Abstract
Microbiological analyses of sediments located near a point source for petrogenic chemicals resulted in the isolation of a pyrene-mineralizing bacterium. This isolate was identified as a Mycobacterium sp. on the basis of its cellular and colony morphology, gram-positive and strong acid-fast reactions, diagnostic biochemical tests, 66.6% G + C content of the DNA, and high-molecular-weight mycolic acids (C58 to C64). The mycobacterium mineralized pyrene when grown in a mineral salts medium supplemented with nutrients but was unable to utilize pyrene as a sole source of carbon and energy. The mycobacterium grew well at 24 and 30 degrees C and minimally at 35 degrees C. No growth was observed at 5 or 42 degrees C. The mycobacterium grew well at salt concentrations up to 4%. Pyrene-induced Mycobacterium cultures mineralized 5% of the pyrene after 6 h and reached a maximum of 48% mineralization within 72 h. Treatment of induced and noninduced cultures with chloramphenicol showed that pyrene-degrading enzymes were inducible in this Mycobacterium sp. This bacterium could also mineralize other polycyclic aromatic hydrocarbons and alkyl- and nitro-substituted polycyclic aromatic hydrocarbons including naphthalene, phenanthrene, fluoranthene, 3-methylcholanthrene, 1-nitropyrene, and 6-nitrochrysene. This is the first report of a bacterium able to extensively mineralize pyrene and other polycyclic aromatic hydrocarbons containing four aromatic rings.
Full text
PDF




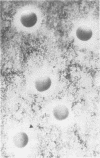
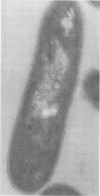
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam H. W., Perry J. J. Microbial degradation and assimilation of n-alkyl-substituted cycloparaffins. J Bacteriol. 1974 May;118(2):394–399. doi: 10.1128/jb.118.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Bartha R. Structure-biodegradability relationships of polycyclic aromatic hydrocarbons in soil. Bull Environ Contam Toxicol. 1986 Oct;37(4):490–495. doi: 10.1007/BF01607793. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Binding of pyrene to DNA, base sequence specificity and its implication. Nucleic Acids Res. 1983 Oct 25;11(20):7231–7250. doi: 10.1093/nar/11.20.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Goodfellow M., Minnikin D. E. A survey of the structures of mycolic acids in Corynebacterium and related taxa. J Gen Microbiol. 1982 Jan;128(1):129–149. doi: 10.1099/00221287-128-1-129. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Isolation of plasmids from mycobacteria. Infect Immun. 1979 Jun;24(3):979–981. doi: 10.1128/iai.24.3.979-981.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W., Whiteside T. L. Mesosomes: membranous bacterial organelles. Bacteriol Rev. 1975 Dec;39(4):405–463. doi: 10.1128/br.39.4.405-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Cerniglia C. E. Mineralization of polycyclic aromatic hydrocarbons by a bacterium isolated from sediment below an oil field. Appl Environ Microbiol. 1988 Jun;54(6):1612–1614. doi: 10.1128/aem.54.6.1612-1614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Cerniglia C. E. Naphthalene biodegradation in environmental microcosms: estimates of degradation rates and characterization of metabolites. Appl Environ Microbiol. 1987 Jan;53(1):129–136. doi: 10.1128/aem.53.1.129-136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Miller D. W., Cerniglia C. E. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl Environ Microbiol. 1988 Oct;54(10):2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Grimmer G., Raab G., Schmoldt A. The metabolism of pyrene by rat liver microsomes and the influence of various mono-oxygenase inducers. Xenobiotica. 1982 Jan;12(1):45–53. doi: 10.3109/00498258209052453. [DOI] [PubMed] [Google Scholar]
- Kiyohara H., Nagao K., Yana K. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl Environ Microbiol. 1982 Feb;43(2):454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H., Sugiyama M., Mondello F. J., Gibson D. T., Yano K. Plasmid involvement in the degradation of polycyclic aromatic hydrocarbons by a Beijerinckia species. Biochem Biophys Res Commun. 1983 Mar 29;111(3):939–945. doi: 10.1016/0006-291x(83)91390-6. [DOI] [PubMed] [Google Scholar]
- LUKINS H. B., FOSTER J. W. METHYL KETONE METABOLISM IN HYDROCARBON-UTILIZING MYCOBACTERIA. J Bacteriol. 1963 May;85:1074–1087. doi: 10.1128/jb.85.5.1074-1087.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Alshamaony L., Goodfellow M. Differentiation of Mycobacterium, Nocardia, and related taxa by thin-layer chromatographic analysis of whole-organism methanolysates. J Gen Microbiol. 1975 May;88(1):200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 1986;8 (Suppl 7):1–119. [PubMed] [Google Scholar]
- Phillips W. E., Jr, Perry J. J. Metabolism of n-butane and 2-butanone by Mycobacterium vaccae. J Bacteriol. 1974 Nov;120(2):987–989. doi: 10.1128/jb.120.2.987-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINOHARA C., FUKUSHI K., SUZUKI J. Mitochondria-like structures in ultrathin sections of Mycobacterium avium. J Bacteriol. 1957 Sep;74(3):413–415. doi: 10.1128/jb.74.3.413-415.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Momma T., Ose Y., Ishikawa T., Kato K. Mutagenicity of Nagara river sediment. Mutat Res. 1983 Sep;118(4):257–267. doi: 10.1016/0165-1218(83)90209-4. [DOI] [PubMed] [Google Scholar]
- Struble V. G., Harmon H. J. Molecular basis for inhibition of mitochondrial respiration by naphthalene. Bull Environ Contam Toxicol. 1983 Dec;31(6):644–648. doi: 10.1007/BF01606040. [DOI] [PubMed] [Google Scholar]