Abstract
Matrix metalloproteinases (MMPs) are implicated in tumour invasion and metastasis. We report the first use of an MMP inhibitor to treat unresectable cholangiocarcinoma. Four men with stage IV cholangiocarcinoma received oral Marimastat (10 mg bd) indefinitely following relief of obstructive jaundice. Monthly measurements of the tumour marker CA 19-9 were used as an indicator of disease response and activity. CA 19-9 levels dropped sharply and stayed low in the two patients who appeared to respond. Mean survival of the four patients was 21.5 months (range 4–48 months). Side effects were well tolerated. A more extensive and detailed examination of MMP inhibitors to treat cholangiocarcinoma is indicated.
Keywords: Cholangiocarcinoma, matrix metalloproteinase inhibitor, marimastat
Introduction
Cholangiocarcinoma is increasing in incidence, with approximately 1000 new cases presenting each year in England and Wales 1. Only surgical resection offers the chance of long-term disease-free survival, yet of those patients who undergo operation, only 10% receive a ‘curative’ resection. Mean survival of patients with unresectable cholangiocarcinoma is 8 months when jaundice is relieved by operative palliation and 5 months in those treated with a biliary endoprosthesis 2. The role of chemotherapy and radiotherapy remains unclear in the therapeutic as well as the adjuvant setting.
Matrix metalloproteinases (MMPs) are a functionally defined family of powerful enzymes, the main action of which is the degradation of the extracellular matrix 3. They have important roles in wound healing, pregnancy and parturition, bone resorption and mammary involution 4. Scientific focus has concentrated on certain disease states in which breakdown of the extracellular matrix is a key feature, e.g. rheumatoid arthritis, periodontal disease and cancer.
The process involved in the metastatic cascade (breakdown of the basement membrane, invasion of surrounding stroma, vascular invasion and extravasation at distant sites) involves proteolysis and remodelling of the extracellular matrix. MMPs have been implicated in this process 3. Tissue inhibitors of matrix metalloproteinases (TIMPs) are important in maintaining a balance between matrix synthesis and degradation caused by MMPs and therefore are found whenever MMPs are present. Factors produced by cancer cause a larger over-expression of MMPs as compared with TIMPs.
In cholangiocarcinoma MMPs have been demonstrated within both tumour cells and the stroma, and their expression is stronger in cases of extensive tumour invasion 5, suggesting that MMPs may play an important role in the degradation of extracellular matrix and facilitate cholangiocarcinoma invasion.
Marimastat (BB-2516) is a synthetic low molecular weight MMP inhibitor (MMPI). It also has angiogenesis inhibiting properties. Marimastat often induces a fibroblastic matrix reaction, which makes monitoring tumour responsiveness to the drug difficult by standard radiological means. The tumour-associated antigen CA 19-9 is elevated in cholangiocarcinoma 6, but it may also be elevated in the presence of obstructive jaundice and cholangitis (100–1000 U/ml) 7, and its serum measurement therefore needs to be considered in combination with tests of liver function. It is postulated that a fall in serum CA 19-9 may reflect decreased tumour cell burden.
Materials and methods
Four men (median age 61; range 48–65 years) with histologically proven stage IV cholangiocarcinoma were offered compassionate treatment with oral Marimastat. Treatment was universally accepted, commenced immediately after surgical or endoscopic intervention, and continued until death. Patient selection was a clinical decision based on certain criteria; notably a life expectancy of >3 months and the relief of jaundice. Tumour characteristics precluding curative resection were bilateral liver and portal vein invasion (patient 1), multiple small hepatic metastases (patient 2), hepatic artery encasement (patient 3) and tumour extension into the hepato-duodenal ligament (patient 4). Palliative bypass procedures were performed on the first three patients, while the fourth patient underwent percutaneous insertion of a metal stent. Blood tests were performed at 1–3-monthly intervals during follow-up. The initial dose of Marimastat was 10 mg twice daily. Side effects – predominantly upper limb joint stiffness, pain, and swelling – occasionally enforced dose reduction to 10 mg once daily. In severe episodes temporary cessation of treatment for 1 week was deemed necessary on one occasion in patient 4 and on two occasions in patient 2.
Results
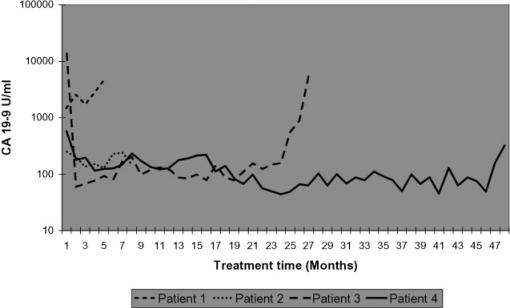
Monthly CA 19-9 measurements are shown in Figure 1. Elevated bilirubin levels, which occurred four times, did not cause an elevation of CA 19-9 levels (data not shown). CA 19-9 levels of patients 1 and 2 did not significantly alter during the treatment period. However, CA 19-9 levels of patients 3 and 4 dropped within 1 month by 14165 U/ml (to 0.4% of original value) and 398 U/ml (31% of original value), respectively, after commencement of Marimastat treatment. In these latter patients these reduced CA 19-9 levels were maintained for 25 and 48 months, respectively.
Figure 1. .
Monthly CA 19-9 levels of patients 1–4.
There was no serious co-morbidity, and none of the patients experienced procedure-related complications. The quality of life on Marimastat was good for patients and 4, who were only affected by their disease close to death. Patient 4 took a short holiday within 6 weeks of his death. Patient 1 survived for 4 months, patient 2 for 8 months, patient 3 for 26 months and patient 4 for 48 months. Repeat axial imaging of patient 4 within a month of his death indicated progression of disease at the hilum of the liver. There were no histological differences between the four patients in this study.
Discussion
Obstructive jaundice was surgically or endoscopically resolved in all four patients immediately before Marimastat treatment and may well have contributed to the fall in CA 19-9. However, after resolution of obstructive jaundice and commencement of Marimastat treatment, CA 19-9 levels in patients 1 (70% rise) and 2 (20% drop) did not mirror those in patients 3 and 4. Furthermore, one episode of cholangitis in patient 3 at 14 months and two episodes of stent blockage in patient 4 at 6 and 15 months did not appreciably affect CA 19-9 levels.
The dose of 20 mg daily has been shown to be sufficiently high to ensure that mean trough levels of Marimastat exceed the desired levels (40 µg/ml) 8, while causing minimal treatment disruption due to side effects and drug holidays.
Survival of patients 1 and 2 (4 and 8 months, respectively) is consistent with the expected prognosis of patients with this stage of disease 2. Patients 3 and 4 show an extended survival (26 and 48 months, respectively) from the expected. Survival of palliated patients above 2 years is rarely reported 9.
Phase II studies of the MMPI Marimastat have shown early promise in the treatment of colorectal, ovarian and prostate cancer. Six studies have been completed, and pooled analysis has demonstrated a dose-dependent biological effect using tumour markers as surrogate indicators of anti-tumour activity. Effects on tumour markers were associated with increased survival 10. Recent phase III studies have shown survival benefit in glioblastoma multiforme 11, malignant melanoma 12 and inoperable gastric cancer 13. In the encouraging latter study there was histological evidence of fibroblastic matrix development. The use of an MMPI to treat unresectable cholangiocarcinoma has not been reported previously.
It is postulated that treatment with Marimastat has contributed to the longevity of patients 3 and 4. A reduction in tumour MMP activity will lessen the capability of the primary tumour to extend locally, regionally and remotely. This negative effect on aggressive activity may result in the observed extended survival. The lack of apparent response in patients 1 and 2 could be due to differing MMP profiles between individuals.
Further studies are needed to address this observation. Larger numbers should be incorporated with frozen tumour tissue available for protein load and enzyme activity analysis. Further results could support and extend this exciting observation and provide scientific backing for this treatment option.
Acknowledgements
Marimastat was kindly provided by British Biotech plc.
References
- 1.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–20. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordback IH, Pitt HA, Coleman J, Venbrux AC, Dooley WC, Yeu NN, et al. Unresectable hilar cholangiocarcinoma: percutaneous versus operative palliation. Surgery. 1994;115:597–603. [PubMed] [Google Scholar]
- 3.Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990;1:99–106. [PubMed] [Google Scholar]
- 4.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–6. [PubMed] [Google Scholar]
- 5.Terada T, Okada Y, Nakanuma Y. Expression of immunor-eactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–4. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- 6.Ahrendt SA, Cameron JL, Pitt HA. Current management of patients with perihilar cholangiocarcinoma. Adv Surg. 1996;30:427–52. [PubMed] [Google Scholar]
- 7.Albert MB, Steinberg WM, Henry JP. Elevated serum levels of tumor marker CA19-9 in acute cholangitis. Dig Dis Sci. 1988;33:1223–5. doi: 10.1007/BF01536670. [DOI] [PubMed] [Google Scholar]
- 8.Millar AW, Brown PD, Moore J, Galloway WA, Cornish AG, Lenehan TJ, et al. Results of single and repeat dose studies of the oral matrix metalloproteinase inhibitor marimastat in healthy male volunteers. Br J Clin Pharmacol. 1998;45:21–6. doi: 10.1046/j.1365-2125.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marvin MR, Lefkowitch J, Ishak KG, Chabot J. Long-term survival of a young woman with peripheral cholangiocarcinoma: a case report. J Clin Gastroenterol. 1999;28:64–6. doi: 10.1097/00004836-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Nemunaitis J, Poole C, Primrose J, Rosemurgy A, Malfetano J, Brown P, et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res. 1998;4:1101–9. [PubMed] [Google Scholar]
- 11.Groves MD, Puduvalli VK, Hess KR, Jaeckle KA, Peterson P, Yung WK, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–8. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 12.Quirt I, Bodurth A, Lohmann R, Rusthoven J, Belanger K, Young V, et al. National Cancer Institute of Canada Clinical Trials G. Phase II study of marimastat (BB-2516) in malignant melanoma: a clinical and tumor biopsy study of the National Cancer Institute of Canada Clinical Trials Group. Invest New Drugs. 2002;20:431–7. doi: 10.1023/a:1020625423524. [DOI] [PubMed] [Google Scholar]
- 13.Bramhall SR, Hallissey MT, Whiting J, Scholefield J, Tierney G, Stuart RC, et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomised trial. Br J Cancer. 2002;86:1864–70. doi: 10.1038/sj.bjc.6600310. [DOI] [PMC free article] [PubMed] [Google Scholar]