Abstract
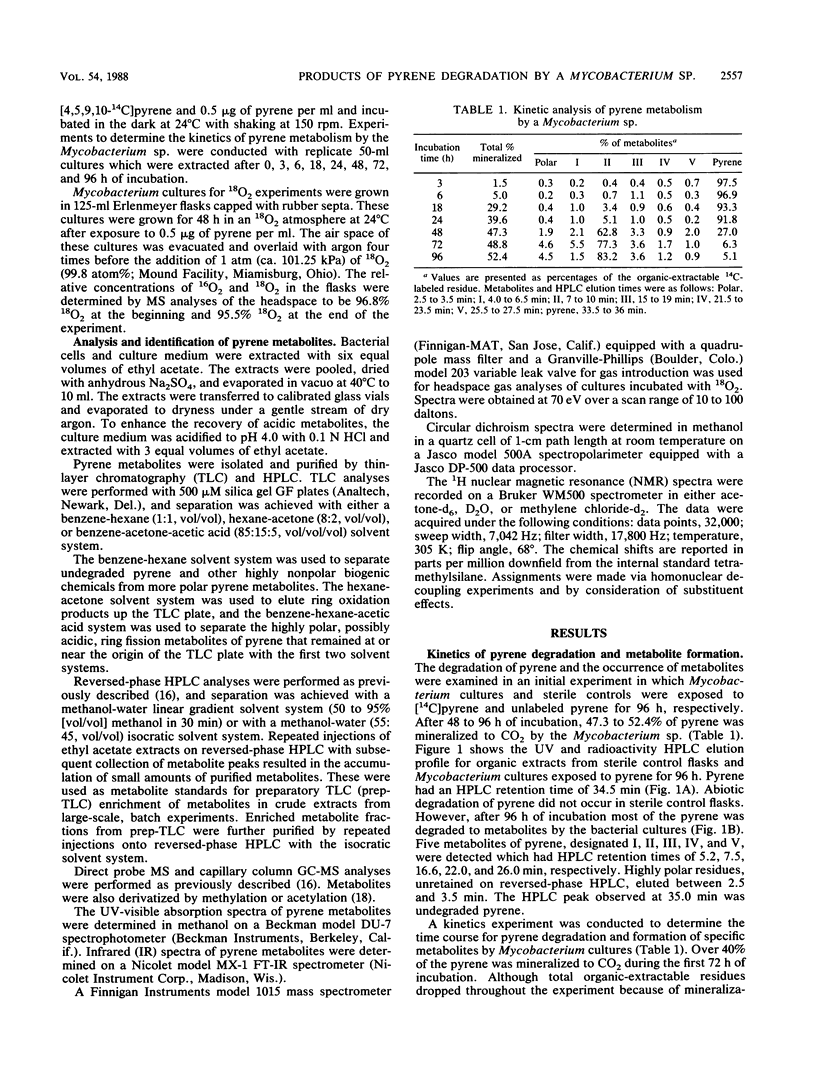
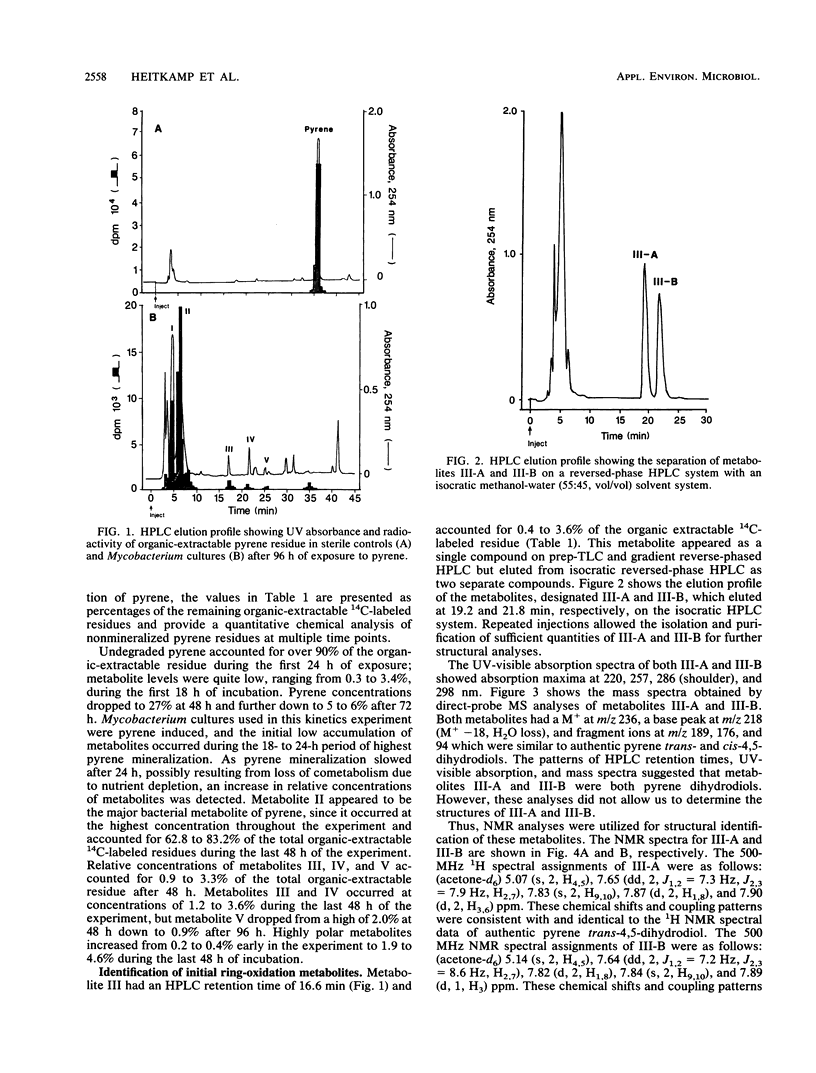
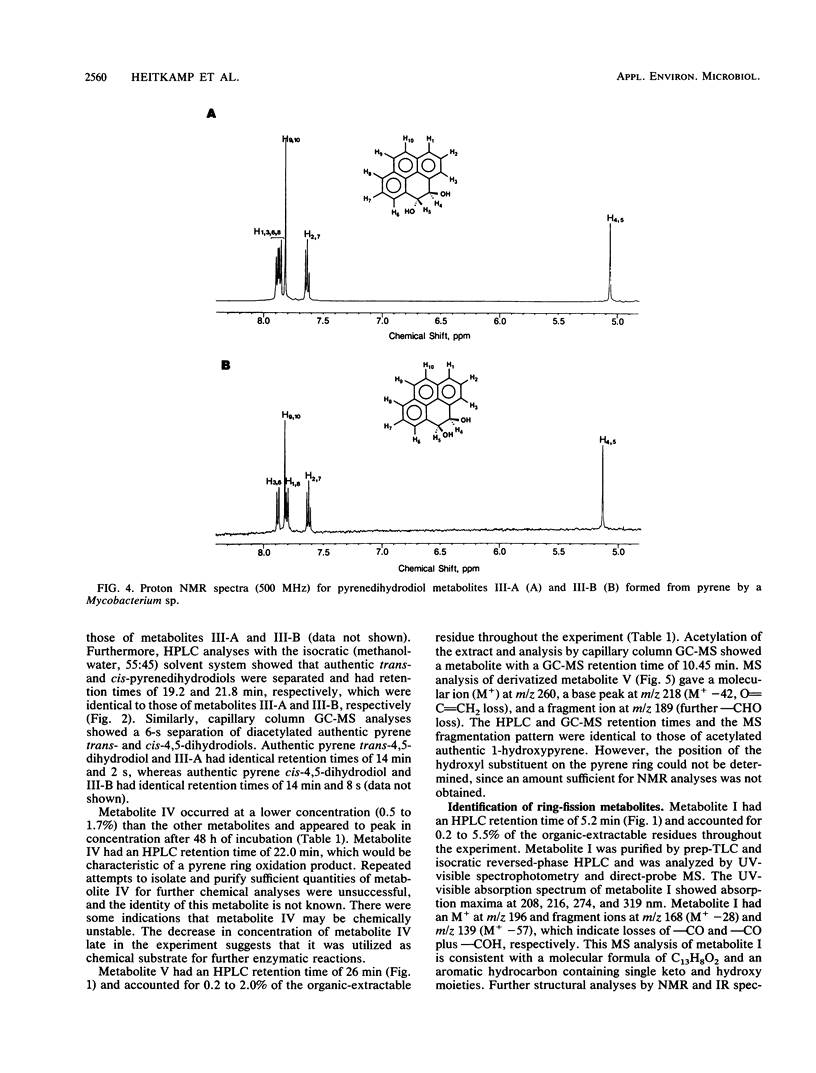
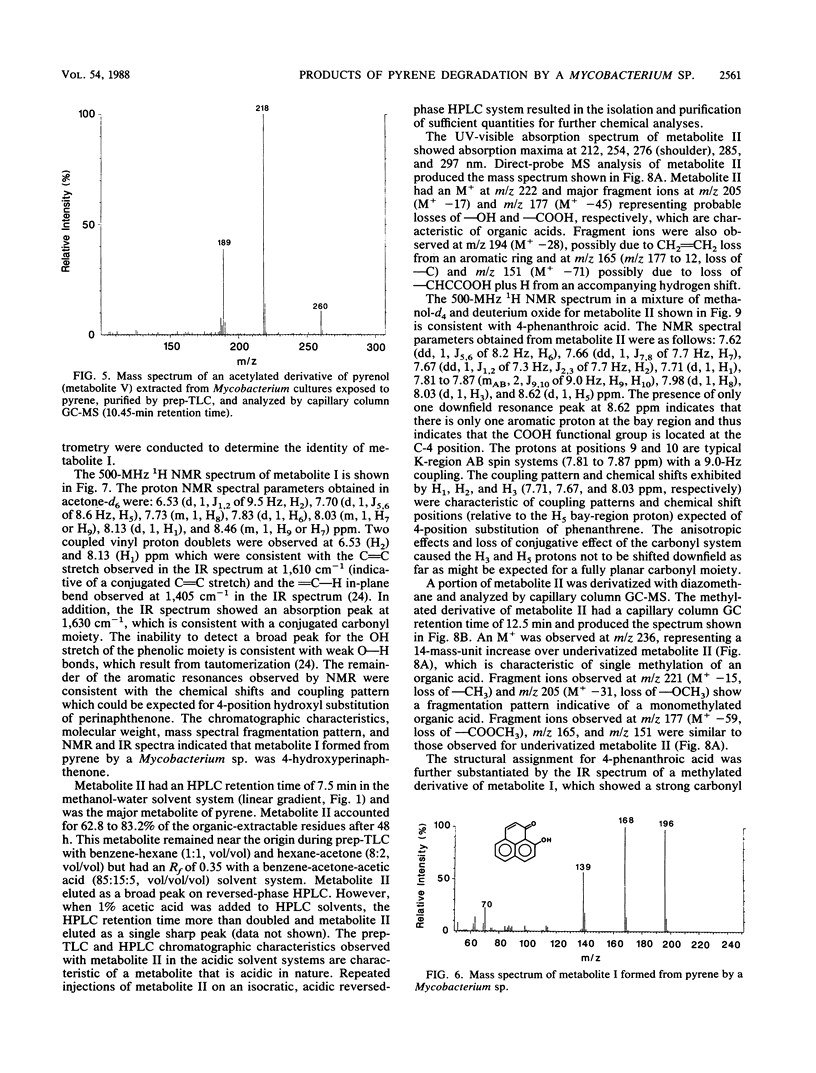
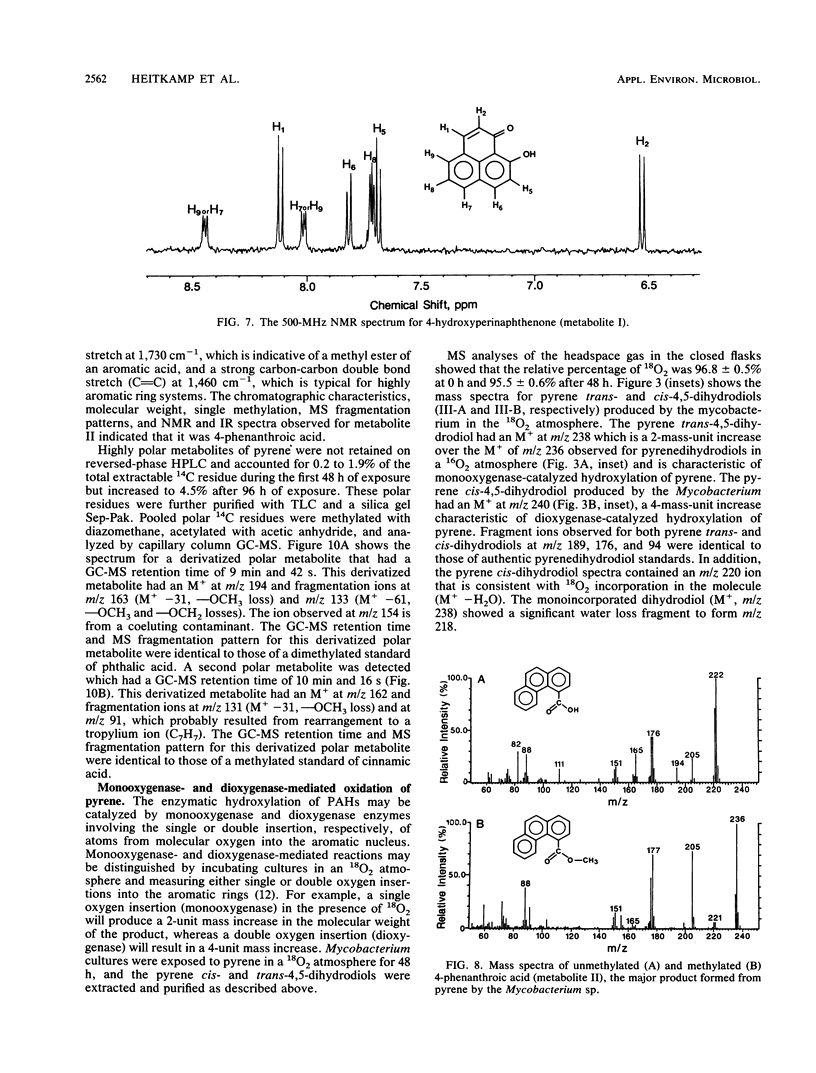
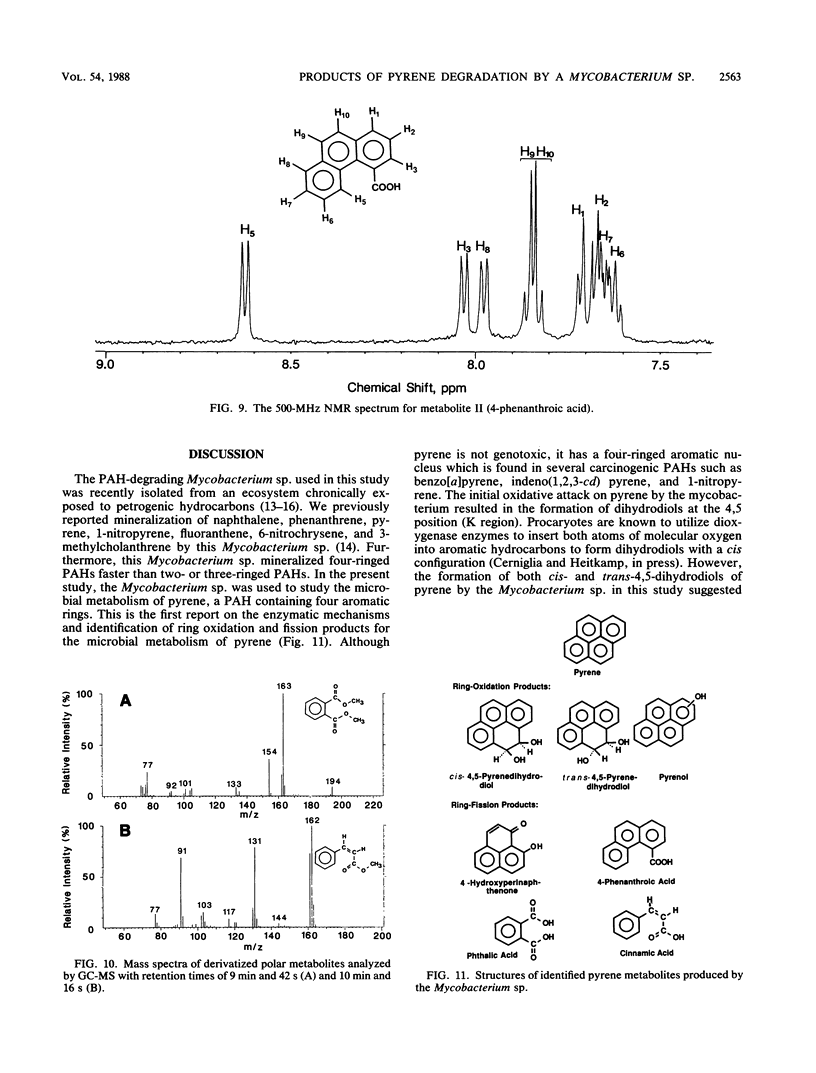
The degradation of pyrene, a polycyclic aromatic hydrocarbon containing four aromatic rings, by pure cultures of a Mycobacterium sp. was studied. Over 60% of [14C]pyrene was mineralized to CO2 after 96 h of incubation at 24 degrees C. High-pressure liquid chromatography analyses showed the presence of one major and at least six other metabolites that accounted for 95% of the total organic-extractable 14C-labeled residues. Analyses by UV, infrared, mass, and nuclear magnetic resonance spectrometry and gas chromatography identified both pyrene cis- and trans-4,5-dihydrodiols and pyrenol as initial microbial ring-oxidation products of pyrene. The major metabolite, 4-phenanthroic acid, and 4-hydroxyperinaphthenone and cinnamic and phthalic acids were identified as ring fission products. 18O2 studies showed that the formation of cis- and trans-4,5-dihydrodiols were catalyzed by dioxygenase and monooxygenase enzymes, respectively. This is the first report of the chemical pathway for the microbial catabolism of pyrene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M. N., Boyd D. R., Thompson N. J., Koreeda M., Gibson D. T., Mahadevan V., Jerina D. M. Absolute sterochemistry of the dihydroanthracene-cis- and -trans-1,2-diols produced from anthracene by mammals and bacteria. J Chem Soc Perkin 1. 1975;(23):2506–2511. [PubMed] [Google Scholar]
- Bernhardt F. H., Pachowsky H., Staudinger H. A 4-methoxybenzoate O-demethylase from Pseudomonas putida. A new type of monooxygenase system. Eur J Biochem. 1975 Sep 1;57(1):241–256. doi: 10.1111/j.1432-1033.1975.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Mitchum R. K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982 May;43(5):1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Kelly D. W., Freeman J. P., Miller D. W. Microbial metabolism of pyrene. Chem Biol Interact. 1986 Feb;57(2):203–216. doi: 10.1016/0009-2797(86)90038-4. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Yang S. K. Stereoselective metabolism of anthracene and phenanthrene by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1984 Jan;47(1):119–124. doi: 10.1128/aem.47.1.119-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Isolation of plasmids from mycobacteria. Infect Immun. 1979 Jun;24(3):979–981. doi: 10.1128/iai.24.3.979-981.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Enzymic studies on the mechanism of double hydroxylation. Pharmacol Rev. 1966 Mar;18(1):71–75. [PubMed] [Google Scholar]
- Heitkamp M. A., Cerniglia C. E. Mineralization of polycyclic aromatic hydrocarbons by a bacterium isolated from sediment below an oil field. Appl Environ Microbiol. 1988 Jun;54(6):1612–1614. doi: 10.1128/aem.54.6.1612-1614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Franklin W., Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterization of a pyrene-degrading bacterium. Appl Environ Microbiol. 1988 Oct;54(10):2549–2555. doi: 10.1128/aem.54.10.2549-2555.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Cerniglia C. E. Naphthalene biodegradation in environmental microcosms: estimates of degradation rates and characterization of metabolites. Appl Environ Microbiol. 1987 Jan;53(1):129–136. doi: 10.1128/aem.53.1.129-136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbes S. E., Schwall L. R. Microbial transformation of polycyclic aromatic hydrocarbons in pristine and petroleum-contaminated sediments. Appl Environ Microbiol. 1978 Feb;35(2):306–316. doi: 10.1128/aem.35.2.306-316.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder C. L., Korfmacher W. A., Slikker W., Jr, Thompson H. C., Jr, Gosnell A. B. Mass spectral characterization of doxylamine and its rhesus monkey urinary metabolites. Biomed Mass Spectrom. 1985 Apr;12(4):151–158. doi: 10.1002/bms.1200120403. [DOI] [PubMed] [Google Scholar]
- Jacob J., Grimmer G., Raab G., Schmoldt A. The metabolism of pyrene by rat liver microsomes and the influence of various mono-oxygenase inducers. Xenobiotica. 1982 Jan;12(1):45–53. doi: 10.3109/00498258209052453. [DOI] [PubMed] [Google Scholar]
- Keimig S. D., Kirby K. W., Morgan D. P., Keiser J. E., Hubert T. D. Identification of 1-hydroxypyrene as a major metabolite of pyrene in pig urine. Xenobiotica. 1983 Jul;13(7):415–420. doi: 10.3109/00498258309052279. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 1986;8 (Suppl 7):1–119. [PubMed] [Google Scholar]
- Okamoto H., Yoshida D. Metabolic formation of pyrenequinones as enhancing agents of mutagenicity in Salmonella. Cancer Lett. 1981 Jan;11(3):215–220. doi: 10.1016/0304-3835(81)90110-5. [DOI] [PubMed] [Google Scholar]
- Von Tungeln L. S., Fu P. P. Stereoselective metabolism of 9-methyl-, 9-hydroxymethyl- and 9,10-dimethylanthracenes: absolute configurations and optical purities of trans-dihydrodiol metabolites. Carcinogenesis. 1986 Jul;7(7):1135–1141. doi: 10.1093/carcin/7.7.1135. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. The purification and properties of cytochrome o from Vitreoscilla. J Biol Chem. 1966 Jul 25;241(14):3308–3315. [PubMed] [Google Scholar]


