Abstract
In human infancy, 2 criteria for intentional communication are (a) persistence in and (b) elaboration of communication when initial attempts to communicate fail. Twenty-nine chimpanzees (Pan troglodytes) were presented with both desirable (a banana) and undesirable food (commercial primate chow). Three conditions were administered: (a) the banana was delivered (successful communication), (b) half of the banana was delivered (partially successful communication), and (c) the chow was delivered (failed communication). The chimpanzees exhibited persistence in and elaboration of their communication in every condition except when the banana was delivered. Thus, their communication was about a specific item, demonstrating that both intentionality and nonverbal reference are capacities shared by humans with our nearest living relatives, the great apes.
A significant milestone in human development is the transition to intentional communication, traditionally held to begin at about 10 to 12 months of age (e.g., Butterworth, 2001; Lock, 2001). One of the most replicated observations from studies of human communicative development is that sometime around the end of the 1st year of life, babies begin to point to distant objects (Franco & Butterworth, 1996; Leung & Rheingold, 1981). Until recently, pointing was held to be a uniquely human capacity (Donald, 1991; Povinelli & Davis, 1994; Povinelli, Bering, & Giambrone, 2003), but anecdotal reports of pointing by our nearest living relatives, the chimpanzees, have existed in the scientific literature for almost 90 years (e.g., Furness, 1916; reviewed by Leavens & Hopkins, 1999). More recently, experimental investigations have demonstrated that chimpanzees in captivity frequently point to distal objects in the absence of any explicit training (e.g., Krause & Fouts, 1997; Leavens & Hopkins, 1998, 1999; Leavens, Hopkins, & Bard, 1996; Leavens, Hopkins, & Thomas, 2004).
We characterize pointing by apes as a referential activity (Hopkins & Leavens, 1998; Leavens & Hopkins, 1998; Leavens et al., 1996; Leavens, Hopkins, et al., 2004), meaning simply that apes and humans who use their outstretched arms and fingers to indicate distant objects or events are referring to specific entities. Because we differ in this use of the term reference from the more typical usages in developmental psycholinguistics, a brief explanatory digression is warranted. In symbolic reference, the term refers to the arbitrary nature of the relationship between an entity and the label used to signify that entity. Thus, the word big is not, in fact, bigger than the word little. For most linguists, the term reference is synonymous with symbolic reference; however, developmental psychologists interested in human communicative development have identified pointing as a class of behavior that functionally constitutes nonverbal reference (e.g., Bates, O’Connell, & Shore, 1987; Camaioni, 2001). Thus, nonverbal reference differs from symbolic reference in that the relationship between the signal (pointing) and its referent is not arbitrary but is dictated by the spatial relationships among the signaler, the recipient of the signal, and the item indicated.
There is a further distinction, however, in the human developmental literature between pointing to request delivery of objects and pointing to share attention in middle to late infancy. Protoimperative gestures serve to request objects or actions (e.g., raising arms above head in an apparent bid to be picked up by a caregiver or pointing to an otherwise unreachable toy) and protodeclarative gestures apparently serve to co-orient a baby and a caregiver toward the same object or event (e.g., pointing to a dog while uttering “doggie!”; Baron-Cohen, 1999; Bates, Camaioni, & Volterra, 1975). In addition to its status as an example of intentional communication, protodeclarative pointing (commenting on the world), but not protoimperative pointing (requesting something), is held to exemplify the dawning, preverbal capacity for nonverbal reference (Bates et al., 1987; Camaioni, 2001). Hence, pointing in which the goal appears to be the co-orientation of visual attention to some distal object or event by the signaler and a social partner is differentiated in the current literature from pointing to request that a social partner retrieve or deliver some specific object, in terms of the presumed psychological underpinnings of these behaviorally similar acts (e.g., Baron-Cohen, 1999; Legerstee & Barillas, 2003; Tomasello, 1999). Pointing to share attention to distant events or objects is widely held to be diagnostic of an infant’s abilities to represent others as psychological agents with perspectives that differ from that of the infant. In this perspective, pointing to request things signifies only that babies perceive others as causal (as opposed to mental) agents. Thus, protoimperative gestures are held to constitute babies’ attempts to manipulate others’ behaviors or to manipulate the world through others’ behaviors, whereas protodeclarative gestures are held to constitute evidence of babies’ attempts to manipulate others’ minds or states of knowledge and therefore imply the possession by the signaler of a nascent theory of mind.
The most extreme statement on this distinction, to our knowledge, is that by Baron-Cohen (1999), who explicitly excluded protoimperative gestures from the category of intentional communication. Related arguments have been proffered by Povinelli and his colleagues (e.g., Povinelli, Bering, & Giambrone, 2000, 2001, 2003; Povinelli & Giambrone, 1999). These authors argued that similarities in communicative behavior between our nearest living relatives, chimpanzees, and human children are accompanied by “striking psychological differences” (Povinelli & Giambrone, 1999, p. 170). In this statement and in subsequent publications, the authors failed to describe the avenue or channel through which they can identify psychological differences between human children and apes other than overt, publicly observable behavior (e.g., Leavens, 2002, 2004, in press). Thus, recent theoretical formulations that emphasize a hierarchical relationship between protoimperative and protodeclarative gestures, with protodeclarative gestures taken to imply the application of higher order representational processes by human babies, all suffer from an inability to pinpoint any objectively observable behavior that can unambiguously implicate these same putative higher order representational processes (e.g., Leavens, Hostetter, Wesley, & Hopkins, 2004). This recent theoretical emphasis on mentalistic implications of gestural communication in human babies ignores a decades-old axiom of developmental psychology; to wit, researchers agree that babies acquire the capability to communicate intentionally and referentially, not by virtue of any unambiguous manifestation of their internal goal states but by their display of a series of novel behaviors and patterns of behavior that are (a) objectively measurable and (b) widely replicated (e.g., Cazden, 1977; Leavens, 2002; Sugarman, 1984; for more nuanced evidence in support of this mentalistic framework, see recent work by Camaioni, Perucchini, Bellagamba, & Colonnesi, 2004; Liszkowski, Carpenter, Henning, Striano, & Tomasello, 2004).
A competing theoretical stance characterizes protodeclarative pointing in terms of the affective consequences of the pointing gesture (Dunham & Dunham, 1995; Moore & Corkum, 1994). Numerous researchers have noted the manifest glee that accompanies joint attention in babies and their caregivers (e.g., Adamson, 1996; Adamson & Bakeman, 1985), and this theoretical perspective emphasizes the importance of these emotional consequences of signaling about distant events or objects to the motivation of babies to engage in joint attention. According to this view, babies exhibit protodeclarative pointing because their caregivers reliably respond with intense bursts of positive emotion to the babies’ communicative efforts, such as smiling and verbalizing with very high pitch contours (motherese). This theoretical interpretation of the onset of declarative pointing concedes that babies will eventually develop representational capacities relevant to the understanding of others as mental agents but suggests that at the onset of protodeclarative pointing, at around 12 months, babies are motivated by the expectation that their pointing will elicit appealing behavior from their social partners. The relevance of this interpretation for the present study is that if the early motivations for babies’ pointing behavior in both protoimperative and protodeclarative contexts is the manipulation of the behavior of their social partners (delivery of objects in protoimperative pointing and elicitation of emotional engagement in protodeclarative pointing), there is no compelling logical basis to postulate fundamentally different psychological processes underlying the two kinds of pointing. Hence, when we use the term nonverbal reference to denote pointing both to request delivery of objects and to share attention to distant objects or events, this is not to claim that chimpanzees exhibit the same representational bases for communication that are widely held to underpin human infant protodeclarative pointing but to signal our skepticism that protodeclarative pointing constitutes compelling evidence for human infants’ acquisition of conceptions of their social partners in terms of their partners’ abstract mental states.
With respect to intentionality, because communicative intent cannot be objectively measured, Bates and her colleagues (e.g., Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979; Bates et al., 1975), among others, developed several operational criteria for defining human infants’ developing behavioral capacity to capture visual attention of others and to manipulate others to act on the world (e.g., see also Bard, 1992; Rolfe, 1996/1999; Sugarman, 1984). Among the behavioral criteria for defining intentional communication in humans are (a) signals are used socially (i.e., an audience is required for the display of a signal; Franco & Butterworth, 1990); (b) an influence of the attentional status of an observer on the propensity to exhibit gestures (Bakeman & Adamson, 1986; Franco & Butterworth, 1990; O’Neill, 1996); (c) successive visual orienting between social partners and distant objects or events (also known as gaze alternation or visual checking; Bates et al., 1975; Franco & Butterworth, 1996; Tomasello, 1995, 1999); (d) deployment of apparent attention-getting behavior (e.g., vocalizations; Bates et al., 1975; Lock, 2001); and (e) persistence in and (f) elaboration of communicative behavior when apparent attempts to manipulate others fail (Bates et al., 1975; Golinkoff, 1986, 1993; Lock, 2001). The first four of these criteria (a–d) are well established in the communication of our nearest living relatives, the great apes. Because these data are relatively recent, and therefore they may not be familiar to readers of this journal, a brief review is warranted.
With respect to the necessity of an audience (Criterion a), to our knowledge, no published study of the influence of observer presence exists for human infants, although there are several unpublished studies (Franco & Butterworth, 1990; Leavens & Todd, 2002). In contrast, the necessity of an audience for the display of manual gestures is well established for chimpanzees (Hostetter, Cantero, & Hopkins, 2001; Leavens et al., 1996; Leavens, Hopkins, et al., 2004; see Call & Tomasello, 1994, for related findings with two orangutans, Pongo pygmaeus). Table 1 summarizes the data from our laboratory on the influence of observer presence on the display of manual gestures by chimpanzees in the context of unreachable food. Clearly, chimpanzees in captivity do not gesture toward unreachable food in the absence of an observer, a robust finding that is far better established for chimpanzees than it is for human infants.
Table 1.
Chimpanzees at the Yerkes National Primate Research Center Almost Never Gesture Outside Their Cages in the Absence of an Audience: Summary of Experimental Studies on the Influence of (Human) Observer Presence on the Display of Gestures by Chimpanzees
| N (Subjects) | N (Gestures) | N (Absence) | % (Presence) | |
|---|---|---|---|---|
| Leavens, Hopkins, & Bard (1996)a | 3 | 256 | 2 | 99 |
| Hostetter, Cantero, & Hopkins (2001)b | 49 | 229 | 6 | 97 |
| Leavens, Hopkins, & Thomas (2004)b | ||||
| Visible banana conditionc | 101 | 50 | 1 | 98 |
| Hidden banana conditionc | 101 | 51 | 1 | 98 |
| Experiment 2c | 35 | 17 | 0 | 100 |
All gestures reported in Leavens et al. (1996) were points, including pointing with the whole hand.
Gestures of all kinds, including pointing, begging, and holding hand out toward observer.
Gestures exhibited as an experimenter approached each subject’s cage, within 4 s of arriving into a position centered on their cage, entering their field of view, are categorized here as occurring in the presence of the experimenter; in the original publication, these gestures were conservatively classified as occurring in the absence of the experimenter for statistical analyses.
With respect to sensitivity to observer visual attention (Criterion b), Call and Tomasello (1994), Hostetter et al. (2001), Krause and Fouts (1997), and Leavens, Hostetter, et al. (2004), among others, have reported influences of observer visual orientation on the propensity to exhibit manual gestures in orangutans and chimpanzees. The significance of these findings is that they imply that apes, like human infants, and with no special training, discriminate the behavioral correlates of visual attention in their human communicative partners. Whether these discriminations are based on particular features, such as eye orientation, head orientation, and so forth, is currently the topic of considerable scrutiny (e.g., Butterworth & Itakura, 2000; Doherty & Anderson, 2001; Moore & Corkum, 1998; Povinelli & Eddy, 1996), but whether discriminations are based on eye direction, head orientation, or other postural cues is irrelevant to the cognitive implications, which are simply that young humans and great apes discriminate the behavioral correlates of different states of visual attention in others (see also Tomasello, Hare, & Agnetta, 1999, for evidence that chimpanzees follow eye gaze geometrically).
Gaze alternation between distant food and a human communicative partner (Criterion c) has been reported to accompany gestural communication in great apes (Table 2) and demonstrates that an organism’s visual orienting behavior is under the stimulus control of both a distant object and a communicative partner (gaze alternation; e.g., Leavens & Hopkins, 1998; Leavens et al., 1996; Leavens, Hopkins, et al., 2004). It is a cardinal feature of intentionality and (nonverbal) reference that publicly observable behavior, such as gaze alternation, disambiguates the meaning of a communicative episode. For some theorists, this bridging of a distant object or event with a particular social partner, through alternation of gaze, implies that the signaler recognizes others as intentional beings whose perspective can differ from that of the signaler (i.e., that others are intentional beings, too; e.g., Tomasello, 1995). Whatever the theoretical interpretation of gaze alternation, it is clear from Table 2 that the proportion of individual chimpanzees who exhibit gaze alternation with their manual gestures is very high, between 76% and 91%, which is substantially higher than has been observed in human infant samples younger than about 2 years of age (see Leavens & Hopkins, 1999, for review).
Table 2.
Percentage of Chimpanzee Gestures Accompanied by Gaze Alternation (GA) Between Food and an Experimenter When Food and Experimenter Are Presented at Some Angular Separation
| N (Subjects) | N (Gestures) | N (With GA) | % (With GA) | |
|---|---|---|---|---|
| Leavens, Hopkins, & Bard (1996) | 1 | 167 | 127 | 76 |
| Leavens & Hopkins (1998) | 115 | 78 | 68 | 87 |
| Leavens, Hopkins, & Thomas (2004) | ||||
| Visible banana condition | 101 | 76 | 65 | 86 |
| Hidden banana condition | 101 | 73 | 62 | 85 |
| Experiment 2 | 35 | 11 | 10 | 91 |
Experimental evidence that chimpanzees exhibit more attention-getting behavior (auditory or tactile signaling; Criterion d) when a human observer is not looking at the communicating chimpanzee were presented by Hostetter et al. (2001) and Leavens, Hostetter, et al. (2004). Observational evidence that representatives of all African great ape species, including gorillas (Gorilla gorilla), bonobos (Pan paniscus), and chimpanzees, exhibit attention-getting behavior in accordance with the visual focus of conspecifics has been provided by Tomasello and his colleagues (Pika, Liebal, & Tomasello, 2003, in press; Tomasello, Call, Nagell, Olguin, & Carpenter, 1994; Tomasello et al., 1997). Observational evidence of attention-getting behavior deployed as a function of the state of attentiveness of a human observer in sign-language-trained chimpanzees has been presented by Krause and Fouts (1997) and Bodamer and Gardner (2002). Deployment of attention-getting behavior demonstrates the procedural awareness of the necessity of capturing the attention of a communicative partner.
With respect to persistence and elaboration of communication (Criteria e and f), however, to our knowledge, no previous study has adequately posed the question: Do chimpanzees persist in or elaborate their communicative behavior in the face of failures to communicate, as human infants do after about 1 year of age? We manipulated the success of chimpanzee communicative bids by simultaneously presenting very desirable but unreachable food (a banana) with relatively undesirable and also unreachable food (commercial standard primate chow) at a large angular separation. After a 30-s period of observation, an experimenter delivered either (a) the banana (banana condition: successful communication); (b) only half of the banana, with the experimenter retaining the other half (half-banana condition: partially successful communication); and (c) the chow (chow condition: failure of communicative bid—construed as a failure because the chimpanzees gestured or oriented visually, or both, to the banana, not the chow). Thus, we deliberately misinterpreted the communicative signals of the chimpanzees in the chow condition and, to a lesser degree, in the half-banana condition. Chimpanzees could repair failures in one of two, not mutually exclusive ways: (a) they could persist in exhibiting a particular response (e.g., gestural or vocal) and (b) they could elaborate on their communicative behavior. We reasoned that if chimpanzees exhibit persistence or elaboration in their communicative behavior after unsuccessful communicative bids (did not get the banana), relative to their behavior after successful communicative bids (got the banana), this demonstrates that the communication is “about” the banana; this “aboutness” is quintessentially intentional communication. The ability to pick out and refer to specific items in the environment, especially through manual gesture—an ability that defines the human transition to intentional communication—apparently is not widespread in the animal kingdom; therefore, chimpanzees are of particular importance in our understanding of human cognitive evolution because we shared a common ancestor perhaps as recently as 6 or 7 million years ago (e.g., Carroll, 2003). If chimpanzees exhibit the same behavioral criteria for intentional communication as do human babies up to approximately years of age, there is no empirical basis for concluding that there are fundamental cognitive differences between young humans and older chimpanzees in the display of their communicative repertoires in requestive contexts. This, in turn, would imply that whatever cognitive capacities are implied by the successful manipulation of a social partner through intentional communication by human infants are also present in our nearest living relatives, the chimpanzees. The relevance to understanding human psychological development is that such evidence strongly implicates neurobiological adaptations that predate the development in our own lineage of bipedal locomotion, very large brains, sophisticated tool manufacture, art, and language. Hence, the sweeping evolutionary changes that have occurred in the human lineage in these domains may build on capacities for the capture and redirection of attention that are shared with great apes. If these behavioral capacities in apes are subject to environmental manipulation of rearing history, we would expect early joint attentional competencies in human infants also to be subject to variations in their life experiences (Leavens, in press).
Method
Subjects
Subjects were 29 adolescent and adult chimpanzees (12 females, 17 males) housed at the Yerkes National Primate Research Center (YNPRC, formerly the Yerkes Regional Primate Research Center), Atlanta, Georgia. All subjects were treated in accordance with the APA guidelines on the ethical treatment of animals (American Psychological Association, 1992). YNPRC is fully accredited by the American Association for Laboratory Animal Care. No chimpanzee was food deprived to elicit its participation in the present study. To our knowledge, none of these chimpanzees has been language trained. Subjects were nonrandomly selected from the larger colony on the basis of their propensities to engage with experimenters in past studies; this selection procedure was necessitated by time and budgetary constraints on data collection in the present study.
Procedure
One experimenter placed a banana and a small pile of four pieces of commercial primate chow approximately 60 to 70 cm from the extreme left and right sides of each subject’s cage (side of food placement counterbalanced across subjects), 2.5 m apart, and then departed. At the onset of testing, a second experimenter arrived and centered himself or herself approximately 1 m from the front of the subject’s home cage, maintaining visual contact with the subject. This experimenter noted whether or not the subject gestured, vocalized, exhibited cage bangs, attempted to barter detritus for food, or exhibited gaze alternation between either of the food items and the experimenter. This predelivery phase ensued for 30 s (elapsed time was monitored by reference to a handheld stopwatch). It should be noted that during the predelivery phase, the experimenter did not deliver any food to the chimpanzees; hence, the chimpanzees’ communicative attempts will have had no apparent effect on the experimenter’s behavior until after the 30-s observation interval had elapsed. Therefore, the predelivery phase may be seen as one of a relatively protracted interval in which chimpanzees’ attempts to communicate were ineffective.
At the end of the predelivery phase, the experimenter responded in one of three ways, constituting one of three conditions: (a) the experimenter delivered the banana to the chimpanzee (banana condition), (b) the experimenter delivered half of the banana to the chimpanzee and placed the other half in his or her lab coat pocket (half-banana condition), and (c) the experimenter delivered chow to the chimpanzee (chow condition). As in the predelivery phase, the experimenter coded the presence and absence of manual gestures, vocalizations, cage bangs, apparent barter attempts, and gaze alternation between the remaining food items and the experimenter for 30 s after food delivery. With respect to the specific food remaining after delivery, in the postdelivery phase, in the banana condition, the four pieces of chow were still present; in the half-banana condition, the chow was present and half of the banana was located in the experimenter’s lab coat pocket; and in the chow condition, the banana remained in place. The rationale for each of these conditions is as follows. First, delivery of the banana constitutes a successful communication attempt (in our combined experience of 25 years of observations, the authors have never seen a chimpanzee evince a preference for chow over a banana). As such, it serves as the baseline against which responses in the other conditions are compared. Second, delivery of half of the banana constitutes an only partially successful communication attempt. This condition serves as a control condition; we reason that if possession of a banana, by itself, inhibits the emission of communicative behavior (particularly gestural responses), possession of half of a banana also should suppress communicative behavior. Conversely, if possession of half of a banana does not suppress communication, reduced communicative behavior in the banana condition cannot be attributed to the exigencies of holding food. Finally, the chow condition represents a complete failure to communicate. All three conditions were administered to every subject in randomized order across subjects. Each subject received only one trial in each condition; therefore, all results reported here constitute first-trial observations.
The behaviors recorded were whether the subjects (a) gestured (using a well-established coding scheme; e.g., Hostetter et al., 2001; Leavens & Hopkins, 1998); (b) vocalized; (c) banged their cages with an extremity (a noise-making behavior); (d) apparently attempted to barter with the experimenter, offering a piece of detritus or chow for something in the experimenter’s possession (bartering by representatives of this population of chimpanzees was described in detail by Hyatt & Hopkins, 1998); and (e) alternated their gaze between one of the food items and the experimenter (as in our previous studies, when a subject looks between both of two foci and the experimenter, the target of gaze alternation is deemed to be the focus toward which the subject exhibited the preponderance of gaze; see Leavens & Hopkins, 1998; Leavens, Hopkins, et al., 2004). For gestures only, all occurrences of gestures were recorded. For analyses related to persistence, subjects were categorized dichotomously in terms of whether they exhibited a manual gesture. In analyses of elaboration of gesture, subjects were categorized dichotomously in terms of whether they exhibited multiple manual gestures. Note that consummatory vocalizations, which were evident only after delivery of the banana in the banana condition during actual consumption of the banana and which comprise repetitive, energetic squeals, were not coded in any condition; these vocalizations are only exhibited by chimpanzees during actual consumption of highly desired food. The presence and absence of all other vocalizations were recorded (see Leavens, Hostetter, et al., 2004, for further discussion of types of vocalizations). Gestures were characterized as being directed toward the experimenter or toward the banana (no chimpanzee gestured toward the chow). Chimpanzees usually point with the whole hand, (de Waal, 1982; Leavens & Hopkins, 1998, 1999; Leavens et al., 1996), though sometimes they point with the index finger. Pointing with the index finger seems to be the predominant form of pointing for apes who have been subject to language training (Krause & Fouts, 1997; Leavens & Hopkins, 1999). Gestures directed toward the banana in the present study included pointing both with the index finger (0–2 subjects per condition pointed with the index finger) and with the whole hand (the remainder of subjects). Gestures directed toward the experimenter were “begs” and “hold hand out” (e.g., Goodall, 1986). This latter gesture is structurally similar to pointing with the whole hand but directed to a social partner, whereas begs are highly stereotypical gestures common both in the wild and in captivity, and involved extension of the supinated hand, often in a cupped posture, toward an observer.
An issue raised during the review process was why we did not videotape the experiments. The filming conditions in the great ape wing at YNPRC are poor. When filming indoors it is dark, we film through cage mesh, and subjects are often backlit by bright light entering from an open door leading to the outdoor parts of their enclosures. It is difficult to code film taken under these circumstances. When we film from the outdoor portions of the chimpanzees’ enclosures, we film through obscuring cage mesh with the added complication that, if it is particularly sunny, the sunlight reflects from the cage mesh, further obscuring the images of the animals behind the mesh. We have previously evaluated reliability on the coding of these behaviors in three circumstances: (a) both coders coding from videotape (Leavens et al., 1996); (b) one coder coding live on a data sheet, the other coder coding from videotape (Leavens, Hopkins, et al., 2004); and (c) both coders coding live on data sheets simultaneously (e.g., Leavens, Hostetter, et al., 2004). It is clear from the reliability estimates reported in these previous studies that live coding of behavior results in substantially higher reliability estimates than coding from videotapes, given the poor filming conditions at YNRPC. In terms of reliability, live coding is therefore far superior to coding from videotape, a fact that may not be obvious to researchers familiar with typical laboratory facilities for observation of human children, where lighting is generally good and the children are not obscured behind cage mesh. It is a secondary consequence of this approach to data collection that we chose to measure the presence and absence of behaviors rather than frequencies or sequences of behavior; presence and absence of five behaviors can be reliably coded with live scoring, but to score frequencies and sequences of five behaviors places too great a burden on the observer. The measures chosen for analysis are adequate to answer the questions we posed, although we recognize that interesting questions that might be answered with different behavioral measures remain unanswered.
Analyses
All statistical tests were two-tailed with alpha specified at .05. For each phase (predelivery and postdelivery), individuals were characterized as either having exhibited a particular behavior of interest, or not having exhibited that behavior. Thus, these analyses involved repeated observations on dichotomous variables. Cochran’s Q was therefore employed to determine whether these behaviors were randomly or nonrandomly distributed across conditions because it is a nonparametric test appropriate for dichotomous variables in repeated measures designs (Siegel & Castellan, 1988). To assess whether response consequences had any impact on the display of each of the behaviors of interest, binomial tests are reported for all pairwise comparisons involving nonparametric data. Because post hoc comparisons involved multiple statistical tests on the same dependent variables, a correction procedure was applied in all cases so that nominal alpha (.05) was divided by the number of tests performed. In some cases, this resulted in p values that were less than .05 but reported as nonsignificant.
Because the behavioral categories were not mutually exclusive, in any given observation interval chimpanzees could exhibit from zero to four of the behaviors of interest (e.g., a chimpanzee might vocalize and bang the cage, thereby exhibiting two of the behaviors of interest or a chimpanzee might exhibit none of the behaviors of interest, and so on). The number of categories of behavior exhibited served as the dependent variable in a 2 (phase: pre-delivery and postdelivery) × 3 (condition: banana, half-banana, chow) repeated measures analysis of variance (ANOVA). Post hoc comparisons were performed with Bonferroni-corrected paired t tests (i.e., nominal alpha, .05, was divided by the number of post hoc tests).
Reliability
At the insistence of the reviewers, reliability estimates were calculated on an additional four administrations of each of the three experimental conditions. This resulted in 12 predelivery and 12 postdelivery observation intervals, for a total of 24 observations conducted by two observers (Jamie Russell and William Hopkins), who coded behavior on data sheets independently from two different observation posts (one coder was centered on the cage, as in the original experiment, and the other coder stood behind and slightly to one side of the first coder during reliability trials). These 24 observations are equivalent to 14% of the 174 observations in the original study (29 subjects times three predelivery periods times three postdelivery conditions). Because of issues related to restricted animal availability at the time of the reliability trials, we were unable to assess the reliability of the coding scheme with additional subjects. Therefore, the reliability trials did not increase our initial sample size; that is, reliability trials were conducted opportunistically on subjects that happened to be available on any given day. Reliability for the presence and absence of the specific measures related to persistence and elaboration were: gestures, Cohen’s κ = .75; vocalizations, Cohen’s κ = 1.00; cage bangs, Cohen’s κ = 1.00; and barter attempts, Cohen’s κ = 1.00. Hence, reliability on the presence or absence of these behaviors is very high.
Two measures characterize the directionality of behavior: gaze alternation between the experimenter and food, and the target of gestures (either toward the banana or toward the experimenter). Reliability with respect to gaze alternation was calculated in the conditions in which there was high angular separation between the experimenter and the food (banana and chow conditions), for a total of 16 observations (Cohen’s κ = .63). When data were included from the half-banana condition, during which half of a banana was placed into the experimenter’s lab coat pocket and therefore there was little angular separation between the half-banana and the experimenter, reliability decreased (Cohen’s κ = .58). In accordance with the guidelines of Fleiss (1981), kappas of .40 to .60 are characterized as fair, .60 to .75 are good, and >.75 are excellent. Landis and Koch (1977) characterized kappas of .41 to .60 as evidence of moderate agreement, .61 to .80 as substantial agreement, and .81 to 1.00 as almost perfect agreement. Hence, in accordance with these criteria, we have excluded from analysis gaze alternation that occurred during the half-banana condition because reliability falls short of good (Fleiss, 1981) or substantial agreement (Landis & Koch, 1977) when those data are included. Reliability on measures relevant to referentiality of the gestures (i.e., the target of the gestures, either at the banana or the experimenter) were calculated as follows: Given that the observers agreed that a gesture did occur, they further agreed on the target of the gesture 100% of the time (Cohen’s κ = 1.00, 100% agreement). As for gaze alternation, there is, however, an ambiguity in the target of a gesture exhibited toward an experimenter who had half of a banana in his or her lab coat pocket; therefore, gestures exhibited in the half-banana condition were excluded from analyses of the target location of gestures. To put this another way, we do not wish to assert that we believe the present research design is adequate to discriminate reliably between gaze or gesture directed to either (a) an experimenter or (b) half of a banana that is located in that same experimenter’s pocket. Although we do believe that high reliability on these measures could be obtained with different data-collection techniques, these measures are not central to the research questions posed here (i.e., whether chimpanzees in the half-banana condition gaze at or gesture at the experimenter or the half-banana in the experimenter’s pocket is irrelevant to the question of whether chimpanzees persist in or elaborate their communicative bids). On the other hand, reliability of the target of gaze or gesture in the conditions in which there is high angular separation between the banana and the experimenter (the banana and chow conditions) is high.
Results
Predelivery
Frequency distributions for all four behaviors in both predelivery and postdelivery phases are depicted in Figure 1. All four behaviors were distributed randomly across conditions in the predelivery phase: for gestures, Cochran’s Q(2, N = 29) = 2.67, p = .26; for vocalizations, Cochran’s Q(2, N = 29) = 2.33, p = .31; for cage bangs, Cochran’s Q(2, N = 29) = 3.46, p = .18; and for barter attempts, Cochran’s Q(2, N = 29) = 2.33, p = .31. The significance of this pattern of results is that the chimpanzees apparently did not discriminate between the conditions before food delivery, suggesting that the differences reported in the postdelivery conditions were attributable to the different response consequences.
Figure 1.
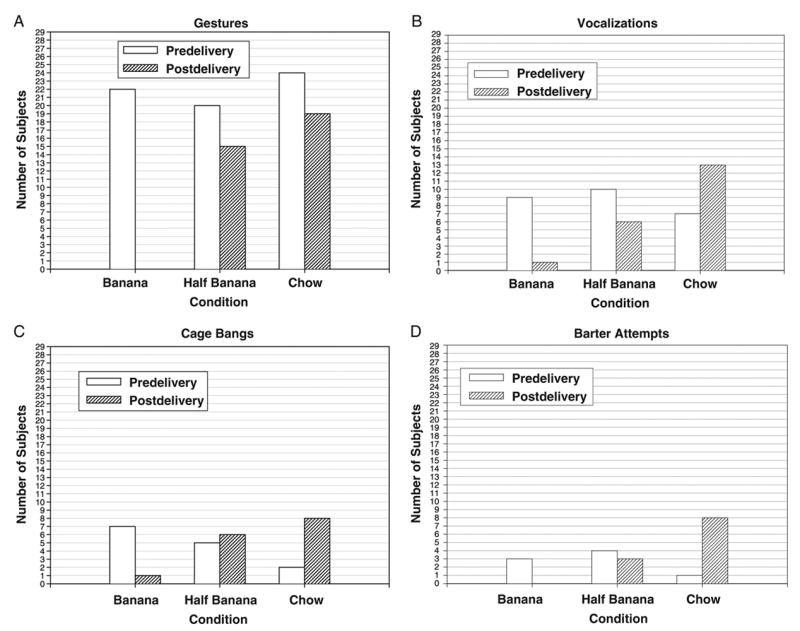
Frequency distributions for the communicative behaviors by phase and condition for 29 chimpanzees.
Postdelivery
In contrast, all four behaviors were nonrandomly distributed across the three conditions in the post-delivery phase: for gestures, Cochran’s Q(2, N = 29) = 28.67, p < .001; for vocalizations, Cochran’s Q(2, N = 29) = 16.77, p < .001; for cage bangs, Cochran’s Q(2, N = 29) = 6.50, p = .039; and for barter attempts, Cochran’s Q(2, N = 29) = 10.89, p = .004. Thus, in every behavior recorded during the postdelivery phase, the chimpanzees exhibited a nonrandom pattern of communication, and visual inspection of Figure 1 demonstrates the substantially higher propensity to communicate in the half-banana and chow conditions relative to the banana condition.
It is important to note that, relative to the predelivery phase, delivery of half of the banana had no apparent suppressive effect on any of the behaviors of interest during the postdelivery phase (binomial tests: gestures, p = .125; vocalizations, p = .125; cage bangs, p = 1.00; barter attempts, p = 1.00—all pairwise tests corrected for multiple comparisons with the Bonferroni procedure; i.e., nominal alpha, .05, was divided by 3, corresponding to the three comparisons of the same dependent variable across the three postdelivery experimental conditions; hence, corrected alpha for these tests = .017). Thus, possession of half of a banana in no way inhibited the display of any of the behaviors of interest. Parallel results were obtained in the chow condition (binomial tests with corrected alpha = .017: gestures, p = .063; vocalizations, p = .070; cage bangs, p = .070; barter attempts, p = .039); hence, possession of chow did not inhibit any of the behaviors recorded. In contrast, the chimpanzees exhibited marked suppression of gestural and vocal behavior when the banana was delivered (binomial tests: gestures, p < .001; vocalizations, p = .008), although this pattern was not statistically significant for the less numerous cage bangs and bartering attempts (binomial tests: cage bangs, p = .070; barter attempts, p = .25). In fact, no chimpanzee exhibited a manual gesture after delivery of the banana.
Elaboration
For analysis of gestural elaboration, subjects were dichotomously classified as having either exhibited multiple manual gestures or not (range = 0 to 4 manual gestures per subject). Because no chimpanzee gestured in the postdelivery phase of the banana condition, there was no variance and hence we performed a nonparametric analysis. In the three predelivery phases, the proportion of chimpanzees who exhibited multiple gestures was randomly distributed, Cochran’s Q(2, N = 29) = 2.33, p = .311 (Figure 2). In contrast, the proportion of individuals who exhibited multiple manual gestures was non-randomly distributed in the postdelivery, Cochran’s Q(2, N = 29) = 14.92, p = .001. Systematic binomial post hoc tests, corrected for multiple comparisons, reveal that significantly more chimpanzees exhibited multiple gestures after delivery of the chow, compared with the banana and half-banana conditions (banana vs. half-banana, p = .250; banana vs. chow, p = .001; half-banana vs. chow, p = .016; please note that the critical value of p is .017 with Bonferroni correction).
Figure 2.
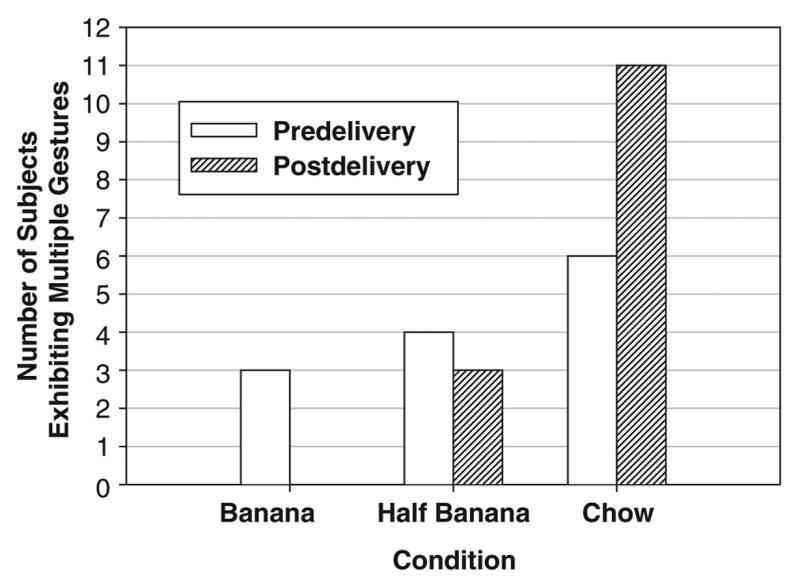
Number of subjects exhibiting multiple manual gestures by phase and condition for 29 chimpanzees.
A 2 (phase) × 3 (condition) repeated measures ANOVA, in which the number of different kinds of behaviors (which could vary from zero to four; thus, if a subject gestured, vocalized, banged its cage, and attempted to barter with the experimenter, it would exhibit four different kinds of behavior) served as the dependent variable, revealed a main effect for phase, F(1, 28) = 15.91, p < .001; partial η2 = .362, such that there were more communicative behaviors exhibited in the predelivery than in the postdelivery phase. There was also a main effect for condition, F(2, 56) = 14.24, p < .001; partial η2 = .337, such that there were fewer communicative behaviors exhibited during the banana condition than during both the half-banana and chow conditions, but the latter two conditions did not differ from each other (i.e., pairwise comparisons: banana and half-banana conditions, p = .001; banana and chow conditions, p < .001; half-banana and chow conditions, p = .085). This demonstrates that the chimpanzees exhibited more elaborate communicative behavior in the half-banana and chow conditions than in the banana condition. Finally, there was a Phase × Condition interaction, F(2, 56) = 23.27, p < .001; partial η2 = .454, which is depicted in Figure 3. Paired t tests, adjusted for multiple comparisons (alpha, .05, divided by 3; corrected alpha = .017), confirmed that there was no apparent suppression of the number of different kinds of communicative behaviors exhibited after delivery of half of a banana compared with the predelivery phase of the half-banana condition, t(28) = 1.61, p = .119, and parallel results were obtained for the chow condition, t(28) = − 2.46, p = .020. Note that this last t statistic is not significant under the Bonferroni procedure for which the critical value of t = 2.55; if no Bonferroni procedure were adopted, this would demonstrate a significant increase in the elaboration of communication in the chow condition. In contrast, after delivery of the banana, chimpanzees exhibited marked suppression in the number of different behaviors exhibited: t(28) = 8.45, p < .001. Thus, by this aggregate measure, as for the individual response types reported earlier, possession of half of a banana or chow apparently did not suppress communication in this study, suggesting that the suppression in communicative behavior observed in the banana condition is not attributable to the mechanics of handling food but rather to the chimpanzees’ perceived success in obtaining the desired communicative outcome.
Figure 3.

Interaction between phase and condition in the number of kinds of communicative behaviors exhibited by 29 chimpanzees. Relative to successful communicative bids (banana condition, postdelivery phase), the chimpanzees exhibited elaborated communicative bids in the postdelivery phases of the half-banana and chow conditions.
Gaze Alternation
To demonstrate that the behaviors reported here are consistent with established behavioral criteria for intentional communication, we analyzed the subjects’ gaze-orienting behavior. Because the undelivered half of each banana in the postdelivery phase of the half-banana condition was in the lab coat of the experimenter, with a relatively small angular displacement between the face of the experimenter and the undelivered half of the banana, and because reliability estimates on gaze alternation were fairly modest when that condition is included (see the Method section), we did not analyze data on gaze alternation in this condition. Gaze alternation was nonrandomly distributed across the experimental conditions: Cochran’s Q(3, N = 29) = 57.22, p < .001. Figure 4 depicts the percentage of subjects who exhibited gaze alternation in these four observational periods. Post hoc tests comparing the predelivery and postdelivery phases of the banana and chow conditions reveals what is obvious from Figure 4: (a) there is a substantial reduction in gaze alternation in the postdelivery phase, relative to the predelivery phase, of the banana condition (binomial test, p < .001), and (b) there is no significant difference in the display of gaze alternation in the predelivery and postdelivery phases of the chow condition (binomial test, p = .688). Only 1 subject (Suwannee) exhibited gaze alternation between the chow and the experimenter in any condition (postdelivery phase of the banana condition); otherwise, the subjects alternated their gaze between the banana and the experimenter. Thus, delivery of the chow had no influence on gaze-alternating behavior, but delivery of the banana resulted in a near-total cessation of this activity. Hence, communication in this experimental context is about the banana, using the same operational measure employed in studies of human infant communication.
Figure 4.
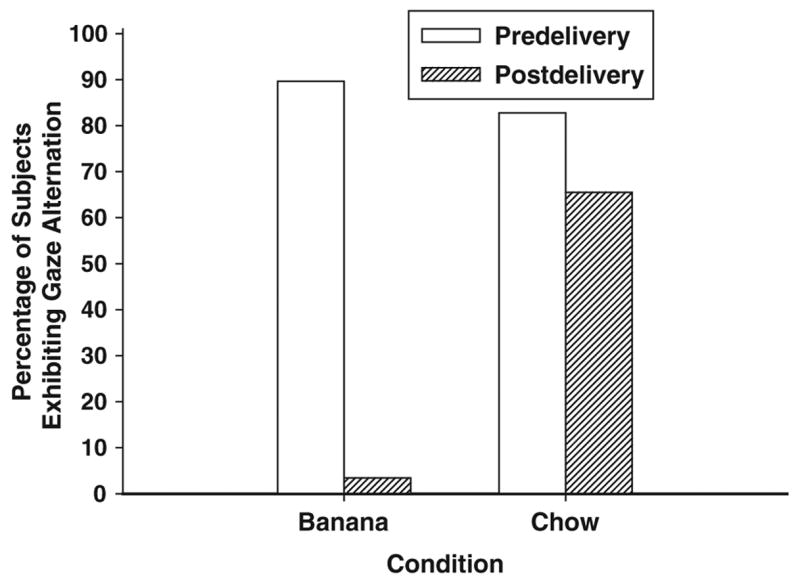
Percentage of chimpanzees that exhibited gaze alternation between food and the experimenter in the banana and chow conditions. Only one subject exibited gaze alternation between the experimenter and the chow (Suwannee in the postdelivery phase of the banana condition). Data on gaze alternation from the half-banana condition are excluded because of relatively modest interobserver reliability estimates in this condition (see the Method section).
Referentiality
Finally, we examined the target of the chimpanzees’ gestures to establish the referential nature of these communicative bids. We reasoned as follows: If chimpanzees’ communicative bids are about a specific food item (i.e., the banana), delivery of the chow in the chow condition should not influence the target of gestures (the banana) relative to any of the predelivery phases. Because no chimpanzee gestured in any direction after delivery of the banana and because in the postdelivery phase of the half-banana condition the banana and the experimenter occupy the same distance and angular displacement from the subject, no comparison of target of gestures is meaningful in the postdelivery phases of the banana and half-banana condition. Sixteen chimpanzees gestured toward the banana in each of the predelivery phases of the banana, half-banana, and chow conditions, representing 73%, 80%, and 67% of all gestures exhibited, respectively (all other gestures were directed toward the experimenter). In the postdelivery phase of the chow condition, 63% of the subjects who gestured, gestured toward the banana. There was no significant difference in the number of individuals who gestured toward the banana in these four phases, Cochran’s Q(3, N = 29) = 2.88, p = .410. Thus, on average, 71% of the chimpanzees’ manual gestures were directed toward the banana, and delivery of the chow did not suppress the display of these manifestly referential gestures, indicating that these communicative bids are truly about a specific food item. This is a higher incidence of pointing than we have seen in previous studies (Table 3).
Table 3.
Percentage of Subjects That Pointed in Several Studies of Chimpanzee Gestural Communication
| N (Subjects) | N (Gestured) | N (At food) | % (At food, point) | |
|---|---|---|---|---|
| Leavens & Hopkins (1998) | 115 | 78 | 53 | 68 |
| Leavens, Hopkins, & Thomas (2004) | ||||
| Visible banana condition | 101 | 68 | 28 | 41 |
| Hidden banana condition | 101 | 66 | 32 | 48 |
| Experiment 2 | 35 | 14 | 6 | 43 |
| Present study | ||||
| Predelivery | ||||
| Banana condition | 29 | 22 | 16 | 73 |
| Half-banana condition | 29 | 20 | 17 | 80 |
| Chow condition | 29 | 24 | 17 | 67 |
| Postdelivery | ||||
| Banana condition | 29 | 0 | 0 | 0 |
| Half-banana condition | 29 | 15 | —a | —a |
| Chow condition | 29 | 19 | 13 | 63 |
It is ambiguous whether gestures exhibited in the postdelivery phase of the half-banana condition are under the stimulus control of the food or the experimenter, or both (because the half-banana retained by the experimenter and the experimenter himself occupy the same locus); therefore, no data on target of gesture are reported for this phase.
Discussion
Four substantive conclusions are warranted by these data. First, the chimpanzees persisted in communication when faced with only partial success or complete failure in their communicative attempts (Figure 1). Second, the chimpanzees elaborated their communicative repertoires in the face of both partial success and complete failure to achieve their putative communicative goals (Figures 2 and 3). Third, the communicative behaviors exhibited by these chimpanzees were about the banana, as evidenced by their gaze-orienting behavior (Figure 4). Finally, the chimpanzees gestured predominantly toward the banana in each of the three predelivery phases and after delivery of the chow, demonstrating that their manual gestures are about specific items in the environment (i.e., the chimpanzees pointed; Table 3). These findings are all the more striking because each subject experienced each experimental condition only one time. These chimpanzees clearly communicated about a specific food item, the banana. Persistence and elaboration of communicative bids by these chimpanzees add substantially to the number of objective behavioral criteria for intentional communication already demonstrated to exist in the communicative repertoire of this species. These are the same criteria by which we deem human babies to have made the transition to intentional communication. Previous studies have demonstrated the necessity of an audience (Leavens et al. 1996; Leavens, Hopkins, et al., 2004; Table 1), sensitivity to the attentional status of a human observer (Hostetter et al., 2001; Leavens, Hostetter, et al., 2004), high rates of concomitant gaze alternation between food and an experimenter while gesturing (Leavens & Hopkins, 1998; Leavens et al., 1996; Leavens, Hopkins, et al., 2004; Table 2), and tactical deployment of attention-getting behavior (Leavens, Hostetter, et al., 2004). The aboutness that characterizes intentional communication in humans, and as measured by these objective behavioral criteria, is present in the non-verbal communication of these chimpanzees. It is as if these chimpanzees had told us, “No, not the chow, you idiot, the banana!” There is very little more a nonlinguistic organism can do to disambiguate the particular topic of a communicative bid than these chimpanzees have done in the present study.
With respect to the proportion of chimpanzees that pointed, Table 3 lists the percentage of subjects that pointed in the present study compared with the percentage of chimpanzees that pointed in several recent studies in our laboratory, considered in relation to the total number of subjects that exhibited manual gestures in all of these studies. Compared with these previous studies, the present study has elicited the highest rates of pointing. It should be emphasized that because we selected subjects on the basis of past propensities to communicate with experimenters, it is ambiguous whether it was (a) this selection procedure, (b) features of the present experimental procedure, or (c) a combination of these factors that accounts for this high rate of pointing. With respect to procedural factors, in previous studies only one food item was presented during any given trial, whereas in the present study, two different food types were presented at a wide angular separation. Thus, the presence of the less desirable chow may elicit more explicit reference from the chimpanzees. Future studies in which the specificity of pointing is measured within subjects and across conditions in which two types of food are presented compared with other conditions in which only one of the two types is presented will clarify this issue. Nevertheless, in accordance with the most general definition of the term reference, meaning “to draw attention to,” these chimpanzees exhibited referential communication; their pointing identified a specific item in the context of multiple items (e.g., Leavens, Hopkins, et al., 2004). This does not constitute symbolic reference or semantic reference (e.g., Cheney & Seyfarth, 1990) and therefore does not demonstrate the same kind of psychological relation implied between an arbitrary signal and its referent. It does demonstrate, however, that the capacity for nonverbal reference, defined as the ability to direct the attention of a social partner to some specific entity without language (e.g., Adamson, 1996; Bates et al., 1987), emerges spontaneously in captive chimpanzees with no explicit training.
The six observation intervals depicted in Figures 2 and 3 can be characterized as one interval of successful communication (banana: postdelivery), one interval of partial success (half-banana: postdelivery), and four additional intervals of communicative failure (all three predelivery intervals and the chow: postdelivery interval). Post hoc analyses of the distribution of results revealed that there was no statistically significant difference between the partially successful interval (half-banana: postdelivery) and the other intervals characterized by communicative failure. The chimpanzees, therefore, exhibited two states: (a) persistence in and elaboration of communication in the face of failure (i.e., intentional communication) and (b) consummatory behavior when the banana was delivered.
Virtually all laboratory studies of the communicative competence of apes and humans suffer from a lack of knowledge about these organisms’ preexperimental histories. For the most part, researchers can only estimate (rather than precisely characterize) the degree to which a change in experimental conditions constitutes a presentation of a novel context. This widespread ignorance also pertains to studies of communication by human babies. It is therefore typically impossible to characterize adequately the amount of task-relevant experience organisms as long lived as humans and apes bring to any given experimental context (e.g., Leavens et al., 1996; Leavens, Hopkins, et al., 2004; Leavens, Hostetter, et al., 2004). What we can say is that by the time we administered these experiments to these chimpanzees, they had already developed tactics of repetition and elaboration to repair failed communication—this conclusion is warranted by the fact that no chimpanzee in this study experienced more than one trial in any one condition in the present experiment. This interpretation is consistent with long-term observational studies of both captive and wild chimpanzees interacting among themselves (de Waal, 1982; Goodall, 1986).
These data constitute the first experimental evidence in any ape species for repair of communication in the face of communication breakdowns (in which experimenters purposefully misinterpreted the chimpanzees’ communicative signals) by chimpanzees who have not been subject to language training. Furthermore, these data demonstrate that the capacity for nonverbal reference, for communication about a specific item—long considered unique to humans (e.g., see Adamson, 1996, p. 140)—emerges easily in captive populations of chimpanzees with no special training. To the extent that nonverbal reference, as exemplified, for example, by pointing, may help human infants form a bridge to verbal and other symbolic reference (e.g., Baldwin, 1995; Butterworth, 2003), these data suggest that the evolutionary origins of this capacity predate the last common ancestor of humans and chimpanzees, approximately 7 million years ago (Leavens, 2003). Future research designed to clarify how caregiving practices shape joint attentional competencies in humans and apes may illuminate the evolutionary history of the capacity for nonverbal reference with possible implications for constraining theories of the evolution of symbolic reference. Specifically, because the ability to acquire words for objects may require the foundational capacity to direct and follow attention to these objects (Baldwin, 1995) and because these joint attentional competencies emerge so easily in our nearest living relatives in the absence of overt training, the human capacity for nonverbal reference is probably not derived solely from human species-specific cognitive or neurophysiological adaptions for speech but instead reflects a shared behavioral capacity with great apes (e.g., Leavens, 2003, in press; Leavens & Hopkins, 1998; Leavens et al., 1996; Leavens, Hopkins, et al., 2004). This is not to claim that it is completely implausible to suggest that human babies and representatives of our nearest living relatives, the chimpanzees, exhibit similar kinds of behaviors in joint attention contexts that are predicated on unrelated cognitive and neurophysiological processes; rather, we suggest that this latter claim is simply less parsimonious than the interpretation that two very similar patterns of activity in two very closely related organisms are derived from shared (homologous) adaptive histories (cf. Suddendorf & Whiten, 2001). It is far more parsimonious to suggest that pointing, predicated as it is on the shared anatomical substrates of humans and chimpanzees (both species have similar body plans; both species have dextrous fingers), is elicited by epigenetic processes (predicated on homologous neurobehavioral adaptations) than it is to suggest that two entirely different adaptive histories account for very similar deployments of the same suite of anatomical features in two closely related species.
This claim is rendered more plausible by the fact that apparent pointing behavior is rare in wild populations of apes (e.g., Leavens & Hopkins, 1999; the only reported instances of pointing in wild ape populations, to our knowledge, are those by Inoue-Nakamura & Matsuzawa, 1997, and Veá & Sabater-Pi, 1998, for chimpanzees and bonobos, respectively). Hence, a further implication of the repeated observations that pointing emerges spontaneously in captive ape populations (Call & Tomasello, 1994; Leavens & Hopkins, 1998; Leavens et al., 1996; Leavens, Hopkins, et al., 2004; the present study) is that as yet poorly specified aspects of captive environments facilitate pointing in apes (Leavens, in press). Thus, if pointing is so variably expressed in chimpanzees (rare in the wild, common in captivity), chimpanzees constitute important animal models for our understanding of how genetic and behavioral adaptations interact to produce organisms capable of discriminating visual attention, capturing visual attention, and manipulating visual attention in their social partners, in the absence of any explicit training.
Two recent hypotheses have been put forth to account for the development of pointing behavior in captive apes. Call and Tomasello (1994, 1996; Tomasello & Call, 1997) hypothesized that through continued close association with human caregivers, captive apes achieve an insight that their caregivers have independent agency (i.e., are intentional beings). According to this Promethean hypothesis, “human-raised apes understand the intentions of others in ways that their wild conspecifics do not” (Tomasello & Call, 1997, p. 393). In other words, exposure to humans invests captive apes with a novel cognitive capacity, the capacity to recognize and manipulate simple intentional states in others.
In a competing hypothesis, the social tool use hypothesis, pointing by apes reflects the cognitive capacity for means–ends reasoning (coordinated secondary circular reactions or tertiary circular reactions, in Piagetian terminology; e.g., Bard, 1990, 1992; Leavens et al., 1996). According to this hypothesis, captive apes will have had long experience with humans as “food-delivery machines”; that is, they will have experienced so many instances in which caregivers have delivered food that when faced with a situation in which both desirable, but unreachable, food and a human are present, pointing emerges as a simple solution to a common problem faced in captivity (but not in the wild). This solution involves coordinating means (human observer) with an end (obtaining the food); in other words, pointing reflects the same cognitive capacities employed when using one object (e.g., a stick) to obtain another object (e.g., food). The ability to use a tool to retrieve otherwise unreachable food is commonplace in both wild and captive ape populations (e.g., Goodall, 1986; Köhler, 1925), and according to the social tool use hypothesis, apes simply generalize their excellent problem-solving capacities to the social domain.
Both the Promethean and social tool use hypotheses are viable on the present evidence and both have implications for our understanding of human cognitive development. According to the Promethean hypothesis, human species-specific caregiving practices inculcate the abstract concept of intentionality in young humans (and captive apes), and this concept permits flexible manipulation of social partners. According to the social tool use hypothesis, intentional communication reflects cognitive capacities for problem solving through tool use, which are shared by both humans and apes and generalized to the social domain in particular contexts that facilitate this generalization. For captive apes these contexts involve physical barriers to obtaining desirable objects, whereas for human infants these contexts involve endogenous limitations on their ability to locomote to graspable proximity with desirable objects (Leavens et al., 1996). (As this article was going to press, we became aware that Tomasello and Call (2004) have substantially amended their hypothesis: they now conclude that association with humans does not induce novel discriminative capacities in captive chimpanzees, at least with respect to the discrimination of simple intentional and attentional cues in others; that is, they now argue now that most reasonably normal rearing circumstances are sufficient to elicit these simple discriminations in chimpanzees).
In summary, to the extent that indicating a specific item through manual gesture constitutes evidence for the aboutness that defines intentional communication, the present study demonstrates that chimpanzees in captivity with no special training exhibit this skill. The present data, which are based on only one trial of observation per experimental condition, do not permit identification of the learning process by which chimpanzees come to direct the attention of observers (see Leavens, Hopkins, et al., 2004, for an extended discussion of this issue). Nevertheless, the present data refute previous claims to the effect that pointing is a uniquely human behavior (Donald, 1991) or requires explicit training of apes (e.g., Corballis, 2003). To previous demonstrations that chimpanzee gestural communication exhibits the defining criteria of sensitivity to the presence of an audience, audience visual attention, accompaniment by gaze alternation between food and a human observer, and context-appropriate display of attention-getting vocalizations, the present study adds the demonstration that persistence and elaboration of communication are displayed by chimpanzees in the face of communicative failures.
Acknowledgments
We thank Autumn B. Hostetter, Berry College, for assistance with data collection. We thank Kim A. Bard, University of Portsmouth, and several anonymous reviewers for helpful comments on the design and interpretation of this study. We thank Vasudevi Reddy, also of the University of Portsmouth, for her generous and scholarly guidance to the human developmental literature. This study was funded by National Institutes of Health Grants NS-36606 and NS-42867 to William D. Hopkins and RR-00165 to the Yerkes National Primate Research Center.
Contributor Information
David A. Leavens, University of Sussex
Jamie L. Russell, Yerkes National Primate Research Center
William D. Hopkins, Yerkes National Primate Research Center and Berry College
References
- Adamson LR. Communication development during infancy. Boulder, CO: Westview; 1996. [Google Scholar]
- Adamson LR, Bakeman R. Affect and attention: Infants observed with mothers and peers. Child Development. 1985;56:582–593. [Google Scholar]
- American Psychological Association. Ethical principles of psychologists and code of conduct. American Psychologist. 1992;47:1597–1611. [Google Scholar]
- Bakeman R, Adamson LB. Infants’ conventionalized acts: Gestures and words with mothers and peers. Infant Behavior and Development. 1986;9:215–230. [Google Scholar]
- Baldwin DA. Understanding the link between joint attention and language. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 131–158. [Google Scholar]
- Bard KA. “Social tool use” by free-ranging orangutans: A Piagetian and developmental perspective on the manipulation of an animate object. In: Parker ST, Gibson KR, editors. “Language” and intelligence in monkeys and apes: Comparative developmental perspectives. Cambridge, England: Cambridge University Press; 1990. pp. 356–378. [Google Scholar]
- Bard KA. Intentional behavior and intentional communication in young free-ranging orangutans. Child Development. 1992;62:1186–1197. [PubMed] [Google Scholar]
- Baron-Cohen S. The evolution of a theory of mind. In: Corballis MC, Lea SEG, editors. The descent of mind: Psychological perspectives on hominid evolution. Oxford, England: Oxford University Press; 1999. pp. 261–277. [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The emergence of symbols: Cognition and communication in infancy. New York: Academic Press; 1979. [Google Scholar]
- Bates E, Camaioni L, Volterra V. Performatives prior to speech. Merrill-Palmer Quarterly. 1975;21:205–226. [Google Scholar]
- Bates E, O’Connell B, Shore C. Language and communication in infancy. In: Osofsky J, editor. Handbook of infant development. New York: Wiley; 1987. pp. 149–203. [Google Scholar]
- Bodamer MD, Gardner RA. How cross-fostered chimpanzees (Pan troglodytes) initiate and maintain conversations. Journal of Comparative Psychology. 2002;116:12–26. doi: 10.1037/0735-7036.116.1.12. [DOI] [PubMed] [Google Scholar]
- Butterworth G. Joint visual attention in infancy. In: Bremner JG, Fogel A, editors. Blackwell handbook of infant development. Oxford, England: Blackwell; 2001. pp. 213–240. [Google Scholar]
- Butterworth G. Pointing is the royal road to language for babies. In: Kita S, editor. Pointing: Where language, culture, and cognition meet. Mahwah, NJ: Erlbaum; 2003. pp. 9–33. [Google Scholar]
- Butterworth G, Itakura S. How the eyes, head and hand serve definite reference. British Journal of Developmental Psychology. 2000;18:25–50. [Google Scholar]
- Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) Journal of Comparative Psychology. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. The effect of humans on the cognitive development of apes. In: Russon AE, Bard KA, Parker ST, editors. Reaching into thought: The minds of the great apes. Cambridge, England: Cambridge University Press; 1996. pp. 371–403. [Google Scholar]
- Camaioni L. Early language. In: Bremner G, Fogel A, editors. Blackwell handbook of infant development. Oxford, England: Blackwell; 2001. pp. 404–426. [Google Scholar]
- Camaioni L, Perucchini P, Bellagamba F, Colonnesi C. The role of declarative pointing in developing a theory of mind. Infancy. 2004;5:291–308. [Google Scholar]
- Carroll SB. Genetics and the making of Homo sapiens. Nature. 2003;422:849–857. doi: 10.1038/nature01495. [DOI] [PubMed] [Google Scholar]
- Cazden CB. The question of intent. In: Lewis M, Rosenblum LA, editors. Interaction, conversation, and the development of language. New York: Wiley; 1977. pp. 309–313. [Google Scholar]
- Cheney DL, Seyfarth RM. How monkeys see the world. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Corballis MC. From mouth to hand: Gesture, speech, and the evolution of right-handedness. Behavioral and Brain Sciences. 2003;26:199–260. doi: 10.1017/s0140525x03000062. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. Chimpanzee politics: Power and sex among apes. New York: Harper & Row; 1982. [Google Scholar]
- Doherty MJ, Anderson JR. People don’t keep their heads still when looking to one side and other people can tell. Perception. 2001;30:765–767. doi: 10.1068/p2998. [DOI] [PubMed] [Google Scholar]
- Donald M. Origins of the modern mind: Three stages in the evolution of culture and cognition. Cambridge, MA: Harvard University Press; 1991. [Google Scholar]
- Dunham PJ, Dunham F. Optimal social structures and adaptive infant development. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 159–188. [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. New York: Wiley; 1981. [Google Scholar]
- Franco F, Butterworth G. Effects of social variables on the production of infant pointing; Poster presented at the fourth European Conference on Developmental Psychology; University of Stirling. 1990. Aug, [Google Scholar]
- Franco F, Butterworth G. Pointing and social awareness: Declaring and requesting in the second year. Journal of Child Language. 1996;23:307–336. doi: 10.1017/s0305000900008813. [DOI] [PubMed] [Google Scholar]
- Furness WH. Observations on the mentality of chimpanzees and orangutans. Proceedings of the American Philosophical Society. 1916;55:281–290. [Google Scholar]
- Golinkoff RM. “I beg your pardon?”: The preverbal negotiation of failed messages. Journal of Child Language. 1986;13:455–476. doi: 10.1017/s0305000900006826. [DOI] [PubMed] [Google Scholar]
- Golinkoff RM. When is communication a “meeting of minds”? Journal of Child Language. 1993;20:199–207. [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Belknap Press; 1986. [Google Scholar]
- Hopkins WD, Leavens DA. Hand use and gestural communication in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter AB, Cantero M, Hopkins WD. Differential use of vocal and gestural communication in response to the attentional status of a human. Journal of Comparative Psychology. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt CW, Hopkins WD. Interspecies object exchange: Bartering in apes? Behavioural Processes. 1998;42:177–187. doi: 10.1016/s0376-6357(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Inoue-Nakamura N, Matsuzawa T. Development of stone tool use by wild chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1997;111:159–173. doi: 10.1037/0735-7036.111.2.159. [DOI] [PubMed] [Google Scholar]
- Köhler W. The mentality of apes. London: Routledge and Kegan Paul; 1925. [Google Scholar]
- Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: Hand shapes, accuracy, and the role of eye gaze. Journal of Comparative Psychology. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Leavens DA. On the public nature of communication. Behavioral and Brain Sciences. 2002;25:630–631. [Google Scholar]
- Leavens DA. Integration of visual and vocal communication: Evidence for Miocene origins. Behavioral and Brain Sciences. 2003;26:232–233. [Google Scholar]
- Leavens DA. Book review of S. Kita (Ed.), Pointing: Where language, culture and cognition meet. Cognitive Systems Research. 2004;5:157–165. [Google Scholar]
- Leavens DA. Manual deixis in apes and humans. Interaction Studies in press. [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. The whole hand point: The structure and function of pointing from a comparative perspective. Journal of Comparative Psychology. 1999;113:417–425. doi: 10.1037/0735-7036.113.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Thomas RK. Referential communication by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2004;118:48–57. doi: 10.1037/0735-7036.118.1.48. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD. Tactical use of unimodal and bimodal communication by chimpanzees (Pan troglodytes) Animal Behaviour. 2004;67:467–476. [Google Scholar]
- Leavens DA, Todd BK. Audience effects on infant communication. 2002. Unpublished raw data. [Google Scholar]
- Legerstee M, Barillas Y. Sharing attention and pointing to objects at 12 months: Is the intentional stance implied? Cognitive Development. 2003;18:91–110. [Google Scholar]
- Leung EHL, Rheingold HL. Development of pointing as a social gesture. Developmental Psychology. 1981;17:215–220. [Google Scholar]
- Liszkowski U, Carpenter M, Henning A, Striano T, Tomasello M. Twelve-month-olds point to share attention and interest. Developmental Science. 2004;7:297–307. doi: 10.1111/j.1467-7687.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Lock A. Preverbal communication. In: Bremner JG, Fogel A, editors. Blackwell handbook of infant development. Oxford, England: Blackwell; 2001. pp. 379–403. [Google Scholar]
- Moore C, Corkum V. Social understanding at the end of the first year of life. Developmental Review. 1994;14:349–372. [Google Scholar]
- Moore C, Corkum V. Infant gaze following based on eye direction. British Journal of Developmental Psychology. 1998;16:495–503. [Google Scholar]
- O’Neill DK. Two-year-old children’s sensitivity to a parent’s knowledge state when making requests. Child Development. 1996;67:659–677. [Google Scholar]
- Pika S, Liebal K, Tomasello M. Gestural communication in young gorillas (Gorilla gorilla): Gestural repertoire, learning, and use. American Journal of Primatology. 2003;60:95–111. doi: 10.1002/ajp.10097. [DOI] [PubMed] [Google Scholar]
- Pika S, Liebal K, Tomasello M. The gestural repertoire of bonobos (Pan paniscus): Flexibility and use. American Journal of Primatology. doi: 10.1002/ajp.20096. in press. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Bering JM, Giambrone S. Toward a science of other minds: Escaping the argument by analogy. Cognitive Science. 2000;24:509–541. [Google Scholar]
- Povinelli DJ, Bering JM, Giambrone S. Reasoning about beliefs: A human specialization? Child Development. 2001;72:691–695. doi: 10.1111/1467-8624.00307. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Bering JM, Giambrone S. Chimpanzee’s “pointing”: Another error of the argument by analogy? In: Kita S, editor. Pointing: Where language, culture, and cognition meet. Mahwah, NJ: Erlbaum; 2003. pp. 35–68. [Google Scholar]
- Povinelli DJ, Davis DR. Differences between chimpanzees (Pan troglodytes) and humans (Homo sapiens) in the resting state of the index finger: Implications for pointing. Journal of Comparative Psychology. 1994;108:134–139. doi: 10.1037/0735-7036.108.2.134. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. What young chimpanzees know about seeing. Monographs of the Society for Research in Child Development. 1996;62 3, Serial No. 247. [PubMed] [Google Scholar]
- Povinelli DJ, Giambrone S. Inferring other minds: Failure of the argument by analogy. Philosophical Topics. 1999;27:167–201. [Google Scholar]
- Rolfe L. Theoretical stages in the prehistory of grammar. In: Lock A, Peters CR, editors. Handbook of human symbolic evolution. Oxford, England: Blackwell; 1999. pp. 776–792. Original work published 1996. [Google Scholar]
- Siegel S, Castellan J. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill; 1988. [Google Scholar]
- Suddendorf T, Whiten A. Mental evolution and development: Evidence for secondary representation in children, great apes, and other animals. Psychological Bulletin. 2001;127:629–650. doi: 10.1037/0033-2909.127.5.629. [DOI] [PubMed] [Google Scholar]
- Sugarman S. The development of preverbal communication: Its contribution and limits in promoting the development of language. In: Scheifelbush RL, Pickar J, editors. The acquisition of communicative competence. Baltimore, MD: University Park Press; 1984. pp. 23–67. [Google Scholar]
- Tomasello M. Joint attention as social cognition. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 103–130. [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Tomasello M, Call J. Primate cognition. Oxford, England: Oxford University Press; 1997. [Google Scholar]
- Tomasello M, Call J. The role of humans in the congnitive development of apes revisited. Animal Cognition. 2004;7:213–215. doi: 10.1007/s10071-004-0227-x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin K, Carpenter M. The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates. 1994;35:137–154. [Google Scholar]
- Tomasello M, Call J, Warren J, Frost T, Carpenter M, Nagell K. The ontogeny of chimpanzee gestural signals: A comparison across groups and generations. Evolution of Communication. 1997;1:223–253. [Google Scholar]
- Tomasello M, Hare B, Agnetta B. Chimpanzees, Pan troglodytes, follow eye gaze geometrically. Animal Behaviour. 1999;58:769–777. doi: 10.1006/anbe.1999.1192. [DOI] [PubMed] [Google Scholar]
- Veá JJ, Sabater-Pi J. Spontaneous pointing behaviour in the wild pygmy chimpanzee (Pan paniscus) Folia Primatologica. 1998;69:289–290. doi: 10.1159/000021640. [DOI] [PubMed] [Google Scholar]


