival.
Patients and methods
From January 1993 to January 2003, 54 patients underwent resection for hilar cholangiocarcinoma in our institution. Starting in 1998, a more aggressive policy consisting of more extensive, partial liver resections was adopted and these were combined with total excision of segment 1 in a total of 15 patients. Resections were performed in 31 men and 23 women with a mean age of 59.5±1.5 years (range 29–79 years). There was no statistical difference in gender or mean age of patients who had undergone surgery before and after 1998.
Preoperative workup
Preoperative assessment of patients with hilar cholangiocarcinoma showed few changes during the whole study period. The usual imaging techniques in the two time periods included endoscopic retrograde cholangiopancreatography (ERCP), ultrasound with Doppler imaging and computed tomography (CT). Diagnostic laparoscopy was used routinely to detect small liver or peritoneal metastases in both study groups 10. Percutaneous transhepatic cholangiography (PTC) was used in one patient in the first period and in five patients in the second period. After 1998, CT scanning became more valuable and was used more often, owing to improved scanning techniques. It was used in 12 patients before 1998 (48%), and in 22 patients after 1998 (76%). As of the end of 2000, MRCP was performed to assess vascular ingrowth in six patients in total.
Tumour classification
The Bismuth–Corlette classification was used to describe proximal tumour extension, preoperatively as well as intraoperatively in all patients 8 (Table I). Pathological evaluation during and after surgery revealed an accurate preoperative diagnosis in 45 patients (83%). These patients were equally divided between the two time periods.
Table I. Bismuth–Corlette classification, types of resection and outcome of patients who had undergone resection for Klatskin tumors, divided into two 5-year periods.
| Total | 1993–1998 25 | 1998–2003 29 | p value | Total 54 |
|---|---|---|---|---|
| Bismuth type | NS | |||
| Type I | 4 (16%) | 2 (7%) | 6 (11%) | |
| Type II | 5 (20%) | 5 (17%) | 10 (19%) | |
| Type IIIa | 9 (36%) | 12 (41%) | 21 (39%) | |
| Type IIIb | 7 (28%) | 8 (28%) | 15 (28%) | |
| Type IV | 0 (0%) | 2 (7%) | 2 (4%) | |
| Type of resection | NS | |||
| Local resection | 12 (48%) | 8 (28%) | 20 (37%) | |
| Hilar resection + partial liver resection | 13 (52%) | 21 (72%) | 34 (63%) | |
| (Extended) right hemihepatectomy | 7 (6) (54%) | 11 (7) (52%) | 18 (53%) | |
| Left hemihepatectomy | 6 (47%) | 10 (48%) | 16 (47%) | |
| Complete segment 1 resection | NA | |||
| Local resection | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hilar resection + partial liver resection | 0 (0%) | 15 (52%) | 15 (28%) | |
| Portal vein reconstruction | NS | |||
| Yes | 1 (4%) | 6 (21%) | 7 (13%) | |
| No | 24 (96%) | 23 (79%) | 47 (87%) | |
| In-hospital morbidity | NS | |||
| Yes | 13 (52%) | 19 (66%) | 32 (59%) | |
| No | 12 (48%) | 10 (34%) | 21 (41%) | |
| In-hospital mortality | ||||
| Yes | 3 (12%) | 3 (10%) | 6(11%) | |
| No | 22 (89%) | 26 (90%) | 48 (89%) | |
| Margin-negative resections (R0) | 8 (32%) | 17 (59%) | >0.05 | 25 (46%) |
| Local resection | 3 (38%) | 5 (29%) | ||
| Hilar resection + partial liver resection | 5 (63%) | 12 (71%) | ||
| Complete segment 1 resection | 0/0 (0%) | 10/15 (67%) |
NS, not significant; NA, not applicable.
Preoperative biliary drainage
To relieve jaundice and to optimize future remnant liver function, preoperative biliary drainage was performed in 48 patients (89%). ERCP was used in 46 (96%) patients and PTC was used in 6 patients (13%); both techniques were used in four patients (8%). The percentage of patients who had undergone preoperative biliary drainage was 96% before 1998 and 83% after 1998.
Additional therapy
Low dose preoperative radiotherapy (3×3.5 Gy) was given to all patients to prevent implantation metastases 11. Patients routinely underwent postoperative radiotherapy (55 Gy) in both time periods 12.
Statistical analysis
The χ2 Fisher's exact test were used to assess the significance of differences between groups. Numeric data were evaluated using independent-samples t test and were expressed as mean±standard error of the mean (SEM). The Kaplan-Meier method was used to construct survival curves and the log-rank significance test was used for comparison of survival between groups. SPSS 10.0.7 for Windows (SPSS Inc., Chicago, IL, USA) was used as statistical software and a p value <0.05 was considered significant.
Results
Bismuth types
Table I shows the definitive Bismuth types obtained during or after surgery. There were no statistically significant differences in Bismuth type between patients in the two study groups. Most partial liver resections were performed in patients with type III tumours (67%).
Types of resection
The types of resection that were performed were as follows: local resection for type I tumours, local resection with partial excision of segment 1 and/or 4 for type II tumours and hilar resections in combination with (extended) right or left hemihepatectomy for type IIIa and IIIb tumours, respectively. Extended right hemihepatectomy was performed in 13 patients. The two type IV tumours were treated with hilar resection and left hemihepatectomy and in one of these patients a portal vein reconstruction was performed. Portal vein reconstruction was performed in seven patients in total. As of 1998, complete excision of segment 1 was performed in combination with hilar resection and partial hepatectomy in 15 patients (52%). Apart from concomitant segment 1 excision, no statistical differences were found in types of resection in the two study periods.
Morbidity and mortality
Overall, postoperative complications, including minor and major procedure-related complications, occurred in 59% of the patients (Table I). No significant differences were found in the rate of postoperative complications in the two study periods. The most frequently occurring complications were liver failure, bile leakage and liver abscess or intra-abdominal abscesses (data not shown). The hospital mortality was 12% in the first and 10% in the second period (Table I). All six patients ultimately died due to multi-organ failure. In five of these patients, multi-organ failure was preceded by liver failure, whereas in one patient sepsis was the main cause of multi-organ failure and subsequent death.
Histopathological examination
The microscopic resection and dissection margins were analysed, along with tumour differentiation, invasion into perineural tissue and lymph node involvement. Significantly more patients had undergone a margin-negative (R0) resection in the second period, compared with the first period (59% vs 32%, respectively, p<0.05). There were no differences in tumour differentiation, invasion into perineural tissue or lymph node involvement between the two study groups (data not shown).
Survival
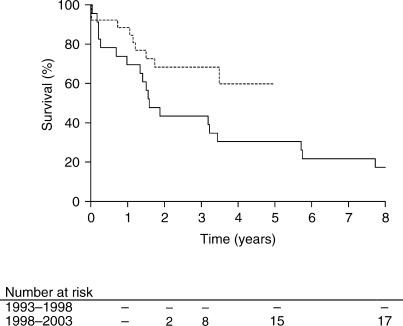
The mean overall survival of the two patient groups was 4.8 years with a 95% confidence interval (CI) of 3.4–6.1 years. The overall 1-, 2- and 5-year survival rates were 78%, 54% and 38%, respectively. Figure 1 shows the survival curves for the two periods. The mean survival time in group 1 and 2 was 3.6 years (CI 2.1–5.2) and 3.7 years (CI 3.0–4.5), respectively. Survival was significantly longer in the second period (p<0.05). A total of 21 patients were still alive at the completion of the study: 4 in the first and 17 in the second period. As more than 50% of patients in the second period are still alive, the median survival times were not assessed.
Figure 1. .
Survival curves of all patients, divided into two 5-year periods. The solid line represents the period 1993–1998 while the dotted line represents the period 1998–2003. A significantly better survival was found in the period 1998–2003 (p<0.05).
Discussion
The outcome of surgical treatment for hilar cholangiocarcinoma has improved during the last 10–15 years. This is largely due to the fact that more resections are combined with partial hepatectomy, leading to more margin-negative resections 5,6,7. In addition, Japanese authors in the late 1990s and early 1990s reported that combining hilar resection and partial hepatectomy with complete caudate lobe resection further increased the rate of margin-negative resections 3,4. Tsao and colleagues stated that this procedure can be performed safely, in the hands of experienced surgeons who are familiar with caudate lobe anatomy 9. These findings resulted in implementation of this concept by other Japanese and some Western surgeons 13,14.
The caudate lobe, designated as segment 1 in Couinaud's segmental classification of the liver, constitutes 5–10% of the entire liver mass. Segment 1 is composed of three portions, i.e. the Spiegel lobe located to the left and dorsally to the inferior vena cava, the paracaval portion lying anteriorly to the inferior caval vein and the caudate process traversing between the inferior vena cava and the portal vein, from the paracaval portion to segment 7 of the right liver 15. The blood supply to segment 1 is independent and originates mainly from the left portal vein and left hepatic artery. Bile ducts from segment 1 may drain into any part of the hepatic duct confluence, but most frequently enter the left hepatic duct near the hepatic duct confluence. Because of the proximity of segment 1 posterior to the hepatic duct confluence, the segment 1 biliary ducts are frequently involved in Klatskin tumours, necessitating en bloc removal of the entire lobe along with the hepatic duct confluence in curative resections. Total excision of the caudate lobe was advocated, since the incidence of caudate bile duct invasion by hilar cholangiocarcinoma was indeed found in 31–98% of cases 3,4,9. Incomplete removal, usually of the caudate process alone, does not suffice for radical excision of all tumour. In the present series, all segment 1 excisions were performed in conjunction with partial liver resection in the second 5-year period of the study. Although local excision, along the same lines, may require en bloc removal of segment 1, most local excisions in the second period of this series were performed for Bismuth type I or II tumours, in which tumour infiltration into the segment 1 bile ducts is less frequent. The type III and IV tumours that were treated by local excision were intentionally palliative resections. In case of type II Klatskin tumours, we now choose to undertake local resection in combination with excision of segment 1, preferably in combination with partial liver resection.
As of 1998, we applied the concept of complete excision of segment 1 along with partial hepatectomy in our institution. In this study, patients who had undergone resection for hilar cholangiocarcinoma were analysed retrospectively during a 10-year period and were divided into two 5-year periods, the second starting in 1998. No significant differences were found in tumour types according to the Bismuth–Corlette classification or types of resection between these groups, although more hilar resections in combination with partial liver resections were performed in the later period (72% vs 52% in the first period) 8. There were also no differences in accuracy of preoperative imaging or other general characteristics of patients, such as age and gender. The surgical team remained the same in both periods.
The overall postoperative morbidity rate of 59% in this study is comparable to that found in recent series, in which postoperative morbidity varies from 37% to 85% 7,15,16. When recent literature is compared to more than 5-year-old literature, a slight increase in postoperative morbidity has occurred, which is in keeping with a generally adopted, more aggressive surgical approach in the treatment of Klatskin tumours 1,2,3,4. The addition of complete excision of segment 1 in the second period did not lead to an increase in postoperative complications or mortality in this study, even though more portal vein resections were also performed in this period.
In recent literature, overall mortality rates vary from 0% to 10% 7,16,17. In the first 5-year period, mortality in this series was 12% whereas in the second 5-year period, mortality was 10%. Liver failure was the most important cause of multi-organ failure and subsequent death in this study, underscoring the need for methods to accurately predict and to optimize future remnant liver function.
Significantly more margin-negative resections were performed in the second 5-year period. Apart from the addition of complete segment 1 resection, increased surgical experience and the tendency to perform more extended resections, i.e. hilar resections in combination with partial liver resection, may have contributed to this improved outcome. Despite the short follow-up of patients in the second period, a significantly increased survival was found compared with the first period. The increased rate of margin-negative resections obviously is the most significant factor to determine improved survival, as has repeatedly been shown in the literature 4,6,9. Because of the small number of patients in this study, univariate and multivariate analysis were not performed.
In conclusion, concomitant complete excision of segment 1 for patients with hilar cholangiocarcinoma did not lead to increased morbidity or mortality. The addition of complete excision of segment 1 therefore, is a safe procedure that can lead to a higher rate of R0 resections and increased survival.
References
- 1.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–6. doi: 10.1001/archsurg.1995.01430030040006. [DOI] [PubMed] [Google Scholar]
- 2.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–62. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 3.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–43. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Mizumoto R, Tabata M, Matsuda S, Kusuda T. Surgical treatment of carcinoma of the hepatic duct confluence: analysis of 55 resected carcinomas. World J Surg. 1993;17:85–93. doi: 10.1007/BF01655714. [DOI] [PubMed] [Google Scholar]
- 5.Gerhards MF, van Gulik TM, Bosma A, ten Hoopen-Neumann H, Verbeek PCM, Gonzales Gonzales D, et al. Long-term survival after resection of proximal bile duct carcinoma (Klatskin tumors) World J Surg. 1999;23:91. doi: 10.1007/s002689900571. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–8. [PubMed] [Google Scholar]
- 9.Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma. Comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–74. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilleman EHBM, de Castro SMM, Busch ORC, Bemelman WA, van Gulik TM, Obertop H, et al. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg. 2002;6:426–30. doi: 10.1016/s1091-255x(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 11.Gerhards MF, Gonzales Gonzales D, ten Hoopen-Neumann H, van Gulik TM, de Wit LT, Gouma DJ. Prevention of implantation metastases after resection of proximal bile duct tumours with pre-operative low dose radiation therapy. Eur J Surg Oncol. 2000;26:480–5. doi: 10.1053/ejso.1999.0926. [DOI] [PubMed] [Google Scholar]
- 12.Gerhards MF, van Gulik TM, Gonzalez GD, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–9. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- 13.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–8. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, et al. Complete resection of the caudate lobe of the liver: technique and experience. Hepatogastroenterology. 2001;48:808–11. [PubMed] [Google Scholar]
- 16.Gerhards MF, van Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma – a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 17.Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277–83. doi: 10.1007/s00268-001-0110-8. [DOI] [PubMed] [Google Scholar]