Abstract
Genetic mechanisms have been proposed to explain the pervasive representation of right-handedness in humans, whereas random, nongenetic factors have been posited to explain the lack of population-level right-handedness in nonhuman primates. We report evidence that hand preferences in chimpanzees are heritable, even among related individuals raised in different environments. Furthermore, we report that the degree of heritability is modified by factors associated with developmental instability, notably, offspring parity. The data are interpreted to reconcile both genetic models for handedness and hypotheses suggesting that developmental instability influences variation in handedness.
Approximately 85 to 90% of humans report themselves as being right-handed (Annett, 1985; Porac & Coren, 1981). The evolution of population-level handedness has been linked to the emergence of a number of complex human behaviors, including tool use, complementary motor skill, intelligence, and language (see Bradshaw & Rogers, 1993, for a review). Therefore, understanding the phylogeny of handedness in nonhumans may provide insights into a host of allegedly unique human psychological functions.
The pervasiveness of right-handedness in humans has led to numerous debates about the mechanisms involved in the expression of hand preference (B. Hopkins & Ronnqvist, 1998). Some genetic models propose one or two loci coding for handedness (or related lateralized brain functions, i.e., language; Annett, 1985; McManus & Bryden, 1992), whereas others have proposed that multiple loci are involved (Laland, Kumm, Van Horn, & Feldman, 1995; Yeo & Gangestad, 1993). The principle empirical support of all single-gene or multiple-loci genetic models of hand preference comes from the fact that it runs in families, and that offspring typically exhibit patterns of hand preference more similar to their biological than adoptive parents (Carter-Saltzman, 1980; Curt, De Agostini, Maccario, & Dellatolas, 1995; Hicks & Kinsbourne, 1976; McGee & Cozad, 1980; McManus & Bryden, 1992). One problem with genetic models, particularly those proposing a single locus or double loci, is that they cannot account for all the phenotypic variation in handedness. To explain these findings, several authors have invoked social learning or social pressure, prenatal hormone exposure, in utero fetal position, birth season, birth order, and maternal age as factors influencing the development of hand preference in humans (Geschwind & Galaburda, 1985; Porac & Coren, 1981; Previc, 1991; Provins, 1997).
Such in utero perturbations and perinatal factors are believed to cause shifts in the genetic predisposition for right-handedness toward ambidexterity or left-handedness (i.e., when they create some critical level of developmental instability). From this perspective, right-handedness is perceived as being the normative path of development, and non-right-handedness reflects a deviation from normal development that is due to pathological factors or perturbations that disturb the canalizing or buffering processes of normal left-hemisphere development in hemispheric specialization.
The general view is that laterality in nonhumans, in contrast to human handedness, is influenced by random environmental rather than genetic factors (Warren, 1980). The principle support for a nongenetic basis for limb preferences in nonhumans is the failure to demonstrate selective breeding of direction of paw preference in mice and the equivocal results on heritability of hand preference in nonhuman primates (Brinkman, 1984; Brooker, Lehman, Heinbuch, & Kidd, 1981; Byrne & Byrne, 1991; Collins, 1985; W.D. Hopkins, 1999; W.D. Hopkins, Bales, & Bennett, 1993; Kubota, 1990; Matoba, Masataka, & Tanioka, 1991; Westergaard & Suomi, 1997; but see Bisazza, Facchin, & Vallortigara, 2000; Waters & Denenberg, 1994). However, few heritability studies have been performed in nonhuman primates, and rarely can the influence of genetic and nongenetic factors be dissociated in explaining the observed patterns of hand use.
In the current study, we report the first evidence that genetic factors play a significant role in the manifestation of directional biases in hand preference in a nonhuman species. We further report that phenotypic variation from the genetic prediction for hand preference is probably consequent to increased levels of developmental instability experienced by the offspring. Specifically, it has recently been reported that first- and latter-born chimpanzees show significantly higher incidences of left-handedness than middle-born chimpanzees (W.D. Hopkins & Dahl, 2000; W.D. Hopkins, Dahl, & Pilcher, 2000). It has also been reported that higher incidences of abnormal pregnancies with pathological outcomes in chimpanzees cluster at low and high parities (Dahl, 1999). One interpretation of these findings is that at least some of the left-handedness observed in first-born and high-parity offspring is caused by developmental instability induced by reproductive factors associated with parity. It follows that if much of the phenotypic variation in handedness associated with developmental instability is accounted for in heritability analyses for handedness, then any genetic mechanism will become clear, if it exists. To test this hypothesis, we examined whether concordance in hand preference is significantly greater in genetically related individuals born at “nonrisk” parities (with low developmental instability) than in genetically related individuals born at “risk” parities (pregnancies with relatively high developmental instability).
Method
Subjects
A sample of chimpanzees (Pan troglodytes) housed at the Yerkes Regional Primate Research Center (YRPRC) served as subjects in this study. The number of individuals used in the analyses varied according to the familial comparisons being made.
Procedure
To assess hand preferences in a sample of captive chimpanzees, we used a measure referred to as the tube task. This task requires coordinated bimanual actions and has been described in detail elsewhere (W.D. Hopkins, 1995, 1999). Briefly, peanut butter is smeared on the inside surfaces of a polyvinyl chloride (PVC) pipe (approximately 9 cm in length and 2 cm in diameter). The PVC pipe is then handed to the subject in its home cage, and the finger or hand used in removing the peanut butter is recorded by the observer. The hand active in removing the peanut butter is defined as the dominant hand. Observations continue until the subject eats all the food, drops the tube, or hands the tube back to the experimenter.
A minimum of 20 responses was recorded for each subject. Although other measures of hand preference have been collected in the YRPRC colony, the tube measure was used to classify subjects as left-or right-handed because (a) it induces the largest magnitude of hand preference in the chimpanzees, and (b) it is a highly reliable measure, with test-retest measures exceeding .70 over a 2-year period (W.D. Hopkins, 1999).
On the basis of their hand use in this task, the chimpanzees' hand preferences were characterized in the following way. First, a handedness index (HI) was determined for each subject following the formula HI = [(R − L)/(R + L)], where R and L are the number of right- and left-hand responses, respectively. Values on this index can range from − 1.0 to 1.0. Subjects with a negative score or a score of zero were classified as left-handed, and all others were classified as right-handed. Chimpanzees with the same classification of hand preference were considered concordant; all others were considered disconcordant.
Two sets of data were analyzed. First, percentages of concordance in hand preference were compared in mother-infant dyads. For this analysis, 134 mother-infant dyads with known hand preferences and parities were used. In the second set of analyses, we compared percentages of concordance in hand preference in 155 maternal half-sibling dyads for which parity was known. All of a female's stillbirths and live births were considered in the assignment of offspring parity. Spontaneous abortions were not included in the evaluation of parity.
In the analysis of mother-infant dyads, as in our previous studies, chimpanzees with a parity of one (first-born) or of six or higher (late-born) were considered the risk group. This group had a relatively high incidence of left-handedness (W.D. Hopkins & Dahl, 2000) and also showed signs of hormonal irregularity associated with puerperal pathologies (Dahl, 1999). These signs are indicators of relatively high levels of developmental instability. Chimpanzees with parities from two to five were considered a nonrisk group for developmental instability because they did not show high incidences of puerperal pathology or left-handedness. We also recorded whether each subject was mother- or nursery-reared (mother vs. human). Chimpanzees that stayed with their biological mother for more than 30 days were considered mother-reared. All other chimpanzees were considered nursery-reared (Bard, 1996). Specifically, of the 134 mother-infant dyads, 58 offspring were in the risk group and 76 were in the nonrisk group. Within the risk group, there were 19 mother- and 39 nursery-reared offspring. Within the nonrisk group, there were 34 mother- and 42 nursery-reared chimpanzees.
For the analysis of maternal half-siblings, we characterized each sibling pair according to whether both were raised by their biological mother (mother), both were raised by humans in a nursery setting (human), or one was raised by their biological mother and the other was raised in a nursery setting (cross-fostered). There were 44 cross-fostered, 37 mother-raised, and 74 human-raised sibling dyads. In addition, within each rearing condition, sibling pairs were characterized as both being middle-born (mid-mid), one being middle-born and one being first-born (first-mid), and one being middle-born and one being latter-born (mid-late). Although other parity pairings are feasible (e.g., late-late), they were not included in this analysis because they were not of theoretical interest and they had very low sample sizes (n ≤ 7).
Results
Log-linear analysis was used to assess the effects of parity grouping (risk, nonrisk) and rearing (mother, human) on mother-infant concordance in hand preference. In the initial analysis, the degree of concordance was compared for offspring born to right-handed females (n = 83). A significant main effect for parity was found, χ2(2, N = 83) = 15.23, p < .001. For the nonrisk group, 86% of offspring born to right-handed females were right-handed; in contrast, in the risk group, only 46% of offspring born to right-handed females were right-handed. For left-handed females (n = 51), no effect of parity group or rearing history was found (see Fig. 1). The only percentages of mother-infant concordance that exceeded chance were for offspring in the nonrisk parity group, either mother-reared or nursery-reared, born to right-handed females. No other percentages reached statistical significance.
Fig. 1.
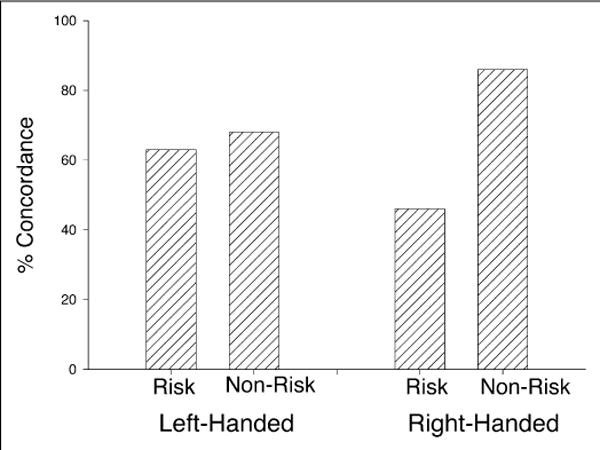
Percentages of concordance in hand preference in mother-infant dyads as a function of parity group and maternal hand preference.
In the initial analyses of the sibling data, the degrees of concordance and disconcordance were compared as a function of birth-order pairing and rearing history. The categorical data were then compared in relation to the birth-order-pairing and rearing-history variables using chi-square analyses. The proportion of concordant and disconcordant pairs was not influenced by the rearing variable. However, a 2 × 3 chi-square test of independence revealed a significant interaction between sibling concordance and birth-order pairing, χ2(2, N = 155) = 15.89, p < .001 (see Fig. 2). Overall, the percentage of concordance in mid-mid pairs was significantly higher than chance, whereas the percentages for the other two birth-order pairings were not. This result was consistent across all three rearing conditions. Separate chi-square tests within each rearing condition revealed significant interactions between concordance percentage and birth-order pairing for the mother-raised subjects, χ2(2, N = 37) = 9.92, p < .01, and cross-fostered subjects, χ2(2, N = 44) = 15.05, p < .001, but not the human-raised subjects, χ2(2, N = 74) = 0.67, n.s.
Fig. 2.
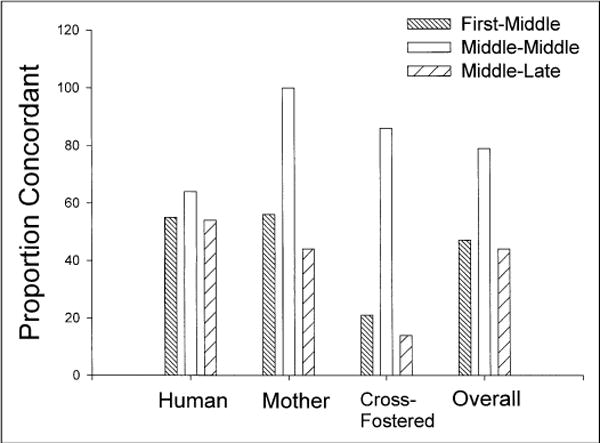
Percentages of concordance in hand preference in siblings reared together with their biological mother, reared together by humans, or reared apart and in a different environment (cross-fostered). Results are shown separately for pairs in which one sibling was first-born and the other was middle-born, both siblings were middle-born, and one sibling was middle-born and one sibling was late-born.
Discussion
In these analyses, when variation in parity was accounted for, the tendency for right-handed females to give birth to offspring that develop right-hand preferences became clear. Similarly, once at least some of the variation in parity and developmental instability was accounted for, maternal half-siblings showed a significant degree of concordance in hand preference, even when raised in different environments.
The results of this study indicate that hand preferences in chimpanzees are probably genetically determined and most closely follow the predictions of the right-shift model originally proposed by Annett (1985). These data do not support the suggestion that a genetic mutation in hominid evolution accounts for the emergence of uniquely human functional or neuroanatomical asymmetries (Corballis, 1997). Rather, these data strongly suggest that a gene or set of genes for handedness (or hemispheric specialization) may have been shared by the common ancestor of humans and chimpanzees. It is important to emphasize that the ratio of right- to left-handedness is much more pronounced in humans (8:1 or 9:1) than in chimpanzees (2:1). One explanation for this finding may be that an additional genetic mutation in hominid evolution accounts for the differences in right-handedness observed in chimpanzees and humans. At present, there are no candidate genes for handedness, but given the genetic similarity between chimpanzees and humans, it is likely that the discovery of any such genes in humans will allow for direct comparative studies in chimpanzees using linkage analyses or other molecular biological techniques.
An underlying question that emerges from these findings is what causes disconcordance in hand preference in related chimpanzees, particularly in those that are genetically predisposed to be right-handed. Parity per se is likely not the causal factor for left-handedness in these chimpanzees, but rather prenatal factors that are marked by parity are probable candidates. We believe that at least some left-handedness is induced by unknown pathological events that take place during the prenatal period of development, particularly at the identified risk parities. Two sets of data support this interpretation. First, studies in humans and to a lesser extent in nonhuman primates indicate that asymmetries in dermatoglyphic ridge counts on homologous fingertips reflect both genetic and nongenetic prenatal perturbations experienced by the developing fetus (Arrieta et al., 1993; Babler, 1991). Recently, we (Pilcher, Dahl, Swetland, & Hopkins, 2000) fingerprinted the YRPRC colony of chimpanzees and measured asymmetries in dermal ridge counts on homologous fingers and found an association between hand preference, birth order, and dermatoglyphic asymmetry. Specifically, significantly more ridges were found on the left than the right hand in first- and middle-born chimpanzees. In contrast, among latter-born chimpanzees, the pattern was reversed, with significantly more ridges on the right compared with the left hand. This finding indicates that prenatal events alter the morphology of the developing fetus, particularly in the offspring that have high parity and are born to relatively older females.
Second, we believe that variation in prenatal hormone concentrations in chimpanzees may be one factor that either influences handedness directly or reflects the developmental instability that induces left-handedness. For chimpanzees, the examination of at least some of the variation in estrogens and progesterone during pregnancy is made possible by the use of an external marker of the genitalia, the perineal sex swelling (PS). It has been well established that PS during intermen-strual intervals is stimulated by estrogens and antagonized by progesterone (Faiman, Reyes, Winter, & Hobson, 1981; Graham, 1981). Recent analyses of PS scores in pregnant chimpanzees indicate a similar correspondence between swelling patterns and the patterns of estrogen and progesterone fluctuations (Dahl, 1999). PS scores during pregnancy vary systematically as a function of the parity of the developing fetus, with abnormally high and prolonged PS in many low-parity pregnancies and significantly reduced PS in multiparous, older females (Dahl, 1999).
For a subsample of chimpanzees in this study, PS scores of the female during pregnancy were known. Therefore, these PS scores could be linked to the hand preferences of offspring produced using retrospective analysis. A comparison of the distribution of unusually high and low PS scores as a function of either offspring or maternal hand preference did not reveal a significant association. This finding does not support the interpretation that unusually high or low PS scores directly influence hand preference. Alternatively, unusually high or low PS scores may reflect some level of stability of the developing feto-placental unit. According to this hypothesis, if unusually high or low PS scores induce greater developmental instability that alters the development of hand preference, then genetically related individuals that are concordant for hand preference would show smaller differences in PS scores during gestation than disconcordant siblings. To test this hypothesis, we calculated the absolute differences in the total PS scores derived from maternal half-siblings and compared this difference among concordant and disconcordant pairs raised in different environments (see Fig. 3). The results indicated that disconcordant dyads experienced significantly different prenatal environments, as reflected in higher differences in PS scores, than concordant siblings. These results are consistent with concordance rates among siblings from different parities. This close association among swelling patterns, parity, and handedness strongly suggests that hormonal variation may either strongly influence the development of laterality in chimpanzees or, more likely, be a clear reflection of some developmental instability.
Fig. 3.
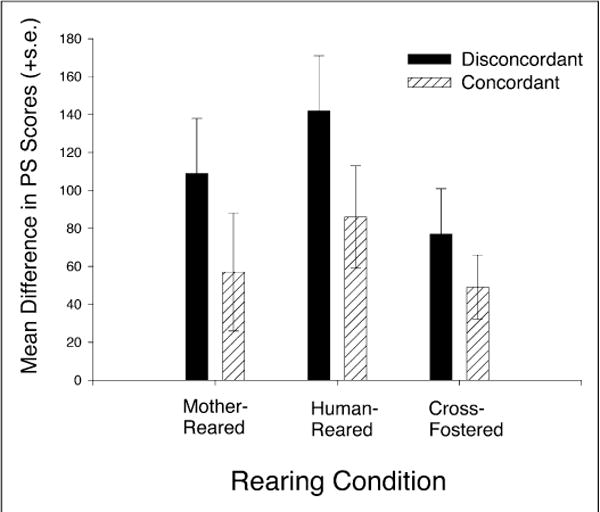
Mean differences in cumulative perineal sex swelling (PS) scores in chimpanzee pregnancies for offspring concordant or disconcordant for hand preference, as a function of rearing condition.
In conclusion, we propose that genetic determination of handedness may rely on a particular stable in utero environment acting as a canalizing phenomenon. Genetic and nongenetic perturbations can be seen as causing developmental instability, a phenomenon recently implicated in the causation of human handedness (Yeo & Gangestad, 1993). In our chimpanzee sample, the evidence suggests that high-parity offspring (and therefore offspring born to older females) manifest higher levels of developmental instability, as reflected in reversed dermatoglyphic asymmetries and unusually low PS scores during gestation. Thus, left-handedness in these individuals may be seen as being induced by pathological prenatal events.
It is not clear what genetic or nongenetic factors are causing prenatal perturbations in our chimpanzee sample, but we emphasize that both sources could play a role. For example, the YRPRC colony of chimpanzees has been self-sustaining and largely self-contained, genetically speaking, for nearly 70 years. Thus, many of the chimpanzees are related, and high levels of developmental instability could reflect increased homozygosity of the developing fetus, which, by itself, would put the offspring at risk for the expression of deleterious recessive genes. Alternatively, captive chimpanzees typically breed at a younger age and produce offspring at older ages than wild chimpanzees. The stress of carrying a pregnancy at the extreme end of the age range may induce some critically higher levels of instability in the developing fetus. Continued studies on the interaction between genetic and nongenetic factors in humans and nonhumans should elucidate the importance of each variable in the development of behavioral and neuroanatomical lateralization.
Acknowledgments
This research was supported by National Institutes of Health Grants NS-29574 and NS-36605 to W.D.H., Grant RR-03587 to K.G. Gould, and Grant RR-00165 to the Yerkes Regional Primate Research Center. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. We would like to thank the animal-care staff and animal-records staff for their help in executing this research. We also appreciate the helpful assistance of Samuel Fernandez-Carriba in collecting the data.
References
- Annett M. Left, right, hand, and brain: The right-shift theory. London: Erlbaum; 1985. [Google Scholar]
- Arrieta M, Criado B, Martinez B, Lobato M, Lostao A, Lostao C. Fluctuating dermatoglyphic asymmetry: Genetic and prenatal influences. Annals of Human Biology. 1993;20:557–563. doi: 10.1080/03014469300002962. [DOI] [PubMed] [Google Scholar]
- Babler WJ. Embryologic development of epidermal ridges and their configurations. Birth Defects: Original Article Series. 1991;27:95–112. [PubMed] [Google Scholar]
- Bard KA. Responsive care: Behavioral intervention for nursery-reared chimpanzees. 1996. Available from the Jane Goodall Institute, Ridgefield, CT 06877. [Google Scholar]
- Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: Concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Brinkman C. Determinants of hand preference in Macaca fascicularis [Abstract] International Journal of Primatology. 1984;5:325. [Google Scholar]
- Brooker RJ, Lehman RAW, Heinbuch RC, Kidd KK. Hand usage in a colony of bonnet monkeys (Macaca radiata) Behavior Genetics. 1981;11:49–56. doi: 10.1007/BF01065827. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Carter-Saltzman L. Biological and sociocultural effects on handedness: Comparison between biological and adoptive parents. Science. 1980 September;209:1263–1265. doi: 10.1126/science.7403887. [DOI] [PubMed] [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral lateralization in nonhuman species. Orlando, FL: Academic Press; 1985. pp. 150–164. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Curt F, De Agostini M, Maccario M, Dellatolas G. Parental hand preference and manual functional asymmetry in preschool children. Behavior Genetics. 1995;25:525–536. doi: 10.1007/BF02327576. [DOI] [PubMed] [Google Scholar]
- Dahl JF. Perineal swelling during gestation and maternal competence in chimpanzees. Journal of Medical Primatology. 1999;28:129–141. doi: 10.1111/j.1600-0684.1999.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Faiman C, Reyes I, Winter JSD, Hobson WC. Endocrinology of pregnancy in apes. In: Graham CE, editor. Reproductive biology of the great apes: Comparative and biomedical aspects. New York: Academic Press; 1981. pp. 45–68. [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization: Biological mechanisms, associations and pathology: I. A hypothesis and program of research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Graham CE. Menstrual cycle of the great apes. In: Graham CE, editor. Reproductive biology of the great apes: Comparative and biomedical aspects. New York: Academic Press; 1981. pp. 1–43. [Google Scholar]
- Hicks R, Kinsbourne M. Genetic basis for human handedness: Evidence from a partial cross-fostering study. Science. 1976;192:908–910. doi: 10.1126/science.1273577. [DOI] [PubMed] [Google Scholar]
- Hopkins B, Ronnqvist L. Human handedness: Developmental and evolutionary perspectives. In: Simion F, Butterworth G, editors. The development of sensory, motor and cognitive capacities of early infancy: From perception to cognition. East Sussex, England: Psychology Press; 1998. pp. 191–236. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Heritability of hand preference in chimpanzees: Evidence from a partial interspecies cross-fostering paradigm. Journal of Comparative Psychology. 1999;113:1–7. doi: 10.1037/0735-7036.113.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bales S, Bennett AJ. Heritability of hand preference in chimpanzees (Pan troglodytes) International Journal of Neuroscience. 1993;74:17–26. doi: 10.3109/00207459408987225. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF. Birth order and hand preference in chimpanzees (Pan troglodytes): Implications for pathological models of human handedness. Journal of Comparative Psychology. 2000;114:302–306. doi: 10.1037/0735-7036.114.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Birth order and left-handedness revisited: Some recent findings in chimpanzees (Pan troglodytes) and their implications for developmental and evolutionary models of human handedness. Neuropsychologia. 2000;38:1626–1633. doi: 10.1016/s0028-3932(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Kubota K. Preferred hand use in the Japanese macaque troop, Arashiyama-R, during visually-guided reaching for food pellets. Primates. 1990;31:393–406. [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-culture model of human handedness. Behavior Genetics. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- Matoba M, Masataka N, Tanioka Y. Cross-generational continuity of hand-use preference in marmosets. Behaviour. 1991;117:281–286. [Google Scholar]
- McGee MG, Cozad T. Population genetic analysis of human hand preference: Evidence for generation differences, familial resemblance and maternal effects. Behavior Genetics. 1980;10:263–275. doi: 10.1007/BF01067772. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology: Vol 6 Developmental neuropsychology, Part 1. Amsterdam: Elsevier; 1992. pp. 115–144. [Google Scholar]
- Pilcher D, Dahl JF, Swetland M, Hopkins WD. Asymmetries in dermatoglyphics of the fingertips of chimpanzees: Their association with handedness, birth order and sex. 2000. Manuscript submitted for publication. [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer; 1981. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provins KA. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychological Review. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Waters NS, Denenberg VH. Analysis of two measures of paw preference in a large population of inbred mice. Behavioural Brain Research. 1994;63:195–204. doi: 10.1016/0166-4328(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Westergaard GG, Suomi SJ. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Developmental Psychobiology. 1997;31:143–147. [PubMed] [Google Scholar]
- Yeo R, Gangestad SW. Developmental origins of variation in human hand preference. Genetica. 1993;89:281–296. [Google Scholar]


